Recent progress in metal-functionalized germanotungstates: from structures to properties
Yanzhou Li
,
Jie Luo
,
Lijuan Chen
* and
Junwei Zhao
*
Henan Key Laboratory of Polyoxometalate Chemistry, Institute of Molecular and Crystal Engineering, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, Henan 475004, P. R. China. E-mail: zhaojunwei@henu.edu.cn; ljchen@henu.edu.cn; Fax: +86 371 23886876
First published on 23rd September 2014
Abstract
As an important ramification of polyoxometalates, germanotungstate (GT) chemistry has gradually developed as a new research focus and a challenging area, and has made great progress in the past decade due to a wide range of potential applications in various areas such as catalysis, magnetism, photochemistry and materials science. In this review, we mainly focus on three categories of metal-functionalized GTs: transition metal (TM)-substituted GTs (TMSGTs), rare earth (RE)-substituted GTs (RESGTs) and GT-based TM–RE heterometallic derivatives (GTTRHDs). We discuss their synthetic strategies, structural features and some properties involving magnetism, electrochemistry and catalysis. Finally, some perspectives on this field are also provided and some possible directions for future work are outlined.
1. Introduction
Polyoxometalates (POMs) have been known for almost two centuries since Berzelius first discovered (NH4)3PMo12O40·nH2O formed by reaction of ammonium molybdate with phosphoric acid.1 From then on, the widespread interest in POMs and the pace of discovering novel species have steadily increased. To date, POMs have developed as a vigorously growing family of metal-oxide clusters with intriguing compositional and structural variability, versatile physical and chemical performances and a wide range of applications such as catalysis, medicine, photochemistry, magnetism, etc.1 Furthermore, POMs not only can undergo reversible and stepwise multi-electron transfer processes without changing their structures, but also the rich oxygen surface of lacunary POM fragments renders them as excellent inorganic multidentate candidates to integrate transition metal (TM), rare earth (RE) or both TM and RE ions, giving rise to a variety of novel POM-based TM, RE and TM–Ln derivatives.Germanotungstates (GTs), as an important subfamily in POM chemistry, have gradually developed as an emerging energetic research field. GTs mainly exist in the form of Keggin-type polyoxoanionic units or derived species. Generally speaking, it is comparatively difficult to separate the saturated Keggin {GeW12O40} precursor under bench conditions.2a Therefore, during the course of preparing GT derivatives, lacunary Keggin precursors are usually selected as reactant materials because of their easy availability and the high yield. These lacunary Keggin precursors can be considered to derive from the {GeW12O40} parent skeleton by removal of one or more {W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O} groups. The commonly employed lacunary GT precursors chiefly include: (i) the mono-vacant [α-GeW11O39]8− that was first communicated by Hervé and Tézé in 1977,2b (ii) the di-vacant [γ-GeW10O36]8− that was first reported by Kortz in 2006,2c and (iii) the tri-vacant [α-GeW9O34]10−, which was first discovered by Hervé and Tézé in 1977.2b It is noteworthy that these vacant sites endow the GT defect shell with enhanced reactivity towards TM or Ln electrophiles. That is to say, these precursors can be used as versatile building blocks to incorporate almost any metal ion to the vacant sites in the backbones giving rise to functional metal-functionalized GTs. Hence, it is known that these lacunary GT species are good, rigid and highly nucleophilic multidentate electronic donors and can induce the aggregation of metal electron accepters.
O} groups. The commonly employed lacunary GT precursors chiefly include: (i) the mono-vacant [α-GeW11O39]8− that was first communicated by Hervé and Tézé in 1977,2b (ii) the di-vacant [γ-GeW10O36]8− that was first reported by Kortz in 2006,2c and (iii) the tri-vacant [α-GeW9O34]10−, which was first discovered by Hervé and Tézé in 1977.2b It is noteworthy that these vacant sites endow the GT defect shell with enhanced reactivity towards TM or Ln electrophiles. That is to say, these precursors can be used as versatile building blocks to incorporate almost any metal ion to the vacant sites in the backbones giving rise to functional metal-functionalized GTs. Hence, it is known that these lacunary GT species are good, rigid and highly nucleophilic multidentate electronic donors and can induce the aggregation of metal electron accepters.
In order to obtain diverse metal-functionalized GTs with aesthetic architectures and interesting underlying properties, the conventional solution synthesis and the hydrothermal synthesis methods as two kinds of synthetic approaches are extensively used. The self-assembly strategy of oxometalates (e.g. Na2WO4, GeO2) or lacunary GT fragments with metal ions in conventional solution (ambient pressure, T < 100 °C) has been established as a general method for making GT derivatives because of the manipulation convenience and relatively undemanding experimental requirements. Up to now, a vast variety of GT derivatives have been made by this method. However, this conventional solution synthetic method has some disadvantages: (i) the precursors or organic components with low solubility cannot effectively participate in the reaction; (ii) the formation of large amounts of precipitates in the reactions usually makes the growth of crystals difficult, which prevents the structural determination of desired products; (iii) the crystallization time usually is rather long such as a few weeks or several months. To overcome these disadvantages or difficulties, hydrothermal synthesis is also widely used considering its merits: (i) hydrothermal conditions can effectively reduce the viscosity of solvents, greatly improving the diffusion processes and favoring the formation of good-quality crystals;3a–d (ii) hydrothermal conditions can promote a reaction to shift from the thermodynamic to the kinetic so that the equilibrium phases at room temperature are replaced by structurally more complicated meta-stable phases;3e–g (iii) the solubility of materials can be efficiently increased, and, as a result, those slightly soluble or even insoluble precursors and organic components in the conventional aqueous solution may be imported to the hydrothermal system.3d,h To date, the combination strategy of the hydrothermal technique and lacunary POM precursors has been largely explored as an effective method by Yang and other research groups in order to make inorganic–organic hybrid TM-substituted POMs.3h,4 The successful preparations of a large number of inorganic/inorganic–organic hybrid GTs have also demonstrated that the hydrothermal technique combined with lacunary GT segments is a feasible avenue in the field of metal-functionalized GTs.4c–e Thus this method has been widely used in our lab as a general synthetic procedure to prepare novel inorganic–organic hybrid GT-based TM–RE heterometallic derivatives.5 Overall, both methods are effective for the development of metal-functionalized GTs. Hitherto, a large number of TM-substituted GTs (TMSGTs), RE-substituted GTs (RESGTs) and GT-based TM–RE heterometallic derivatives (GTTRHDs) have been synthesized. Fig. 1 summarizes the numbers of these three kinds of metal-functionalized GTs prepared in the past ten years.
 | ||
| Fig. 1 A statistics histogram of three kinds of metal-functionalized GTs: TMSGTs, RESGTs and GTTRHDs. | ||
In the past decade, TMSGTs have constituted one of the most exciting developments. The synthetic motivation not only originates from their intriguing structures but also their magnetic and electrochemical properties.4c–e,6 Compared with TMSGTs, since Liu et al. reported a series of bis(undecatungstogermanate) lanthanides in 1987,7 the development of RESGTs has been slow, the main reason for which is that the combination of RE cations with lacunary GT precursors often results in precipitation instead of crystallization. In this branch of research, the main synthetic method focuses on conventional solution synthesis while the hydrothermal synthetic technique is less used. From Fig. 1 we can see that the synthesis and exploration of GTTRHDs have shown great potential in the GT field since 2008, which suggests that GTTRHDs are also a promising field.
Notably, the reported metal-functionalized GTs display a variety of stable or meta-stable lacunary GT secondary structural units that primarily comprise mono-vacant {α-GeW11}, di-vacant {α-GeW10}, {β-GeW10}, {γ-GeW10}, tri-vacant {α-GeW9}, {β-GeW9}, tetra-vacant {α-GeW8}, {β-GeW8} and multi-vacant {α-GeW6} (Fig. 2). Among these secondary structural units, some are derived from the self-assembly of simple oxometalates and others come from the degradation or rearrangement of lacunary GT precursors in the reaction procedure. A metal-functionalized GT can contain one kind of secondary structural unit or two kinds of secondary structural units. These secondary structural units not only play a crucial role in the diversity in structure and quick expansion in the number of metal-functionalized GTs, but also enrich the research objects of GT chemistry and accelerate the rapid development of POMs.
In the past decade, remarkable progress has been made in metal-functionalized GTs with various structures and interesting properties. In the following, we will discuss in turn TMSGTs, RESGTs and GTTRHDs, and some typical compounds involved in synthetic approaches, structural description and related properties. Finally, an outlook and personal viewpoints on future research in this field are also provided.
