Surface charge modification for improvement of photocatalytic H2 production over a La2Ti2O7/graphene nanocomposite†
Sujuan Hu,
Bo Chi*,
Jian Pu and
Li Jian
Center for Fuel Cell Innovation, State Key Laboratory of Materials Processing and Die and Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: chibo@hust.edu.cn; Fax: + 86 27 87558142; Tel: + 86 27 87558142
First published on 28th October 2014
Abstract
A La2Ti2O7/graphene (GR) nanocomposite with intimate interfacial contact and a large contact area is synthesized by a facile electrostatic self-assembly approach. The nanocomposite is characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV-Vis diffuse reflectance spectroscopy (DRS), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy and photoluminescence (PL) spectroscopy. The La2Ti2O7/GR nanocomposite exhibits the highest H2 production rate when used in photocatalytic water splitting, with an improvement of 5.7 and 4.9 times relative to pure La2Ti2O7 and La2Ti2O7/GR-H that was prepared without surface charge modification, respectively. In addition, the transient photocurrent responses and electrochemical impedance spectroscopy (EIS) results indicate that the La2Ti2O7/GR nanocomposite exhibits more effective separation of photogenerated electron–hole pairs and faster interfacial electron transfer compared to pure La2Ti2O7 nanosheets and the La2Ti2O7/GR-H nanocomposite. The enhanced photocatalytic H2 production rate and photo-electrochemistry activity can be ascribed to the intimate interfacial contact and large contact area between the La2Ti2O7 nanosheets and GR, which help to make full use of the electron conductivity of GR for transferring photogenerated electrons, prolonging the lifetime of charge carriers and improving the rate of water splitting to form H2.
1. Introduction
Hydrogen (H2) as a clean, zero carbon emission, high energy density (140 MJ kg−1) fuel has been considered as the major energy source for the future.1–3 The traditional production of H2 is through consuming non-renewable and increasingly depleted fossil fuels, and this method is seriously limited by its low productivity, high cost and environment-unfriendliness. Photocatalytic water-splitting for H2 evolution has become a promising approach to realize solar-fuel conversion since Fujishima and Honda first reported the photoelectrochemical splitting of water into H2 and O2 on a TiO2 semiconductor electrode in 1972.4–7 However, the rapid recombination of photogenerated electron–hole pairs in semiconductors restricts the enhancement of their photocatalytic H2 production activity.8–10 To overcome this shortcoming and enhance the photocatalytic H2 production efficiency, many studies have been devoted to reducing the recombination rate of charge carriers by coupling photocatalysts with other materials, such as noble metals, semiconductors or carbon materials.11–15 Recently, graphene (GR or RGO) as an excellent functional carbon material has been comprehensively researched because of its unique two-dimensional (2D) network of hexagonal structured sp2-hybridized carbon atoms.16–20 It has been demonstrated that coupling of a semiconductor with the conjugative π structure of graphene is an effective means of improving the photocatalytic H2 production efficiency of the semiconductor. This improvement can be ascribed to the excellent electron conductivity of GR, which acts as an electron reservoir for accepting and shuttling photogenerated electrons over the semiconductor.21–24 For example, Wang et al. reported that a RGO/AgBr composite exhibited enhanced photocatalytic H2 evolution activity compared to bare AgBr.25 Dong et al. measured that the mean life time of photogenerated electron–hole pairs of a RGO–TiO2 nanocomposite was prolonged from ∼10−7 to ∼10−5 s, in comparison with that of TiO2.26(110) layered-structure perovskites, such as M2N2O7 (M = Ca, Sr, La; N = Nb, Ti), have received increasing attention because of their unique electronic configuration, high chemical resistance and interlayer spatial structure enabling the use of different reaction sites for water oxidation and reduction.27,28 Among the perovskites, lanthanum titanate (La2Ti2O7, LTO) has received major attention because of its efficiency for photocatalytic H2 production from water splitting.29–31 The conventional preparation methods of La2Ti2O7 are solid-state reaction (SSR) and sol–gel methods. However, neither of these methods is convenient or economical. In addition, the particles obtained are large with undesirable low specific surface areas. These characteristics are not favorable for efficient and low-cost photocatalytic H2 production. For an efficient photocatalyst, the presence of a large specific surface area is desirable. To obtain such an area for the catalysts reported in this work, a hydrothermal method was used to prepare two-dimensional La2Ti2O7 nanosheets, because this morphology is more effective than zero-dimensional La2Ti2O7 particles from the viewpoint of the mobility and recombination rate of charge carriers.
Interfacial contact and the contact area between GR and a semiconductor are crucial for influencing the electron transfer efficiency from the semiconductor to GR.32–36 Intimate interfacial contact and a large contact area help to make full use of the electron conductivity of GR for promoting the transfer rate of photogenerated electrons and prolonging the life span of charge carriers, thus result in the improvement of a catalyst’s photoactivity for H2 evolution.37,38
Many attempts have been made to modify La2Ti2O7 photocatalyst for application in the degradation of organic dyes and H2 production from water-splitting.39–42 However, few studies have been conducted on La2Ti2O7/GR nanocomposite. Herein, to effectively take advantage of graphene as an electron acceptor and transporter, we used a facile electrostatic self-assembly approach to prepare an intimate interfacial contact and large contact area La2Ti2O7/GR nanocomposite and investigate the synergistic effects of the GR and La2Ti2O7 combination on the photocatalytic H2 production activity. The La2Ti2O7/GR nanocomposite exhibits a higher photocatalytic H2 production activity and photo-electrochemistry performance than that of pure La2Ti2O7 nanosheets. For comparison, both of the property measurements were conducted on a LTO/GR-H nanocomposite that was prepared without surface charge modification. The results revealed that spontaneous attraction of electrostatic forces helps to reinforce the interfacial contact between the La2Ti2O7 nanosheets and GR and the intimate interfacial contact helps to make full use of the electron conductivity of GR for transferring photogenerated electrons and prolonging the lifetime of charge carriers, and thus results in improvement of the photoactivity.
2. Experimental
2.1 Materials
All chemicals were of analytical grade, purchased from Sinopharm Chemical Reagent Co., Ltd. and used without further purification. High-purity deionized (DI) water was used throughout our experiments.2.2 Preparation of graphene oxide (GO) nanosheets
Graphite oxide (GO) nanosheets were synthesized by chemical exfoliation of graphite powder according to a modified Hummers’ method.43,44 Specifically, 2.0 g of graphite powder was added to 46 mL of concentrated H2SO4 in an ice-bath, after which 6.0 g of KMnO4 was gradually added under vigorous stirring. The temperature of the mixture was maintained below 20 °C during this process. The mixture was then further stirred at 35 °C for 30 min, after that 92 mL of distilled water was added to the system, followed by stirring of the resultant mixture at 98 °C for 15 min. The reaction was terminated by adding 280 mL of distilled water, which was followed by 10 mL of 30% H2O2 solution. The solid product was separated by centrifugation and washed repeatedly with 5% HCl solution until sulfate could not be detected with BaCl2. Then the sample was dried in a vacuum oven at 40 °C overnight.2.3 Synthesis of La2Ti2O7 nanosheets
La2Ti2O7 nanosheets were prepared using a hydrothermal method.15 Specifically, 5 mmol of Ti(OBu)4 was mixed with 50 mmol of CH3COOH while stirring vigorously for 30 min and 5 of mmol La(NO3)3·6H2O was dissolved in 10 mL of deionized water. This La(NO3)3·6H2O solution was added dropwise to the Ti(OBu)4–CH3COOH mixture, giving a clear aqueous solution. 30 mL of a 1.8 M NaOH solution was then added to this solution. The resulting solution was transferred to a 100 mL Teflon-lined stainless steel autoclave and heat-treated at 200 °C for 24 h. The product was collected after centrifugation, washed with water and ethanol repeatedly, and then dried at 80 °C for 12 h.2.4 Synthesis of the La2Ti2O7/GR nanocomposite