2. The advances in TMSGTs
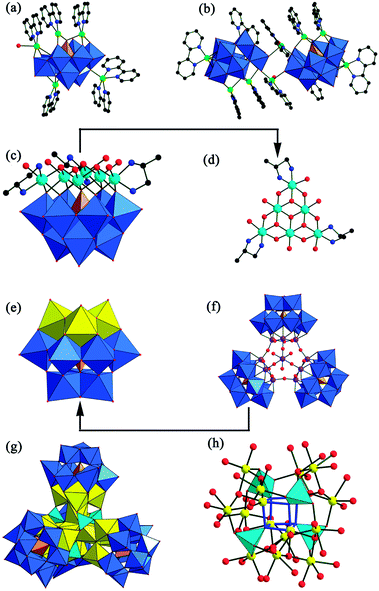
In the realm of TMSGTs, various TM cations are often used as addenda to link or incorporate GT fragments (including plenary, mono-vacant, di-vacant, tri-vacant, tetra-vacant or their mixtures) to construct larger TMSGT clusters. Currently, the most effective and useful approach to preparing TMSGTs is the self-assembly reaction of GT precursors with TM cations in conventional aqueous solution or under hydrothermal conditions. Compared with plenary Keggin GT fragments, as we know, lacunary Keggin GT fragments are easily available and possess higher reaction activity. Moreover, lacunary Keggin GT fragments have well-defined vacant sites that can act as structure-directing agents to induce the formation of TM clusters and large aggregates. As a result, lacunary Keggin GT precursors are often chosen as the starting materials to make novel TMSGTs with a variety of TM nuclearities and configurations and interesting properties. Notice that, in a given reaction system, multiple factors such as the concentration and type of reactants, the size and shape of precursors, solvent, pH, counter cations, temperature and pressure influence the crystallization process and the structures of products.In the case of the mono-vacant {GeW11} and di-vacant {GeW10} Keggin fragments, they can usually form from discrete to polymeric architectures through several TM–O–W connections (Fig. 3).6,8,9 In this case, the nature of TM cations is the main consideration when synthesizing these TMSGTs with expected properties. Within the d-block in the periodic table of the elements, ruthenium is a more advantageous candidate due to its unique electronic and redox properties and catalytic oxidation activities towards organic substrates by O2 and H2O2.10 Therefore, some Ru-substituted GTs have been synthesized.6,8a,9a,b In this respect, Kortz's group made great achievements and they prepared two different RuII-supported GTs: [Ru(dmso)3(H2O)GeW11O39]6− (Fig. 3a)6 and [{Ru(C6H6)(H2O)}{Ru(C6H6)}(γ-GeW10O36)]4− (Fig. 3d)9a by using the clean, well-defined, soluble, and air-stable RuII precursors cis-Ru(dmso)4Cl2 and [Ru(C6H6)Cl2]2 and studied their electrochemistry by cyclic voltammetry. For the former, the result of electrochemistry indicates that it is very stable in solution at least from pH 0 to 7, even in the presence of dioxygen. In the negative potential range, two well-behaved two-electron W-waves are observed, which may open the way for electrochemical study on two-electron system in a broad pH range without any change in structure. For the latter, two of the four tungsten centers at the lacunary site are not involved in bonding to the Ru(C6H6)(H2O) group, which is interesting for the catalytic aspect. Its electrochemical behavior indicates a chemically reversible wave of the Ru center. By the same group, in an aqueous acidic medium (pH 4.8), the dimeric, peroxo-containing GT [Zr2(O2)2(GeW11O39)2]12− (Fig. 3b) was synthesized,8b and its cyclic voltammetry and exhaustive electrolysis manifested fast reductive release of the peroxo ligands upon reduction of it. Kortz et al. also obtained dimeric TMSGTs containing {GeW10} units,9c–e such as [{β-GeNi2W10O36(OH)2(H2O)}2]12− (Fig. 3e),9c [K(H2O)(β-Fe2GeW10O37(OH))(γ-GeW10O36)]12−,9e and [{β-Fe2GeW10O37(OH)2}2]12−.9e In [{β-GeNi2W10O36(OH)2(H2O)}2]12−, the polyanion skeleton consists of two lacunary (β2,3-GeW10O37) fragments, each of which accommodates two Ni ions in the defect sites that lead to the formation of a complete β-Keggin ion. Magnetic measurements suggest predominantly antiferromagnetic couplings in the Ni4 spin cluster in the GT,9c which is obviously distinct from the ferromagnetic behavior observed in tetra-NiII substituted GT {[Ni(dap)2(H2O)]2[Ni(dap)2]2[Ni4(Hdap)2(α-B-HGeW9O34)2]}·6H2O.11 In addition, the magnetic behaviors of the two FeIII substituted GTs have been quantitatively analyzed and both exhibit antiferromagnetic interactions with a magnetic exchange constant of J = −14.2 cm−1 for [K(H2O)(β-Fe2GeW10O37(OH))(γ-GeW10O36)]12− and J = −26.0 cm−1 for [{β-Fe2GeW10O37(OH)2}2]12−.9e In 2011, our lab reported the trimeric TMSG [Co(H2O)6]2[Co(H2O)3(α-GeW11CoO38)3]36− (Fig. 3c) constructed from three mono-CoII substituted Keggin [α-GeW11CoO38]4− fragments linked by six W–O–Co/W bridges and a capping [Co(H2O)3]2+ bridge.8c Interestingly, similar trimeric cyclic structures [Rb ⊂ (GeW10Mn2O38)3]17−,9f {K ⊂ [(Ge(OH)O3)(GeW9Ti3O38H2)3]}14−,9g and [(Mn(H2O)3)2(K ⊂ {α-GeW10Mn2O38}3)]19− (ref. 9h) (Fig. 3f) have already been reported by other groups.
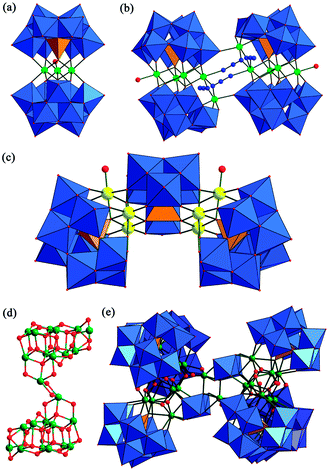
Because the tri-vacant Keggin GT {α-GeW9} structural unit is readily accessible, is highly stable and has six and/or seven unsaturated oxygen atoms available for coordination to positive metal cations, a large number of its TMSGT derivatives have already been discovered.4c,d,11–15 Since the first example of a tetra-TM sandwiched POM, [Co4(H2O)2(PW9O34)2]10−, was reported by Weakley et al. in 1973,16 the sandwich-type TM-substituted POMs have developed as a large branch in POM chemistry. As a matter of course, the nature of lacunary GT building blocks means the sandwich-type TMSGTs have a rich landscape in the GT field. Among them, the most common sandwich-type TMSGTs can be viewed as a fusion of two tri-vacant Keggin GT segments anchoring a tetra-TM group. Within the known sandwich-type TMSGTs, their structures can range from isolated, purely inorganic molecules to inorganic–organic hybrid extended architectures. In 2004, Kortz et al. for the first time reported a class of tetra-TM sandwiched GTs, [M4(H2O)2(GeW9O34)2]12− (M = MnII, CuII, ZnII, CdII) (Fig. 4a), obtained by the reaction of GeO2, Na2WO4·2H2O and TM salts (TM = MnII, CuII, ZnII, CdII) in buffer solution, which consist of two tri-vacant Keggin [B-α-GeW9O34]10− moieties linked via a rhomboid-like TM4O16 group.12a Later, similar analogues containing Co2+ and Ni2+ were also made by Niu et al.12b,c Recently, inorganic–organic hybrid isolated tetra-TM sandwiched GTs have been isolated such as (C6N2H18)3H2[{Co(2,2′-bpy)}2Co4(H2O)2(α-GeW9O34)2]·4H2O,12b [Ag(phen)2]6H2[{Mn(phen)}2 Mn4(H2O)2(α-GeW9O34)2]·3H2O,12b Na3H(C3H5N2)4[{Cu(C3N2H4)2}2 Cu4(H2O)2(GeW9O34)2]·27H2O,12d and [{Ni(2,2′-bpy)2(H2O)}2{Ni(2,2′-bpy)}2{Ni4(H2O)2(B-α-GeW9O34)2}]4−.12e In 2013, Chen reported the organic–inorganic hybrid 1-D GT (H3O)4Cu(H2O)6[K6(H2O)17 Cu2(EGTA)(H2O)2{Cu4(H2O)2(B-α-GeW9O34)2}]·9H2O.12f In addition, under hydrothermal conditions, inorganic–organic hybrid Fe4/Co4/Ni4-substituted sandwich-type GTs can be further extended into 1-D chains and 2-D networks by organic amine or TM-complex connectors.11,12g,h For example, in [enH2]8[Fe4(en)(α-GeW9O34)2][Fe4(en)2(α-GeW9O34)2]·en·14H2O, en as a linker supersedes two water ligands from two neighboring {Fe4(H2O)2O14} in the tetra-sandwich belt constructing the 1-D [Fe4(en)(α-GeW9O34)2]n8n− polymeric chain (Fig. 5a),12g which indicates that two coordination water ligands on {Fe4(H2O)2O14} are the active sites. If the reaction conditions are appropriate, the two active sites can also be substituted by other O- and N-containing organic ligands. In {[Ni(dap)2(H2O)]2[Ni(dap)2]2[Ni4(Hdap)2(α-B-HGeW9O34)2]}·6H2O, hexa-supporting {[Ni(dap)2(H2O)]2[Ni(dap)2]2[Ni4(Hdap)2(α-B-HGeW9O34)2]} subunits are expanded into 2-D (4,4) network (Fig. 5b).11