The La2Ti2O7/GR nanocomposite was prepared using an electrostatic self-assembly approach, along with a hydrothermal reduction method, as shown in Scheme 1.45 In detail, 0.2 g of La2Ti2O7 was first dispersed in 200 mL of ethanol using sonication for 30 min. Then, 2 mL of (aminopropyl)trimethoxysilane (APTMS) was added into the above mixture and the mixture was heated at 100 °C for 4 h with vigorous stirring. The redundant APTMS was removed by washing with ethanol. A self-negatively charged GO suspended aqueous solution (0.2 mg mL−1) was added into the positively charged functionalized La2Ti2O7 dispersion aqueous solution (1 mg mL−1) at a weight ratio of GO to La2Ti2O7 of 5% to form a suspended aqueous solution. After mixing for 30 min, the resulting precipitate was washed with deionized water. A hydrothermal method was adopted for reduction of GO to GR in a 100 mL Teflon-lined stainless steel vessel at 120 °C for 12 h. The resulting precipitate was collected after centrifugation, washed and then dried at 80 °C for 12 h. In addition, samples of La2Ti2O7/GR with different weight ratios of GO (1 wt% and 10 wt%) were also prepared using the same method. To investigate the influence of surface charge modification on the interfacial interaction between GR and the La2Ti2O7 nanosheets, a sample without surface charge modification was prepared, according to the above procedure but with the process for APTMS-modification of the La2Ti2O7 omitted, and the sample was labelled as LTO/GR-H.2.5 Deposition of Pt cocatalyst on the photocatalyst
Deposition of Pt cocatalyst (1.0 wt%) on the photocatalyst surface was achieved through typical in situ photoreduction of H2PtCl6 with UV-light irradiation.46 0.2 g of the photocatalyst was suspended in 200 mL of an aqueous solution containing 20% (v/v) methanol as the sacrificial donor. The appropriate amount of H2PtCl6 was added to the mixture. Following this, the suspension was stirred and purged with N2 continually to ensure anaerobic conditions. After that, the suspension was illuminated with UV light for 4 h. The resulting precipitate was collected by centrifugation and dried at 80 °C for 12 h.2.6 Characterization
X-ray diffraction (XRD) measurements were carried out using an X-ray diffractometer (XRD, X’Pert pro. PANalytical B.V) with Cu Kα radiation. Both scanning electron microscopy (FE-SEM, FEI, Sirion 200) and transmission electron microscopy (TEM, JEM-2100F) were used to characterize the morphologies of the samples. X-ray photoelectron spectroscopy (XPS) was performed on a KRATOS AXIS165 X-ray photoelectron spectrometer. The UV-Vis diffuse reflectance spectra were recorded using a Perkin-Elmer Lambda 35 UV-Vis spectrophotometer. The Fourier transform infrared (FT-IR) spectroscopy was performed on a Bruker VERTEX 70. The Raman spectra and photoluminescence (PL) spectra were recorded on a laser confocal Raman microscope (LabRAM HR800, Horiba Jobin Yvon) with excitation wavelengths of 536 nm and 325 nm, respectively.2.7 Photocatalytic activity
The photocatalytic H2 production experiments were performed in a quartz reactor under solar light irradiation at ambient temperature and atmospheric pressure. A 500 W Xe lamp (100 mW cm−2, CHF-XM 500, Beijing Trusttech Co., Ltd.) was used as the light source. In a typical photocatalytic experiment, 0.1 g of photocatalyst was suspended in 150 mL of an aqueous solution containing 25% (v/v) methanol. Before all photocatalytic H2 production experiments, the reaction vessel was evacuated for 30 min to remove dissolved oxygen and ensure anaerobic conditions. Throughout the experiment 1 mL of gas was sampled intermittently and the hydrogen content was analyzed using a gas chromatograph (DongXi GC-A5000, with high purity Ar as the carrier gas) equipped with a thermal conductivity detector.2.8 Photo-electrochemical measurements
Photocurrents and electrochemical impedance spectra were measured using an electrochemical analyzer (Zennium, Zahner) with a standard three-electrode system, using prepared samples as the working electrodes, a platinum plate as the counter electrode and Ag/AgCl (saturated KCl) as the reference electrode in 1 M Na2SO4 aqueous solution. A 500 W Xe lamp (100 mW cm−2, CHF-XM 500, Beijing Trusttech Co., Ltd.) was used as the light source. For preparation of the working electrodes, 0.2 g of the photocatalyst was ground with 0.06 g of polyethylene glycol (PEG, molecular weight: 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and 0.5 mL of water to make a slurry. The slurry was subsequently coated onto a 2 cm × 1.5 cm FTO glass electrode using the doctor-blade method. The resulting electrodes were dried and calcined at 450 °C for 30 min. All electrodes studied had a similar film thickness (10–11 μm).47,48