Interestingly, hybrid discrete TM6-substituted sandwich-type GTs were also made under hydrothermal and conventional conditions.13,14 For example, in 2008, Niu et al. first reported two discrete hexa-CuII substituted sandwich-type GT hybrids, namely [Cu(2,2′-bipy)]2[Cu(2,2′-bipy)2]2[Cu6(2,2′-bipy)2(GeW9O34)2]·3H2O and [Cu6(phen)2(GeW9O34)2]·2H2O.13a Both contain two tri-vacant Keggin GT segments incorporating a unique Cu6O14N2 cluster, in which six Cu2+ ions in the sandwich belt exhibit the 3:3 distribution motif. Subsequently, the novel 3-D framework [Cu(en)2]2[Cu(deta)(H2O)]2[Cu6(en)2(H2O)2(B-α-GeW9O34)2]·6H2O (Fig. 5c) built via a Cu6-sandwiched GT (Fig. 4b) was reported by Yang et al.13b Most intriguingly, it represents the first 3-D TM-substituted POM with 65·8 CdSO4 topology in POM chemistry (Fig. 5d). Notably, the {Cu6} cores in [Cu6(2,2′-bipy)2(GeW9O34)2]8− (ref. 13a) and [Cu6(en)2(H2O)2(B-α-GeW9O34)2]8− (ref. 13b) resemble each other and both belt-like {Cu6} cores contain four {CuO6} octahedra and two {CuO3N2} square pyramids. Additionally, another two dimeric GTs sandwiching mixed valence {Mn4IIIMn2II} and {Fe6III} cores, {[GeW9O34]2[Mn4IIIMn2IIO4(H2O)4]}12− (ref. 14a) (Fig. 4e) and [Fe6(OH)3(A-α-GeW9O34(OH)3)2]11− (ref. 14b) (Fig. 4g), were synthesized via conventional solution method by Kortz and Cronin, respectively. The difference between them is that in the former there is a central mixed-valence {Mn4IIIMn2II} double cubane cluster (Fig. 4f) with Ci symmetry, in which each cubane comprises three MnIII centers and one MnII center, while the Fe6 core in the latter is a trigonal prismatic {Fe6} fragment (Fig. 4h) with idealized D3h symmetry. Furthermore, magnetic properties of both have been systematically studied. The former indicates the presence of competing ferro- and antiferromagnetic exchange interactions (J1 = 6.5 cm−1, J2 = 3.5 cm−1, J3 = −56.0 cm−1) between metal centers and the low-temperature maximum is indicative of a ground state with S = 5. It also shows temperature- and sweep-rate-dependent magnetic hysteresis with steplike features, which correspond to the adiabatic quantum-tunneling transition expected for a genuine single molecule magnet (SMM).14a The latter indicates an antiferromagnetic exchange interaction (J = −11.5 cm−1) between the Fe3+ centers and an ST = 0 ground state.14b
Besides the above-mentioned TM4/TM6 sandwiched GTs, a series of unprecedented hybrid Cu8-substituted sandwich-type GTs were isolated by Yang's group,4c,d which include two 0-D H4[Cu8(dap)4(H2O)2(B-α-GeW9O34)2]·13H2O (ref. 4c) and (H2en)2[Cu8(en)4(H2O)2(B-α-GeW9O34)2]·5H2O,4c the 2-D [Cu2(H2O)2(2,2′-bpy)2]{[Cu(bdyl)]2[Cu8(2,2′-bpy)4(H2O)2(B-α-GeW9O34)2]}·4H2O,4c and the 3-D [Cu(H2O)2]H2[Cu8(dap)4(H2O)2(α-B-GeW9O34)2]4d (Fig. 4c). It should be noted that the rollover metalation of 2,2′-bpy (ref. 17a) was observed in [Cu2(H2O)2(2,2′-bpy)2]{[Cu(bdyl)]2[Cu8(2,2′-bpy)4(H2O)2(B-α-GeW9O34)2]}·4H2O.4c The rollover metalation of 2,2′-bpy led to the bdyl group acting as a twofold-deprotonated anionic N,C^C,N ligand constructing the 2-D architecture (Fig. 5e). This is the first time that such rollover metalation of 2,2′-bpy has been observed in a system containing a copper complex under hydrothermal conditions.4c Remarkably, [Cu(H2O)2]H2[Cu8(dap)4(H2O)2(α-B-GeW9O34)2] reveals an unprecedented 3-D (3,6)-connected framework with two types of helical channels (A and B) along the 42 screw axis (Fig. 5f),4d and the Schäfli symbol of this framework is (4·62)(42·64·87·102) (Fig. 5g). Most importantly, by using mixed aromatic 2,2′-bpy and 4,4′-bpy as organic ligands, the neoteric mixed-valence octa-Cu sandwiched hybrid GT [CuI(2,2′-bpy)(4,4′-bpy)]2[{Cu2I(2,2′-bpy)2(4,4′-bpy)]2[Cu2ICu6II(2,2′-bpy)2(4,4′-bpy)2(B-α-GeW9O34)2}]·2H2O (Fig. 6a) was obtained by Yang's group.4c In this compound, the most remarkable structural characteristic is that the mixed-valence octa-Cu {[Cu2ICu2II(2,2′-bpy)2(4,4′-bpy)2]Cu4IIO14} is extended to an unprecedented hybrid inorganic–organic dodeca–Cu cluster by coordination of two di-CuI [Cu2I(2,2′-bpy)2(4,4′-bpy)]2+ cations through two 4,4′-bpy bridges (Fig. 6b). As far as we know, they still represent the highest number of 3d TM sandwich-type GTs to date. In addition, by comparison, we can see that the TM4/TM6/TM8 cores in TMSGTs have a mutual relationship with each other (Fig. 4d). Obviously, organic ligands play an important role in the formation of these high-nuclear TM sandwiched GTs.
Also, Yang and co-workers made two Cu-complex-substituted GTs, namely [Cu5(2,2′-bpy)6(H2O)][GeW8O31]·9H2O (Fig. 7a) and {[Cu5(2,2′-bpy)5(H2O)][GeW9O34]}2·7H2O (Fig. 7b), by hydrothermal reaction of Na2WO4·2H2O, GeO2, and CuCl2·2H2O in the presence of 2,2′-bpy and/or 4,4′-bpy.17b Surprisingly, the former is a rare tetra-vacant Keggin moiety, [B-β-GeW8O31]10−, supported by copper complexes while the latter consists of two tri-vacant GT units bridged by copper complexes. Moreover, the hexa-NiII-substituted tri-vacant Keggin GTs [{Ni6(μ3-OH)3(L)3(H2O)6}(B-α-GeW9O34)]− (L = en or dap) (Fig. 7c) were also obtained.4e In them, a {Ni6(μ3-OH)3(L)3(H2O)6} cluster (Fig. 7d) caps a tri-vacant [B-α-GeW9O34]10− unit. The {Ni6(μ3-OH)3(L)3(H2O)6} cluster is built by six nearly coplanar NiII ions with a triangle motif linked together by three μ3-OH bridges. Magnetic susceptibility measurements show the presence of ferromagnetic coupling interactions within the hexa-NiII clusters.4e
Compared to monomeric and dimeric TMSGTs, polymeric TMSGTs are rare. In 2012, the trimeric cyclic Ti10-containing GT [(α-Ti3GeW9O37OH)3(TiO3(OH2)3)]17− was synthesized by reaction of [A-α-GeW9O34]10− with [Ti4O4(C2O4)8]8− in a mixture of rubidium and lithium acetate buffers at 60 °C.18a The skeleton anion consists of three tri-TiIV substituted tri-vacant Keggin GT units (Fig. 7e) linked together via three Ti–O–Ti bridges and an octahedral TiO6 cap (Fig. 7f). In 2014, both Zhang and Kortz reported the high-nuclear cobalt–phosphate cluster {Co16(PO4)4(OH)12} encapsulated tetrameric GT [{Co4(OH)3(PO4)}4(GeW9O34)4]32− (Fig. 7g).1g,18b The {Co16(PO4)4(OH)12} cluster comprises a central {Co4O4} cubane unit capped by four {Co3} units via four PO4 ligands and 12 μ3-OH groups (Fig. 7h). The Co4O4 cubane is structurally analogous to the [Mn3CaO4] core of the oxygen-evolving complex in photosystem II. So its photocatalytic water oxidation activities have been systematically investigated by Zhang et al. and it shows highly effective photocatalytic activity towards water oxidation under visible light irradiation.1g Furthermore, Kortz et al. probed its SMM behavior.18b
Because GT structural units can be produced either from the self-assembly of simple oxometalates or from the degradation or rearrangement of lacunary GT precursors in the reaction procedure, it is possible that there exist two types of mixed GT structural units in a TMSGT. To date, several TMSGTs with mixed lacunary GT structural units have been found.19 Typically, Kortz reported three copper-, cobalt- and manganese-containing sandwich-type GTs, [Cu3(H2O)(B-β-GeW9O33(OH))(B-β-GeW8O30(OH))]12−, [Co(H2O)2{Co3(B-β-GeW9O33(OH))(B-β-GeW8O30(OH))}2]22−, and [Mn(H2O)2{Mn3(H2O)(B-β-GeW9O33(OH))(B-β-GeW8O30(OH))}2]22−,19a which all contain two nonequivalent Keggin units: {B-β-GeW8O31} and {B-β-GeW9O34}. The structure of [Cu3(H2O)(B-β-GeW9O33(OH))(B-β-GeW8O30(OH))]12− is shown in Fig. 8a. Wang et al. prepared the tetrameric Cu10(N3)4-containing GT K10Na14[Cu10(H2O)2(N3)4(GeW9O34)2(GeW8O31)2]·30H2O in aqueous solution,19b in which two {B-β-GeW8O31} and two {B-α-GeW9O34} units are connected together by a [Cu10(N3)4O32(H2O)2] cluster (Fig. 8b). What is more, it reveals obvious electrocatalytic activity towards the reduction of nitrate. Yang's group also reported two polymeric TMSGTs, namely [HFe6(B-α-GeW9O34)2(α-GeW6O26)(H2O)2]13− (Fig. 8c)19c and [Zr24O22(OH)10(H2O)2(W2O10H)2(GeW9O34)4(GeW8O31)2]32− (Fig. 8d).19d The former is a hexa-Fe-substituted double sandwich-type GT containing one {α-GeW6O26} and two {B-α-GeW9O34} subunits separated by two Fe3 clusters (Fig. 8c).19c Some other similar TM-containing GT structures have also been found.19e–g The latter is an unprecedented Zr24-cluster substituted GT, which contains the largest [Zr24O22(OH)10(H2O)2] cluster (Fig. 8d) in all Zr-based POM to date.19d The centrosymmetric [Zr24O22(OH)10(H2O)2(W2O10H)2(GeW9O34)4(GeW8O31)2]32− hexamer can be looked on as two symmetry-related [Zr12O11(OH)5(H2O)(W2O10H)(GeW9O34)2(GeW8O31)]16− trimers via six μ3-oxo bridges and it simultaneously includes three different types of POM segments of {B-α-GeW9O34}, {B-α-GeW8O31} and {W2O10} (Fig. 8e). In addition, this complex effectively catalyzes the oxygenation of thioethers using H2O2 as an oxidant. The unique redox property of oxygen-rich POM fragments and Lewis acidity of the Zr cluster embedded in them may provide a sufficient driving force for the catalytic conversion from thioethers to sulfoxides/sulfones.19d
3. The advances in RESGTs
As we know, the removal of a {W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O} group from the [α-GeW12O40]4− unit can form a larger “bite angle” [α-GeW11O39]8− unit, which may allow a RE ion to bind deeper into the defect site.20 To date, Niu's, Xu's and Hussain's groups have introduced RE ions to the defect site of [α-GeW11O39]8− and made various RESGTs with different proportions of RE
O} group from the [α-GeW12O40]4− unit can form a larger “bite angle” [α-GeW11O39]8− unit, which may allow a RE ion to bind deeper into the defect site.20 To date, Niu's, Xu's and Hussain's groups have introduced RE ions to the defect site of [α-GeW11O39]8− and made various RESGTs with different proportions of RE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GeW11 = 1
GeW11 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
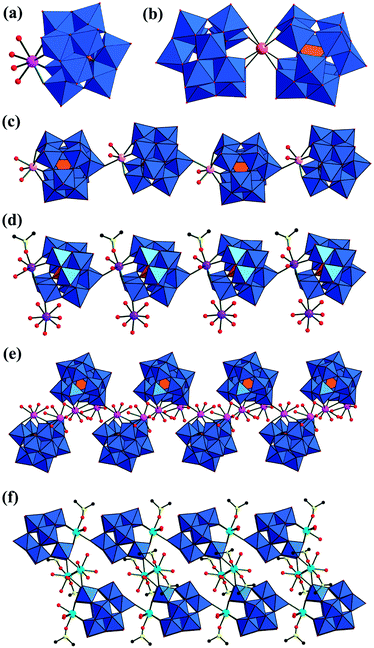
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.21–23 In 2006, Niu's group obtained a series of RESGTs containing {RE(GeW11O39)} units (RE = YIII, NdIII, SmIII, EuIII, TbIII, DyIII, YbIII) (Fig. 9) from a mixture of α-K8GeW11O39·H2O and RE2O3/RECl3 at 70–90 °C.21 When the ratio of RE3+ to [GeW11O39]8− is 1
2.21–23 In 2006, Niu's group obtained a series of RESGTs containing {RE(GeW11O39)} units (RE = YIII, NdIII, SmIII, EuIII, TbIII, DyIII, YbIII) (Fig. 9) from a mixture of α-K8GeW11O39·H2O and RE2O3/RECl3 at 70–90 °C.21 When the ratio of RE3+ to [GeW11O39]8− is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the RE3+ (RE = EuIII, TbIII, YIII, YbIII, DyIII, NdIII) cation resides in the vacancy of the [α-GeW11O39]8− fragment and bonds to the adjacent fragment via the terminal oxygen atoms, constructing 1-D linear chain (Fig. 9c and d) and 1-D zigzag chain arrangements (Fig. 9e).21a–c The introduction of the organic molecule DMSO results in an interesting 1-D double-parallel chainlike structure of [Sm2(GeW11O39)(DMSO)3(H2O)6]2− (Fig. 9f).21a Xu first reported the crystal structures for 1:2 type RESGTs (Fig. 9b),22 after a series of K13[Ln(GeW11O39)2]·nH2O (Ln = CeIII, PrIII, NdIII, SmIII, EuIII, GdIII, TbIII, DyIII, TmIII, YbIII) were found by Liu et al. in 1987.7 In 2010–2011, Hussain's group isolated a series of acetate-bridging 2:2-type RESGTs, [{Y(α-GeW11O39)(H2O)}2(μ-CH3COO)2]12− (ref. 23a) and [{Ln(CH3COO)GeW11O39(H2O)}2]12− (Ln = EuIII, GdIII, TbIII, DyIII, HoIII, ErIII, TmIII, YbIII), by one-pot reaction in an acetate buffer at pH 4.5.23b It should be noted that two acetate chelators in the μ2:η2,η1 coordination mode connect two {LnGeW11O39(H2O)} moieties together (Fig. 10a).
1, the RE3+ (RE = EuIII, TbIII, YIII, YbIII, DyIII, NdIII) cation resides in the vacancy of the [α-GeW11O39]8− fragment and bonds to the adjacent fragment via the terminal oxygen atoms, constructing 1-D linear chain (Fig. 9c and d) and 1-D zigzag chain arrangements (Fig. 9e).21a–c The introduction of the organic molecule DMSO results in an interesting 1-D double-parallel chainlike structure of [Sm2(GeW11O39)(DMSO)3(H2O)6]2− (Fig. 9f).21a Xu first reported the crystal structures for 1:2 type RESGTs (Fig. 9b),22 after a series of K13[Ln(GeW11O39)2]·nH2O (Ln = CeIII, PrIII, NdIII, SmIII, EuIII, GdIII, TbIII, DyIII, TmIII, YbIII) were found by Liu et al. in 1987.7 In 2010–2011, Hussain's group isolated a series of acetate-bridging 2:2-type RESGTs, [{Y(α-GeW11O39)(H2O)}2(μ-CH3COO)2]12− (ref. 23a) and [{Ln(CH3COO)GeW11O39(H2O)}2]12− (Ln = EuIII, GdIII, TbIII, DyIII, HoIII, ErIII, TmIII, YbIII), by one-pot reaction in an acetate buffer at pH 4.5.23b It should be noted that two acetate chelators in the μ2:η2,η1 coordination mode connect two {LnGeW11O39(H2O)} moieties together (Fig. 10a).
Other RESGTs with more than two RE ions based on di-vacant GT fragments have also been reported. Xu et al. reported the tetra-Ln dimer [Ln4(α(1,4)-GeW10O38)2(H2O)6]12− (Ln = DyIII, ErIII) obtained using the conventional solution method, in which each Ln ion resides in the vacant site of the [α(1,4)-GeW10O38]12− fragment through five oxygen atoms, four of which are from the WO6 octahedra and the other is from the central GeO4 tetrahedron (Fig. 10b).24 Kortz and co-workers prepared the gigantic 20-Ce(III)-containing GT [Ce20Ge10W100O376(OH)4(H2O)30]56− by reaction of the trilacunary POM precursor [α-GeW9O34]10− with CeIII ions in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in water at pH 5.0.25 This giant RESGT can be described as a dimeric entity composed of two half units of [Ce10Ge5W50O188(OH)2(H2O)15]28− related by an inversion center (Fig. 10c).
1 ratio in water at pH 5.0.25 This giant RESGT can be described as a dimeric entity composed of two half units of [Ce10Ge5W50O188(OH)2(H2O)15]28− related by an inversion center (Fig. 10c).
4. The advances in GTTRHDs
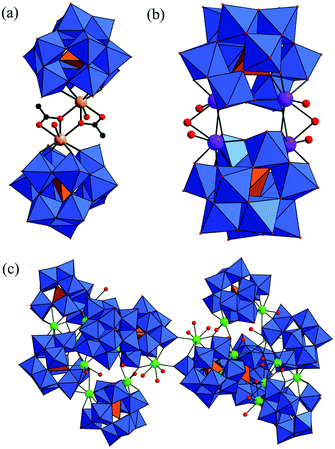
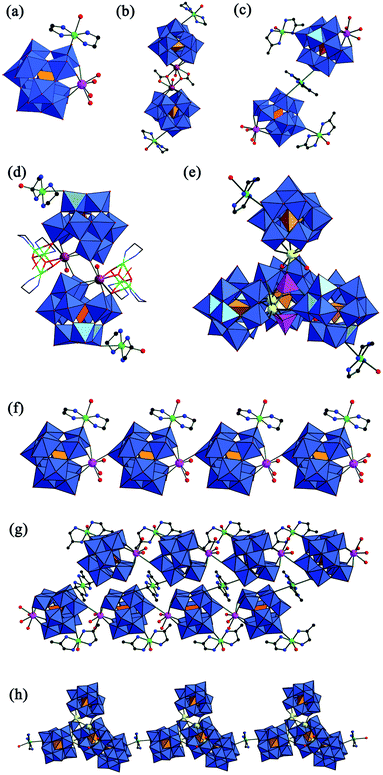
As we know, there exist unavoidable competing reactions among the highly negative POM precursors, strongly oxyphilic Ln cations and less active TM cations in the reaction system;26 therefore, it is comparatively difficult to explore suitable synthetic conditions to prepare POM-based TM–RE heterometallic derivatives. Until now, more than 20 structurally characterized GTTRHDs have been reported. Some typical examples are discussed here.During the course of exploring RESGTs, the mono-vacant [α-GeW11O39]8− fragment is common, which is usually found to form mono-RE-substituted GTs in conventional solution synthesis.21–23 What was surprising is that not only can the conversion of [A-α-GeW9O34]10− to [α-GeW11O39]8− easily occur under hydrothermal conditions,27,28 but also mono-RE-substituted GTs as secondary building units widely exist in most structures of GTTRHDs.5,28 In 2013, Yang and Zhao reported a fascinating GTTRHD, [Cu(en)2(H2O)]2 [Eu(α-HGeW11O39)(H2O)3]·12H2O (Fig. 11a),28 its asymmetrical structural unit consisting of one mono-EuIII-substituted Keggin-type [α-GeW11O39Eu(H2O)3]5− fragment and one pendant [Cu(en)2(H2O)]2+ cation. Interestingly, adjacent structural units are combined with each other via W–O–Eu–O–W bridges, generating a 1-D chain arrangement (Fig. 11f). Additionally, with the help of organic ligands (such as CH3COOH) or metal–organic complexes (such as [Cu(en)2]2+), the mono-RE-substituted GT unit can form different dimers: (i) [Cu(en)2(H2O)]5[Cu(en)2][Tb(α-GeW11O39)(η2,μ-1,1-CH3COO)(H2O)]2·14H2O (Fig. 11b) where two η2,μ-1,1-acetate join two [Tb(H2O)(α-GeW11O39)]5− subunits together;28 (ii) {[Cu(dap)2(H2O)][Ln(H2O)3(α-GeW11O39)]}26− (Ln = LaIII, PrIII, NdIII, SmIII, EuIII, TbIII, ErIII) (Fig. 11c), in which a [Cu(dap)2]2+ cation acts as a linker, and adjacent structural units are interconnected giving rise to a 1-D double chain (Fig. 11g);5a (iii) {[Cu3Ln(en)3(OH)3(H2O)2](α-GeW11O39)}24− (Ln = EuIII, TbIII, DyIII) (Fig. 11d), which is constructed from two {Cu3LnO4} cubane anchored mono-vacant [α-GeW11O39]8− fragments through two W–O–Ln–O–W linkers.5b Considering the presence of {Cu3LnO4} cubane units, their magnetic properties have been investigated. Antiferromagnetic coupling interactions within the {Cu3EuO4} cubane units are observed while dominant ferromagnetic interactions exist in {Cu3Tb/DyO4} cubane units.5b Interestingly, four mono-REIII-substituted Keggin [α-GeW11O39RE(H2O)n]5− (n = 0, 1, 2) moieties can be fused together with the aid of two {WO4} groups, resulting in the unprecedented tetrahedral RE-substituted GT nanocluster {[(α-GeW11O39RE)2(μ3-WO4)(α-GeW11O39RE(H2O))](μ4-WO4)[α-GeW11O39RE(H2O)2]}24− (Fig. 11e).5c It is interesting that adjacent tetrahedral nanoclusters are interconnected with each other via [Cu(en)2]2+ linkers, giving rise to a 1-D chain (Fig. 11h). Furthermore, these compounds show obvious electrocatalytic activities for the reduction of nitrite and bromate. Notice that, due to the connection function of TM complexes or the RE cations, these GTTRHDs can be extended from isolated molecules to 1-D chain structures. To some extent, these findings provide some enlightenment for exploring the extended structures created by TM–RE-containing POM fragments.