000 Da) and 0.5 mL of water to make a slurry. The slurry was subsequently coated onto a 2 cm × 1.5 cm FTO glass electrode using the doctor-blade method. The resulting electrodes were dried and calcined at 450 °C for 30 min. All electrodes studied had a similar film thickness (10–11 μm).47,48
3. Results and discussion
The XRD patterns of pure LTO, LTO/GR and LTO/GR-H nanocomposites are shown in Fig. 1. It can be found that the pure LTO prepared through a hydrothermal method shows a monoclinic phase with a perovskite structure belonging to the P21 space group (JCPDS 01-081-1066). The peaks located at ca. 28.20°, 30.03°, 32.39° and 33.09° can be indexed to (040), (211), (002) and (012) crystal planes of the monoclinic La2Ti2O7 phase and no other impurity phase is detected. For LTO/GR and LTO/GR-H nanocomposites, they show similar XRD patterns to the pure LTO and no GR diffraction peak is observed. This might be due to the small amount and low diffraction intensity of the GR present in the nanocomposites.The UV-Vis DRS of pure LTO, LTO/GR and LTO/GR-H nanocomposites are presented in Fig. 2. The spectra of LTO/GR with different weight ratios of GR (1 wt% and 10 wt%) are shown in Fig. S1.† For pure LTO, the DRS spectrum presents a steep absorption edge at approximately 350 nm, which can be assigned to the intrinsic bandgap absorption of LTO (3.8 eV). After compositing with graphene, the absorption intensities in the visible-light region were all improved. This is mainly due to the background absorption of GR in the visible light region. Besides, the absorption intensity in the visible-light region is strengthened along with an increase of the GR amount.
The photocatalytic activities of pure LTO, LTO/GR and LTO/GR-H nanocomposites were evaluated by measuring H2 production from water splitting under 500 W Xe lamp irradiation, as shown in Fig. 3(A). To improve the photocatalytic H2 production, 1 wt% of Pt cocatalyst was loaded onto the surface of the photocatalysts to provide active sites for the reaction. The photocatalytic H2 evolution rate over pure LTO was only 10.59 μmol h−1 g−1 and the H2 evolution rate was distinctly enhanced to 60.45 μmol h−1 g−1 after compositing with 5 wt% graphene through an electrostatic self-assembly approach. However, the performance decreased to 8.47 μmol h−1 g−1, even lower than that of pure LTO, for LTO composites with 10 wt% graphene (Fig. S2†). This is due to the excessive amounts of graphene absorbing a certain amount of light and weakening the transmittance of incident light.
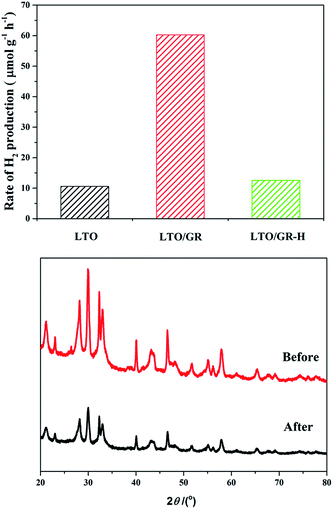 | ||
| Fig. 3 The effect of LTO, LTO/GR and LTO/GR-H nanocomposites on the photocatalytic H2 evolution rate, under 500 W Xe lamp irradition (A) and XRD patterns of LTO/GR before and after the reaction (B). | ||
However, for the LTO/GR-H nanocomposite which was prepared without surface charge modification, the photocatalytic H2 evolution rate is 12.41 μmol h−1 g−1, slightly higher than that of pure LTO but far below that of the LTO/GR nanocomposite. The crystal structure of the LTO/GR nanocomposite after the photoreaction is essentially similar to that of the unused LTO/GR nanocomposite (Fig. 3(B)). No obvious deviation in peak location occurred indicating that the LTO/GR nanocomposite possesses crystalline structure stability. The photostability of the Pt (1 wt%) loaded LTO/GR nanocomposite was investigated through use in two experimental runs of 8 h (Fig. S3†). The average H2 generation rate over the Pt (1 wt%) loaded LTO/GR nanocomposite was about 47.72 μmol g−1 h−1 in the first run of the 8 h photocatalytic reaction, and then slightly decreased to 44.44 μmol g−1 h−1 in the second run. The performance degradation is about 6.9%, indicating that the photocatalyst has good stability.