Besides RESGTs containing {REGeW11} units, RESGTs including dimeric {RE(GeW11)2} units have also been prepared. For example, in 2008, Niu et al. reported two GTTRHDs: [Cu(Hen)(en)]2[Cu(H2O)3]0.5{[Cu(H2en)(Hen)][Cu(H2O)3]0.5[Dy(GeW11O39)2]}·1.25H2O (Fig. 12a)29 and [Cu(en)2]2[Cu(en)2(H2O)]2H3{[Cu(en)2]2[Na2(H2O)1.75][K(H2O)3][Dy2(H2O)2(GeW11O39)3]}·6H2O.30 Notably, the latter consists of a novel 2:3-type trimeric [Dy2(H2O)2(GeW11O39)3]18− cluster (Fig. 12b) based on two DyIII ions and three monolacunary Keggin GT fragments.30 With the help of the [Cu(en)2]2+ complex cation, two [Ln(α-GeW11O39)2]13− can be fused to the particular tetrameric {Cu(en)2[Ln(α-GeW11O39)2]2}24− unit (Ln = LaIII, PrIII, EuIII, ErIII) (Fig. 12c).5b,d More interestingly, dimeric [Dy(GeW11O39)2]13−,29 trimeric [Dy2(H2O)2(GeW11O39)3]18−,30 and tetrameric {Cu(en)2[La(α-GeW11O39)2]2}24− (ref. 5d) units can be all propagated to 1-D extended architectures (Fig. 12d–f).
In addition, the reaction of TM-substituted GTs with RE cations has become a useful method to synthesize novel GTTRHDs. Reinoso et al. noted that the most representative sandwich Weakley-type [M4(H2O)2(B-α-XW9O34)2]n− (X = PV, AsV) dimers have been largely reported,16,31a and two outer {M(H2O)} groups in the sandwich belt in these tetra-substituted dimers appear to be labile, which can be replaced by extraneous metal (M′ = MnII, CoII, NaI) ions forming mixed TM clusters.31b–i So they carried out a systematic study on heterometallic TM–RE POMs starting from the incorporation of RE ions into active Weakley-type skeletons. The first Weakley-like Ce–Mn heterometallic GT [{CeIII(H2O)2}2Mn2III(B-α-GeW9O34)2]8− was separated in 2010,32a from the stoichiometric reaction of CeIV with [Mn4II(H2O)2(B-α-GeW9O34)2]12− (ref. 12a) in aqueous solution (Fig. 13a). [{CeIII(H2O)2}2Mn2III(B-α-GeW9O34)2]8− can be described as the product of the substitution of the two outer MnII atoms in the precursor by two {CeIII(H2O)2} groups, together with internal MnII to MnIII oxidation.32a Magnetic susceptibility data suggest a ground state with six unpaired electrons (S = 3) intermediate between the pure ferromagnetic and antiferromagnetic energy levels and also affected by the spin frustration expected for such a rhomboid-like array with competing magnetic interactions.32a In the following year, the antiferromagnetic Cu–Ce heterometallic GT [{CeIV(OAc)}Cu3II(H2O)(B-α-GeW9O34)2]11− (Fig. 13b) was prepared by the stoichiometric reaction of [Cu4II(H2O)2(B-α-GeW9O34)2]12− (ref. 12a) with CeIV in acid/sodium acetate buffer medium.32b [{CeIV(OAc)}Cu3II(H2O)(B-α-GeW9O34)2]11− can be viewed as the product of the substitution of one outer Cu atom in the [Cu4II(H2O)2(B-α-GeW9O34)2]12− precursor by a {Ce(OAc)}3+ group, where the capping OAc− ligand displays a κ2-O,O′ chelating mode, together with a 60° rotation of one{B-α-GeW9O34} fragment.32b Besides, it is very obvious that sandwich Weakley-type [M4(H2O)2(B-α-XW9O34)2]n− dimers have larger volume and more negative charge, which allow the formation of higher coordination numbers with metal (such as TM or/and RE) cations and should be an ideal class of candidates for constructing high-dimensional extended materials. Thus Yang's team deliberately introduced the [A-α-GeW9O34]10− precursor into the {CeIV/MnII/ox2−} system and made a novel 3-D organic–inorganic hybrid GTTRHD, K4Na4[Ce2(ox)3(H2O)2]2{[Mn(H2O)3]2[Mn4(GeW9O34)2(H2O)2]}·14H2O (Fig. 13c),33 featuring both tetra-MnII-substituted sandwich-type Keggin aggregates and Mn2+ as well as Ce3+ linkers. This not only exemplifies a new type of organic–inorganic hybrid TM–Ln heterometallic POM, but also represents the first 3-D organic–inorganic hybrid framework constructed from sandwich-type TM-substituted polyoxoanions and mixed TM and RE linkers (Fig. 13d). The whole framework possesses a (6,8)-connected topology with a Schäfli symbol of (32·412·58·64·72}{32·48·52·63)2 (Fig. 13e).33
After their first report on GTTRHD derived from the Weakley-type structure,34a Reinoso et al. continued to explore the TM–RE–GT system. Later, the simple one-pot reaction of Ni2+ (1.1):Ce3+ (1.1):GeO2 (1):WO42− (9) led to the giant crown-shaped GTTRHD Na40K6[Ni(H2O)6]3[K ⊂ K7Ce24Ge12W120O456(OH)12(H2O)64]·nH2O (Fig. 14a–c).34 The giant crown-shaped polyanion can be viewed as the product of the K+-directed self-assembly of twelve in situ-formed [Ce2GeW10O38]6− subunits, each of which is composed of a di-vacant Keggin fragment stabilized by coordination of two CeIII ions on the vacant sites through four Ce–O bonds. In the structure, only Ce3+ ions coordinate to the di-vacant fragments while the Ni2+ ions remain as hexaaquo counter cations. To date, it is not only the biggest GTTRHD, but also represents the first giant crown-shaped ring polyoxotungstate with the highest number of 4f metal ions. Furthermore, in 2014, Yang et al. reported a heterometallic hexameric GT containing FeIII–SmIII cations, (enH2)13HK9[Fe6Sm6(H2O)12(α-GeW10O38)6]·42H2O, hydrothermally synthesized by using the tri-vacant [A-α-GeW9O34]10− precursor to react with FeSO4·7H2O and Sm(NO3)·6H2O in the presence of en.19c The trimeric cryptand-type [KFe3Sm3(H2O)6(α-GeW10O38)3]17− aggregate (Fig. 14d) is composed of three [α-GeW10O38]12− subunits connected with each other by three {Sm–(μ3-O)3–Fe} connectors. What is more, two trimeric asymmetric [Fe3Sm3(H2O)6(α-GeW10O38)3]18− units are further linked via five K+ ions generating the hexameric [K7Fe6Sm6(H2O)12(α-GeW10O38)6]29− (Fig. 14e).19c Its magnetic behavior can be explained as antiferromagnetic interactions within {Fe–(μ3-O)3–Sm} clusters or/and the depopulation of the higher energy Kramers doublets of SmIII ions.19c
5. Conclusions and outlook
Apparently, the field of metal-functionalized GTs has indeed made great progress in terms of synthetic chemistry, structural chemistry and the research on physicochemical properties over the past decade and a tremendous number of GT-based compounds with unprecedented structural features (e.g. various sizes, shapes and nuclearities) have been synthesized. This is partially attributed to the ever-growing systematic applications of the conventional solution approach and the hydrothermal treatment to this field of study. During the course of preparation of GT-based compounds, several common synthetic strategies are (a) the self-assembly (or one-pot reaction) of simple oxometalates and metal ions (e.g. Na2WO4, GeO2, TM or/and RE cations, etc.), (b) the building block route (e.g. GT or TMSGT precursors reacting with RE or/and TM cations mainly in conventional aqueous solution), and (c) the combination approach of GT precursors with the hydrothermal technique (introducing GT precursors to hydrothermal environments usually accompanying the participation of organic ligands). The previously reported metal-functionalized GTs are classified into the categories of TMSGTs, RESGTs and GTTRHDs. In this review, we focus on elaborating some synthetic strategies and structural descriptions as well as involving some particular properties. From the viewpoint of structural chemistry, these metal-functionalized GTs can display a rich variety of structures from isolated fragments to extended frameworks, from monomers to oligomers and even giant aggregates, which primarily involve five kinds of stable or meta-stable lacunary Keggin GT secondary structural units: mono-vacant {α-GeW11}, di-vacant {α-GeW10}, {β-GeW10}, {γ-GeW10}, tri-vacant {α-GeW9}, {β-GeW9}, tetra-vacant {α-GeW8}, {β-GeW8} and multi-vacant {α-GeW6}. In the class of GTTRHDs, we can see that RE cations often are encapsulated into the defect sites of lacunary GT building blocks whereas TM cations usually function as supporting pendants or bridging groups. Moreover, the organic components are very limited and mainly focused on organoamines although a few carboxylic ligands are involved. From the viewpoint of structural characterization or investigating properties, most of the characterization methods are related to solid-state investigations such as IR spectra, thermogravimetric analyses, single-crystal X-ray diffraction, magnetic susceptibility and fluorescence, with solution studies being comparatively rarer. As a result, NMR, cyclic voltammetry and electrospray ionization mass spectrometry should be utilized to explore the solution behaviors. Additionally, catalytic studies on metal-functionalized GTs are less developed. On current understanding, we personally speculate that future and perspective work on metal-functionalized GTs may be concentrated on the following several aspects:(i) Besides the traditional conventional solution approach and the hydrothermal technique, other synthetic methods (e.g. mixed solvent diffusion method, ionothermal synthesis, high-temperature solid-state reaction, microwave synthesis, etc.) should be introduced to this domain to overcome the problems encountered in the current experiments and discover novel GT-based materials.