To explore and understand the origins of the improved photocatalytic H2 production of the LTO/GR nanocomposite, a series of characterizations have been conducted. The morphologies of the LTO, LTO/GR and LTO/GR-H nanocomposites are shown in Fig. 4. It is clear to see that pure LTO shows irregular thin nanosheets (Fig. 4(A)). Fig. 4(B) and (C) are typical SEM and HRTEM images of the LTO/GR nanocomposite. It is not easy to perceive, but through careful observation it can still be found that graphene thin layers smoothly and tightly cover the surface of the LTO nanosheets and some wrinkles caused by the GR thin layers are marked with white dotted lines in Fig. 4(B). For better observation of the interfacial contact between the LTO nanosheets and GR, a HRTEM image was obtained and is displayed in Fig. 4(C). The interplanar distance of 0.278 nm is in good agreement with the d-spacing of the (002) plane of monoclinic La2Ti2O7. The graphene thin film, ca. 2.382 nm, is in intimate contact with the LTO and there are no obvious lattice distortions in the interfacial region between LTO and graphene. However, the case is quite different for the LTO/GR-H nanocomposite (Fig. 4(D)). The GR surfaces do not effectively integrate with the LTO nanosheets and the interfacial contact area is relatively small. The morphology of the LTO in the LTO/GR and LTO/GR-H nanocomposites is similar to that of pure LTO nanosheets, indicating that the differences in photocatalytic H2 production over these photocatalysts cannot be caused by a change in LTO morphology. Therefore, we can understand that the large change in photocatalytic H2 production is caused by the introduction of graphene and by different compositing methods. The LTO/GR nanocomposite synthesis involves a process of surface charge modification of the LTO nanosheets and this process leads to LTO nanosheets being electrostatically assembled with negatively charged GO, and results in intimate interfacial contact and a large contact area. This intimate interfacial contact and large contact area help to make full use of the electron conductivity of graphene for transferring the photogenerated electrons and prolonging the lifetime of charge carriers that are favorable to a high photocatalytic H2 production. On the contrary, the tough and poor interfacial contact in LTO/GR-H would not take advantage of graphene for electron transfer.
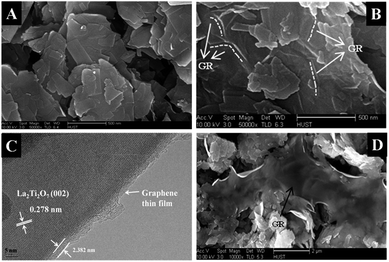 | ||
| Fig. 4 Typical SEM image of LTO (A), SEM (B) and HRTEM (C) images of the LTO/GR nanocomposite, and SEM image of the LTO/GR-H nanocomposite (D). | ||
XPS is used to further investigate the chemical nature of and the interaction between graphene and the LTO nanosheets. The full spectra (Fig. 5(A)) show that elements Ti, La, O and C exist in the LTO/GR nanocomposite. The corresponding high-resolution XPS spectra of C 1s, O 1s, La 3d and Ti 2p are shown in Fig. 5(C)–(F), respectively. Three peaks, at 284.97, 286.18 and 289.94 eV, are observed in the C 1s core level spectra and they are ascribed to C–C (sp2 bonded carbon), C–O (epoxy/hydroxyl) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carboxyl), respectively (Fig. 5(B)).49 Fig. 5(C) presents the high-resolution XPS spectra for O 1s. Two oxygen signals are observed at 532.97 and 530.93 eV, which can be attributed to surface adsorption oxygen and lattice oxygen, respectively.50 Fig. 5(D) reveals the high resolution XPS spectra for La 3d. Seven peaks are observed in the La 3d core level spectra and all of these peaks originate from the spin–orbital splitting of the 3d5/2 and 3d3/2 states of La(III).51 The peaks located at 465.33 and 459.60 eV correspond to Ti 2p1/2 and Ti 2p3/2, respectively (Fig. 5(E)).52
O (carboxyl), respectively (Fig. 5(B)).49 Fig. 5(C) presents the high-resolution XPS spectra for O 1s. Two oxygen signals are observed at 532.97 and 530.93 eV, which can be attributed to surface adsorption oxygen and lattice oxygen, respectively.50 Fig. 5(D) reveals the high resolution XPS spectra for La 3d. Seven peaks are observed in the La 3d core level spectra and all of these peaks originate from the spin–orbital splitting of the 3d5/2 and 3d3/2 states of La(III).51 The peaks located at 465.33 and 459.60 eV correspond to Ti 2p1/2 and Ti 2p3/2, respectively (Fig. 5(E)).52
 | ||
| Fig. 5 XPS spectra of the LTO/GR nanocomposite: (A) full scan spectrum, (B) C 1s, (C) O 1s, (D) La 3d and (E) Ti 2p high-resolution XPS spectra. | ||
Raman spectroscopy is an efficient tool for characterization of carbon materials. As can be seen in Fig. 6, two characteristic peaks in the spectrum of GO are named as the D band (1350 cm−1) and the G band (1590 cm−1) and these two peaks also appear in the LTO/GR and LTO/GR-H nanocomposites.53 The D band is ascribed to local defects or disorders, while the G band arises from the sp2 hybridized graphene domains.54 The intensity ratio (ID/IG) of the D band to the G band in GO is ca. 0.46, while the ID/IG of the LTO/GR and LTO/GR-H nanocomposites are ca. 0.77 and 0.86, respectively. The increase in the ID/IG value is attributed to reduction and restoration of the sp2 network of GO after hydrothermal treatment, which suggests successful reduction of GO to graphene.55,56 In addition, slight red-shifting of the D band and the G band for the LTO/GR nanocomposite compared to GO could be caused by the interaction between the LTO nanosheets and graphene.