(ii) Continuous exploitation and designed synthesis of much higher-nuclear TM or RE cluster-incorporated GTs with structural aesthetic appreciation are very interesting and challenging topics, which are predominantly driven by multiple potential applications related to catalytic, magnetic and photoelectric properties.
(iii) The research on GTTRHDs is an incipient field. In this field, the types of utilized TM cations are very limited (most GTTRHDs involve copper ions because CuII ions exhibit flexible and various coordination modes and the obvious Jahn–Teller distortion). Therefore, many other TM (especially Mn, Ni, Co, Zr, Fe) cations should be tentatively employed to construct metal-directed GTTRHDs.
(iv) The types of organic components used are very limited in the field of metal-functionalized GTs. Thus, many more multi-functional organic ligands should be selected and imported to systems to create new kinds of inorganic–organic hybrid metal-functionalized GTs since functional organic components can alter and tune inorganic GT microstructures and further influence the structural constructions of products.
(v) Recently, chiral POM-based materials have been of particular interest due to a combination of the advantages of POMs with the importance of chirality;35 however, there is no investigation on chiral GTs, which provides an excellent opportunity to perform related studies. By means of the transfer of chirality, chiral ligands (including inorganic and organic) can be introduced to POM systems to make chiral GT derivatives that may be used as asymmetric catalysts, and molecular recognition and nonlinear optical materials.
(vi) To date, the research on metal-functionalized GTs has been less concerned with nanoscience and nanotechnology, which may mean an emerging ramification.
In conclusion, there is no doubt that these research trends will facilitate the continuous and in-depth development of metal-functionalized GTs and promote the interpenetration of multiple disciplines.
Abbreviations
| DMSO | Dimethyl sulfoxide |
| Dap | 1,2-Diaminopropane |
| 2,2′-bpy | 2,2′-Bipyridine |
| Phen | Phenanthroline |
| EGTA | Ethyleneglycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetate tetraanion |
| En | Ethylenediamine |
| Deta | Diethylenetriamine |
| Bdyl | 2,2′-Bipyridinyl |
| 4,4′-bpy | 4,4′-Bipyridine |
| HOAc | Acetic acid |
| Ox | Oxalate |
Acknowledgements
This work was supported by the Natural Science Foundation of China (21101055, 21301049, U1304208), the Natural Science Foundation of Henan Province (122300410106, 102300410093), the Foundation of State Key Laboratory of Structural Chemistry (20120013), 2014 Special Foundation for Scientific Research Project of Henan University, 2012 Young Backbone Teachers Foundation from Henan Province and the 2012, 2013, 2014 Students Innovative Pilot Plans of Henan University.Notes and references
- (a) J. Berzelius and J. Pogg, Ann. Phys. Chem., 1826, 6, 369 CrossRef; (b) C. L. Hill, Chem. Rev., 1998, 98, 1 CrossRef CAS PubMed; (c) P. Gouzerh and M. Che, Actual. Chim., 2006, 298, 9 CAS; (d) M. AlDamen, J. Clemente-Juan, E. Coronado, C. Martí-Gastaldo and A. Gaita-Ariño, J. Am. Chem. Soc., 2008, 130, 8847 CrossRef PubMed; (e) C. Ritchie, A. Ferguson, H. Nojiri, H. N. Miras, Y. F. Song, D. L. Long, E. Burkholder, M. Murrie, P. Kögerler, E. Brechin and L. Cronin, Angew. Chem., Int. Ed., 2008, 47, 5609 CrossRef CAS PubMed; (f) A. Dolbecq, E. Dumas, L. C. Francescoin and M. R. Antonio, Inorg. Chem., 2008, 47, 6889 CrossRef PubMed; (g) X. B. Han, Z. M. Zhang, T. Zhang, Y. G. Li, W. B. Lin, W. S. You, Z. M. Su and E. B. Wang, J. Am. Chem. Soc., 2014, 136, 5359 CrossRef CAS PubMed; (h) B. Nohra, P. Mialane, A. Dolbecq, E. Rivière, J. Marrot and F. Sécheresse, Chem. Commun., 2009, 2703 RSC; (i) S. Reinoso, Dalton Trans., 2011, 40, 6610 RSC; (j) J. T. Rhule, C. L. Hill, D. A. Judd and R. F. Schinazi, Chem. Rev., 1998, 98, 327 CrossRef CAS PubMed; (k) J. M. Clemente-Juan, E. Coronado and A. Gaita-Ariño, Chem. Soc. Rev., 2012, 41, 7464 RSC; (l) Q. Yin, J. M. Tan, C. Besson, Y. V. Geletii, D. G. Musaev, A. E. Kuznetsov, Z. Luo, K. I. Hardcastle and C. L. Hill, Science, 2010, 328, 342 CrossRef CAS PubMed; (m) H. Lv, Y. V. Geletii, C. Zhao, J. W. Vickers, G. Zhu, Z. Luo, J. Song, T. Lian, D. G. Musaev and C. L. Hill, Chem. Soc. Rev., 2012, 41, 7572 RSC; (n) H. Lv, J. Song, Y. V. Geletii, J. W. Vickers, J. M. Sumliner, D. G. Musaev, P. Kögerler, P. F. Zhuk, J. Bacsa, G. Zhu and C. L. Hill, J. Am. Chem. Soc., 2014, 136, 9268 CrossRef CAS PubMed; (o) J. W. Vickers, H. Lv, J. M. Sumliner, G. Zhu, Z. Luo, D. G. Musaev, Y. V. Geletii and C. L. Hill, J. Am. Chem. Soc., 2013, 135, 14110 CrossRef CAS PubMed; (p) Y. Wang and I. A. Weinstock, Chem. Soc. Rev., 2012, 41, 7479 RSC; (q) A. Dolbecq, P. Mialane, F. Sécheresse, B. Keita and L. Nadjo, Chem. Commun., 2012, 48, 8299 RSC; (r) S.-T. Zheng and G.-Y. Yang, Chem. Soc. Rev., 2012, 41, 7623 RSC; (s) R. Yu, X.-F. Kuang, X.-Y. Wu, C.-Z. Lu and J. P. Donahue, Coord. Chem. Rev., 2009, 253, 2872 CrossRef CAS PubMed.
- (a) Z. G. Han, Y. Z. Gao, X. L. Zhai, J. Peng, A. X. Tian, Y. L. Zhao and C. W. Hu, Cryst. Growth Des., 2009, 9, 1225 CrossRef CAS; (b) G. Hervé and A. Tézé, Inorg. Chem., 1977, 16, 2115 CrossRef; (c) N. H. Nsouli, B. S. Bassil, M. H. Dickman, U. Kortz, B. Keita and L. Nadjo, Inorg. Chem., 2006, 45, 3858 CrossRef CAS PubMed.
- (a) R. A. Laudise, Prog. Inorg. Chem., 1962, 3, 1 CrossRef CAS; (b) A. Rabenau, Angew. Chem., Int. Ed., 1985, 24, 1026 CrossRef; (c) R. A. Laudise, Chem. Eng. News, 1987, 65, 30 CrossRef CAS PubMed; (d) P. J. Hagrman, D. Hagrman and J. Zubietua, Angew. Chem., Int. Ed., 1999, 38, 2638 CrossRef; (e) J. Gopalakrishnan, Chem. Mater., 1995, 7, 1265 CrossRef CAS; (f) D. Hagrman, C. Sangregorio, C. J. O'Connor and J. Zubieta, J. Chem. Soc., Dalton Trans., 1998, 3707 RSC; (g) H. Jin, Y. Qi, E. Wang, Y. Li, C. Qin, X. Wang and S. Chang, Eur. J. Inorg. Chem., 2006, 4541 CrossRef CAS; (h) J. W. Zhao, H. P. Jia, J. Zhang, S. T. Zheng and G. Y. Yang, Chem.–Eur. J., 2007, 13, 10030 CrossRef CAS PubMed.