To further verify the interaction between the LTO nanosheets and graphene, FT-IR characterisation was performed, and the spectra are shown in Fig. 7. Five characteristic peaks at ca. 1049, 1269, 1400, 1620 and 1736 cm−1 in the GO spectrum are caused by C–O stretching vibrations of epoxy groups, C–O stretching vibrations of phenolic C–OH, O–H deformation vibrations of tertiary C–OH, H–O–H bending of adsorbed H2O molecules or skeletal vibrations of unoxidized C–C bonding and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of COOH groups, respectively.57,58 However, a dramatic decrease occurred in the intensities of these peaks for the LTO/GR and LTO/GR-H nanocomposites, indicating reduction of the GO to graphene. In addition, a new peak at ca. 1548 cm−1 was observed, which is caused by the skeletal vibration absorption of graphene.59
O stretching vibrations of COOH groups, respectively.57,58 However, a dramatic decrease occurred in the intensities of these peaks for the LTO/GR and LTO/GR-H nanocomposites, indicating reduction of the GO to graphene. In addition, a new peak at ca. 1548 cm−1 was observed, which is caused by the skeletal vibration absorption of graphene.59
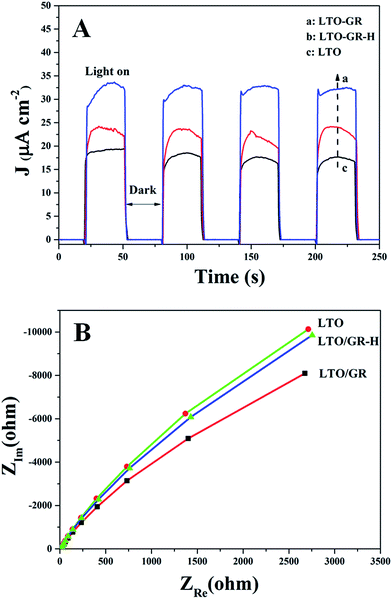
Photo-electrochemical measurements are used to study the interfacial electron transfer of photocatalysts. In our experiments, transient photocurrent responses (J–t) and electrochemical impedance spectroscopy (EIS) spectra were obtained to better understand the photogenerated carrier separation of the LTO/GR nanocomposite (Fig. 8). All of the samples possess relatively stable photocurrent responses (Fig. 8(A)), suggesting that the working electrodes are stable and the photoresponse is quite reversible.60 The photocurrent density obtained over the LTO/GR nanocomposite is obviously enhanced compared to that of LTO/GR-H and pure LTO. Photocurrent is formed mainly by transferring photogenerated electrons to the counter electrode, the higher photocurrent for LTO/GR indicates more effective separation and longer lifetime of the photogenerated electrons on it. In addition, EIS Nyquist analysis was performed from 0.1 Hz to 3000 Hz to investigate the interfacial electron transfer (Fig. 8(B)). As is known, the high-frequency arc corresponds to the charge transfer limiting process and can be attributed to the charge transfer resistance at the contact interface between the electrode and electrolyte solution and the charge transfer resistance can be directly measured from the semicircle diameter.61–63 It can be observed that the arc of the LTO/GR prepared through the electrostatic self-assembly approach is smaller than that of LTO and LTO/GR-H in the high-frequency region, implying that the LTO/GR nanocomposite has a faster interfacial electron transfer and more effective separation of photogenerated electron–hole pairs, which is in accordance with its higher photocatalytic H2 production and transient photocurrent responses. Then, the photoluminescence (PL) spectra of LTO, LTO/GR and LTO/GR-H nanocomposites were measured (Fig. 9). LTO/GR displays a dramatically diminished PL intensity compared to LTO and LTO/GR-H, suggesting that the recombination rate of photogenerated electron–hole pairs is efficiently suppressed. These results are also well in accordance with the photo-electrochemical performances and photocatalytic H2 production activities.