- (a) S. T. Zheng, D. Q. Yuan, H. P. Jia, J. Zhang and G. Y. Yang, Chem. Commun., 2007, 1858 RSC; (b) J. W. Zhao, J. Zhang, S. T. Zheng and G. Y. Yang, Inorg. Chem., 2007, 46, 10944 CrossRef CAS PubMed; (c) J. W. Zhao, C. M. Wang, J. Zhang, S. T. Zheng and G. Y. Yang, Chem.–Eur. J., 2008, 14, 9223 CrossRef CAS PubMed; (d) J. W. Zhao, J. Zhang, S. T. Zheng and G. Y. Yang, Chem. Commun., 2008, 570 RSC; (e) J. W. Zhao, J. Zhang, Y. Song, S. T. Zheng and G. Y. Yang, Eur. J. Inorg. Chem., 2008, 3809 CrossRef CAS; (f) S. T. Zheng, J. Zhang and G. Y. Yang, Angew. Chem., Int. Ed., 2008, 47, 3909 CrossRef CAS PubMed; (g) S. T. Zheng, J. Zhang, J. M. Clemente-Juan, D. Q. Yuan and G. Y. Yang, Angew. Chem., Int. Ed., 2009, 48, 7176 CrossRef CAS PubMed; (h) S. T. Zheng, J. Zhang, X. X. Li, W. H. Fang and G. Y. Yang, J. Am. Chem. Soc., 2010, 132, 15102 CrossRef CAS PubMed; (i) X. X. Li, S. T. Zheng, J. Zhang, W. H. Fang, G. Y. Yang and J. M. Clemente-Juan, Chem.–Eur. J., 2011, 17, 13032 CrossRef CAS; (j) S. T. Zheng and G. Y. Yang, Chem. Soc. Rev., 2012, 41, 7623 RSC; (k) C. Pichon, A. Dolbecq, P. Mialane, J. Marrot, E. Rivièreb and F. Sécheresse, Dalton Trans., 2008, 71 RSC; (l) C. Pichon, A. Dolbecq, P. Mialane, J. Marrot, E. Rivière, M. Goral, M. Zynek, T. McCormac, S. A. Borshch, E. Zueva and F. Sécheresse, Chem.–Eur. J., 2008, 14, 3189 CrossRef CAS PubMed; (m) J. W. Zhao, D. Y. Shi, L. J. Chen, X. M. Cai, Z. Q. Wang, P. T. Ma, J. P. Wang and J. Y. Niu, CrystEngComm, 2012, 14, 2797 RSC; (n) J. W. Zhao, D. Y. Shi, L. J. Chen, P. T. Ma, J. P. Wang and J. Y. Niu, CrystEngComm, 2011, 13, 3462 RSC.
- (a) J. W. Zhao, Y. Z. Li, F. Ji, J. Yuan, L. J. Chen and G. Y. Yang, Dalton Trans., 2014, 43, 5694 RSC; (b) J. W. Zhao, D. Y. Shi, L. J. Chen, Y. Z. Li, P. T. Ma, J. P. Wang and J. Y. Niu, Dalton Trans., 2012, 41, 10740 RSC; (c) J. W. Zhao, D. Y. Shi, L. J. Chen, P. T. Ma, J. P. Wang, J. Zhang and J. Y. Niu, Cryst. Growth Des., 2013, 13, 4368 CrossRef CAS; (d) J. L. Zhang, J. Li, L. J. Li, H. Z. Zhao, P. T. Ma, J. W. Zhao and L. J. Chen, Spectrochim. Acta, Part A, 2013, 114, 360 CrossRef CAS PubMed.
- L. H. Bi, U. Kortz, B. Keitab and L. Nadjo, Dalton Trans., 2004, 3184 RSC.
- C. Y. Rong, J. F. Liu, X. Chen and E. B. Wang, Inorg. Chim. Acta, 1987, 130, 265 CrossRef CAS.
- (a) S. Ogo, N. Shimizu, T. Ozeki, Y. Kobayashi, Y. Ide, T. Sano and M. Sadakane, Dalton Trans., 2013, 42, 2540 RSC; (b) S. S. Mal, N. H. Nsouli, M. Carraro, A. Sartorel, G. Scorrano, H. Oelrich, L. Walder, M. Bonchio and U. Kortz, Inorg. Chem., 2010, 49, 7 CrossRef CAS PubMed; (c) L. J. Chen, D. Y. Shi, J. W. Zhao, Y. L. Wang, P. T. Ma, J. P. Wang and J. Y. Niu, Cryst. Growth Des., 2011, 11, 1913 CrossRef CAS.
- (a) L. H. Bi, E. V. Chubarova, N. H. Nsouli, M. H. Dickman, U. Kortz, B. Keita and L. Nadjo, Inorg. Chem., 2006, 45, 8575 CrossRef CAS PubMed; (b) C. Besson, D. G. Musaev, V. Lahootun, R. Cao, L. M. Chamoreau, R. Villanneau, F. Villain, R. Thouvenot, Y. V. Geletii, C. L. Hill and A. Proust, Chem.–Eur. J., 2009, 15, 10233 CrossRef CAS PubMed; (c) N. H. Nsouli, M. Prinz, N. Damnik, M. Neumann, E. Talik and U. Kortz, Eur. J. Inorg. Chem., 2009, 5096 CrossRef CAS; (d) Y. Liu, J. F. Shang, G. L. Xue, H. M. Hu, F. Fu and J. W. Wang, J. Cluster Sci., 2007, 18, 205 CrossRef CAS PubMed; (e) N. H. Nsouli, S. S. Mal, M. H. Dickman, U. Kortz, B. Keita, L. Nadjo and J. M. Clemente-Juan, Inorg. Chem., 2007, 46, 8763 CrossRef CAS PubMed; (f) S. G. Mitchell, S. Khanra, H. N. Miras, T. Boyd, D. L. Long and L. Cronin, Chem. Commun., 2009, 2712 RSC; (g) Y. H. Ren, S. X. Liu, R. G. Cao, X. Y. Zhao, J. F. Cao and C. Y. Gao, Inorg. Chem. Commun., 2008, 11, 1320 CrossRef CAS PubMed; (h) P. I. Molina, H. N. Miras, D. L. Long and L. Cronin, Inorg. Chem., 2013, 52, 9284 CrossRef CAS PubMed.
- (a) T. Naota, H. Takaya and S. I. Murahashi, Chem. Rev., 1998, 98, 2599 CrossRef CAS PubMed; (b) S. I. Murahashi, Ruthenium in Organic Synthesis, Wiley, New York, 2004 Search PubMed; (c) R. G. Finke and C. X. Yin, Inorg. Chem., 2005, 44, 4175 CrossRef PubMed; (d) Y. Matsumoto, M. Asami, M. Hashimoto and M. J. Misono, J. Mol. Catal. A: Chem., 1996, 114, 161 CrossRef CAS; (e) C. L. Hill and C. M. Prosser-McCartha, Coord. Chem. Rev., 1995, 143, 407 CrossRef CAS; (f) J. C. Bart and F. C. Anson, J. Electroanal. Chem., 1995, 390, 11 CrossRef; (g) R. Neumann and M. Dahan, J. Am. Chem. Soc., 1998, 120, 11969 CrossRef CAS; (h) R. Neumann and M. Dahan, Polyhedron, 1998, 17, 3557 CrossRef CAS; (i) R. Neumann and M. Dahan, Nature, 1997, 388, 353 CrossRef CAS PubMed; (j) R. Neumann, A. M. Khenkin and M. Dahan, Angew. Chem., Int. Ed., 1995, 34, 1587 CrossRef CAS.
- J. W. Zhao, B. Li, S. T. Zheng and G. Y. Yang, Cryst. Growth Des., 2007, 7, 2658 CAS.
- (a) U. Kortz, S. Nellutla, A. C. Stowe, N. S. Dalal, U. Rauwald, W. Danquah and D. Ravot, Inorg. Chem., 2004, 43, 2308 CrossRef CAS PubMed; (b) J. P. Wang, P. T. Ma, Y. Shen and J. Y. Niu, Cryst. Growth Des., 2008, 8, 3130 CrossRef CAS; (c) J. W. Zhao, P. T. Ma, J. P. Wang and J. Y. Niu, J. Cluster Sci., 2009, 20, 671 CrossRef CAS PubMed; (d) N. Jiang, F. Y. Li, L. Xu, Y. G. Li and J. M. Li, Inorg. Chem. Commun., 2010, 13, 372 CrossRef CAS PubMed; (e) P. T. Ma, J. W. Zhao, J. P. Wang, Y. Shen and J. Y. Niu, J. Solid State Chem., 2010, 183, 150 CrossRef CAS PubMed; (f) C. H. Zhang, Y. G. Chen and S. X. Liu, Inorg. Chem. Commun., 2013, 29, 45 CrossRef CAS PubMed; (g) S. B. Tian, Y. Z. Li, J. W. Zhao, P. T. Ma and L. J. Chen, Inorg. Chem. Commun., 2013, 33, 99 CrossRef CAS PubMed; (h) L. J. Chen, D. Y. Shi, J. W. Zhao, Y. L. Wang, P. T. Ma and J. Y. Niu, Inorg. Chem. Commun., 2011, 14, 1052 CrossRef CAS PubMed; (i) X. J. Sang, J. S. Li, L. C. Zhang, Z. J. Wang, W. L. Chen, Z. M. Zhu, Z. M. Su and E. B. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 7876 CrossRef CAS PubMed; (j) R. X. Tan, X. H. Wang, F. Chai, Y. Q. Lan and Z. M. Su, Inorg. Chem. Commun., 2006, 9, 1331 CrossRef CAS PubMed.