On the basis of the above analysis and discussion, it can be concluded that the intimate interfacial contact and large contact area between the La2Ti2O7 nanosheets and graphene are crucial for improving the photo-electrochemical performance and photocatalytic H2 production activity. Because the reduction potential of GR/GR˙− is about −0.08 eV, good interfacial contact and large contact area between La2Ti2O7 nanosheets and graphene could effectively promote photogenerated electron transfer from La2Ti2O7 to graphene, and then these electrons are readily trapped by Pt particles to reduce H2O to H2. Meanwhile, the photogenerated holes are oxidized by methanol, as shown in Fig. 10.
 | ||
| Fig. 10 The proposed photocatalytic H2 evolution mechanism over the La2Ti2O7/GR nanocomposite under simulated solar light irradiation. | ||
4. Conclusions
We adopted a facile electrostatic self-assembly approach to prepare a La2Ti2O7/GR nanocomposite with good interfacial contact and a large contact area. The La2Ti2O7/GR nanocomposite displays a higher photocatalytic H2 production activity and photo-electrochemical performance than those of La2Ti2O7 and the La2Ti2O7/GR-H nanocomposite that was prepared without surface charge modification. The good photocatalytic activity of the La2Ti2O7/GR nanocomposite can be ascribed to intimate interfacial contact and a large contact area between the La2Ti2O7 nanosheets and graphene, that help to make full use of the excellent electron conductivity of graphene for transferring photogenerated electrons and prolonging the lifetime of charge carriers. The electrostatic self-assembly approach plays a significant role in constructing GR-based semiconductors with a high photocatalytic activity for H2 production.Acknowledgements
This work is supported by the National Natural Science Foundation of China (50902056). The authors would like to thank the Materials Characterization Center of Huazhong University of Science and Technology for assistance with the measurements.Notes and references
- A. J. Bard and M. A. Fox, Acc. Chem. Res., 1995, 28, 141 CrossRef CAS.
- Y. Amao, ChemCatChem, 2011, 3, 458 CrossRef CAS.
- J. Barber and B. Andersson, Nature, 1994, 370, 31 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- D. E. Scaife, Sol. Energy, 1980, 25, 41 CrossRef CAS.
- K. Rajeshwar, J. Phys. Chem. Lett., 2011, 2, 1301 CrossRef CAS.
- J. Tang, J. R. Durrant and D. R. Klug, J. Am. Chem. Soc., 2008, 130, 13885 CrossRef CAS PubMed.
- W. Choi, A. Termin and M. R. Hoffmann, Angew. Chem., 1994, 106, 1148 CrossRef CAS.
- K. Domen, A. Kudo, T. Onishi, N. Kosugi and H. Kuroda, J. Phys. Chem., 1986, 90, 292 CrossRef CAS.
- V. H. Houlding and M. Grätzel, J. Am. Chem. Soc., 1983, 105, 5695 CrossRef CAS.
- T. Hirakawa and P. V. Kamat, J. Am. Chem. Soc., 2005, 127, 3928 CrossRef CAS PubMed.
- V. Subramanian, E. E. Wolf and P. V. Kamat, J. Am. Chem. Soc., 2004, 126, 4943 CrossRef CAS PubMed.
- S. H. Elder, F. M. Cot, Y. Su, S. M. Heald, A. M. Tyryshkin, M. K. Bowman, Y. Gao, A. G. Joly, M. L. Balmer, A. C. Kolwaite, K. A. Magrini and D. M. Blake, J. Am. Chem. Soc., 2000, 122, 5138 CrossRef CAS.
- T. Tatsuma, S. Saitoh, P. Ngaotrakanwiwat, Y. Ohko and