- (a) J. P. Wang, J. Du and J. Y. Niu, CrystEngComm, 2008, 10, 972 RSC; (b) J. W. Zhao, S. T. Zheng, Z. H. Li and G. Y. Yang, Dalton Trans., 2009, 1300 RSC; (c) W. J. Niu, D. Y. Shi, J. W. Zhao, X. M. Cai and L. J. Chen, Inorg. Chem. Commun., 2012, 17, 79 CrossRef CAS PubMed; (d) Y. Z. Li, J. Luo, Y. T. Zhang, J. W. Zhao, L. J. Chen, P. T. Ma and J. Y. Niu, J. Solid State Chem., 2013, 205, 82 CrossRef CAS PubMed.
- (a) D. L. Long, E. Burkholder, M. Murrie, P. Kögerler, E. K. Brechin and L. Cronin, Angew. Chem., Int. Ed., 2008, 47, 5609 CrossRef PubMed; (b) L. H. Bi, U. Kortz, S. Nellutla, A. C. Stowe, J. van Tol, N. S. Dalal, B. Keita and L. Nadjo, Inorg. Chem., 2005, 44, 896 CrossRef CAS.
- (a) T. Yamase, T. Ozeki, H. Sakamoto, S. Nishiya and A. Yamamoto, Bull. Chem. Soc. Jpn., 1993, 66, 103 CrossRef CAS; (b) T. Yamase, X. Cao and S. Yazaki, J. Mol. Catal. A: Chem., 2007, 262, 119 CrossRef CAS PubMed; (c) X. H. Wang, J. F. Liu, Y. G. Chen, Q. Liu, J. T. Liu and M. T. Pope, J. Chem. Soc., Dalton Trans., 2000, 1139 RSC; (d) R. X. Tan, D. L. Li, H. B. Wu, C. L. Zhang and X. H. Wang, Inorg. Chem. Commun., 2008, 11, 835 CrossRef CAS PubMed; (e) Y. H. Ren, S. X. Liu, R. G. Cao, X. Y. Zhao, J. F. Cao and C. Y. Gao, Inorg. Chem. Commun., 2008, 11, 1320 CrossRef CAS PubMed.
- T. J. R. Weakley, H. T. Jun. Evans, J. S. Showell, G. F. Tourné and C. M. Tourné, J. Chem. Soc., Chem. Commun., 1973, 139 RSC.
- (a) A. C. Skapski, V. F. Sutcliffe and G. B. Young, J. Chem. Soc., Chem. Commun., 1985, 609 RSC; (b) C. M. Wang, S. T. Zheng and G. Y. Yang, Inorg. Chem., 2007, 46, 616 CrossRef CAS PubMed.
- (a) G. A. Al-Kadamany, B. S. Bassil and U. Kortz, C. R. Chim., 2012, 15, 130 CrossRef CAS PubMed; (b) M. Ibrahim, A. Haider, Y. Lan, B. S. Bassil, A. M. Carey, R. Liu, G. Zhang, B. Keita, W. Li, G. E. Kostakis, A. K. Powell and U. Kortz, Inorg. Chem., 2014, 53, 5179 CrossRef CAS PubMed.
- (a) N. H. Nsouli, A. H. Ismail, I. S. Helgadottir, M. H. Dickman, J. M. Clemente-Juan and U. Kortz, Inorg. Chem., 2009, 48, 5884 CrossRef CAS PubMed; (b) Z. M. Zhang, Y. F. Qi, C. Qin, Y. G. Li, E. B. Wang, X. L. Wang, Z. M. Su and L. Xu, Inorg. Chem., 2007, 46, 8162 CrossRef CAS PubMed; (c) J. Wang, J. W. Zhao, H. Y. Zhao, B. F. Yang, H. He and G. Y. Yang, CrystEngComm, 2014, 16, 252 RSC; (d) L. Huang, S. S. Wang, J. W. Zhao, L. Cheng and G. Y. Yang, J. Am. Chem. Soc., 2014, 136, 7637 CrossRef CAS PubMed; (e) B. Li, J. W. Zhao, S. T. Zheng and G. Y. Yang, Inorg. Chem. Commun., 2009, 12, 69 CrossRef CAS PubMed; (f) T. Ruizhan, L. L. Chen, Y. Liu, B. Liu, G. L. Xue, H. M. Hu, F. Fu and J. W. Wang, Inorg. Chem. Commun., 2010, 13, 98 CrossRef PubMed; (g) N. Jiang, F. Y. Li, L. Xu, Y. G. Li and J. M. Li, Inorg. Chem. Commun., 2010, 13, 372 CrossRef CAS PubMed.
- E. V. Craig, J. C. William and R. C. Gregory, Inorg. Chim. Acta, 2003, 346, 215 CrossRef.
- (a) J. P. Wang, X. Y. Duan, X. D. Du and J. Y. Niu, Cryst. Growth Des., 2006, 6, 2266 CrossRef CAS; (b) J. P. Wang, J. W. Zhao, X. Y. Duan and J. Y. Niu, Cryst. Growth Des., 2006, 6, 507 CrossRef CAS; (c) J. P. Wang, Q. X. Yan, X. D. Du, X. Y. Duan and J. Y. Niu, Inorg. Chim. Acta, 2008, 361, 2701 CrossRef CAS PubMed; (d) J. P. Wang, Q. X. Yan, X. D. Du and J. Y. Niu, Chin. J. Chem., 2008, 26, 1239 CrossRef CAS.
- N. Jiang, L. Xu, F. Y. Li, G. G. Gao and L. H. Fan, Inorg. Chem. Commun., 2008, 11, 24 CrossRef CAS PubMed.
- (a) F. Hussain, A. Degonda, S. Sandriesser, T. Fox, S. S. Mal, U. Kortz and G. R. Patzke, Inorg. Chim. Acta, 2010, 363, 4324 CrossRef CAS PubMed; (b) F. Hussain, S. Sandriesser, M. Speldrich and G. R. Patzke, J. Solid State Chem., 2011, 184, 214 CrossRef CAS PubMed.
- Y. G. Li, L. Xu, G. G. Gao, N. Jiang, H. Liu, F. Y. Li and Y. Y. Yang, CrystEngComm, 2009, 11, 1512 RSC.
- B. S. Bassil, M. H. Dickman, I. Römer, B. von der Kammer and U. Kortz, Angew. Chem., Int. Ed., 2007, 46, 6192 CrossRef CAS PubMed.
- (a) P. Mialane, A. Dolbecq, E. Riviére, J. Marrot and F. Sécheresse, Eur. J. Inorg. Chem., 2004, 33 CrossRef CAS; (b) C. D. Wu, C. Z. Lu, H. H. Zhuang and J. S. Huang, J. Am. Chem. Soc., 2002, 124, 3836 CrossRef CAS PubMed; (c) W. L. Chen, Y. G. Li, Y. H. Wang, E. B. Wang and Z. M. Zhang, Dalton Trans., 2008, 865 RSC.
- J. W. Zhao, S. T. Zheng and G. Y. Yang, J. Solid State Chem., 2008, 181, 2205 CrossRef CAS PubMed.
- H. Y. Zhao, J. W. Zhao, B. F. Yang, H. He and G. Y. Yang, CrystEngComm, 2013, 15, 8186 RSC.
- J. P. Wang, Q. X. Yan, X. D. Du and J. Y. Niu, Chin. J. Chem., 2008, 26, 1239 CrossRef CAS.
- J. P. Wang, Q. X. Yan, X. D. Du and J. Y. Niu, J. Cluster Sci., 2008, 19, 491 CrossRef CAS PubMed.
- (a) R. G. Finke and M. W. Droege, Inorg. Chem., 1983, 22, 1006 CrossRef CAS; (b) X. Zhang, T. M. Anderson, Q. Chen and C. L. Hill, Inorg. Chem., 2001, 40, 418 CrossRef CAS; (c) T. M. Anderson, X. Zhang, K. I. Hardcastle and C. L. Hill, Inorg. Chem., 2002, 41, 2477 CrossRef CAS PubMed; (d) U. Kortz, I. M. Mbomekalle, B. Keita, L. Nadjo and P. Berthet, Inorg. Chem., 2002, 41, 6412 CrossRef CAS PubMed; (e) L. Ruhlmann, J. Canny, R. Contant and R. Thouvenot, Inorg. Chem., 2002, 41, 3811 CrossRef CAS PubMed; (f) I. M. Mbomekalle, B. Keita, M. Nierlich, U. Kortz, P. Berthet and L. Nadjo, Inorg. Chem., 2003, 42, 5143 CrossRef CAS PubMed; (g) I. M. Mbomekalle, B. Keita, L. Nadjo, W. A. Neiwert, L. Zhang, K. I. Hardcastle, C. L. Hill and T. M. Anderson, Eur. J. Inorg. Chem., 2003, 3924 CrossRef CAS; (h) L. Ruhlmann, J. Canny, J. Vaissermann and R. Thouvenot, Dalton Trans., 2004, 794 RSC; (i) Y. Hou, L. Xu, M. J. Cichon, S. Lense, K. I. Hardcastle and C. L. Hill, Inorg. Chem., 2010, 49, 4125 CrossRef CAS PubMed.
- (a) S. Reinoso and J. R. Galán-Mascarós, Inorg. Chem., 2010, 49, 377 CrossRef CAS PubMed; (b) S. Reinoso, J. R. Galán-Mascarós and L. Lezama, Inorg. Chem., 2011, 50, 9587 CrossRef CAS PubMed.
- H. Y. Zhao, J. W. Zhao, B. F. Yang, H. He and G. Y. Yang, Cryst. Growth Des., 2013, 13, 5169 CAS.
- S. Reinoso, M. Giménez-Marqués, J. R. Galán-Mascarós, P. Vitoria and J. M. Gutiérrez-Zorrilla, Angew. Chem., Int. Ed., 2010, 49, 8384 CrossRef CAS PubMed.
- D. Y. Du, L. K. Yan, Z. M. Su, S. L. Li, Y. Q. Lan and E. B. Wang, Coord. Chem. Rev., 2013, 257, 702 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |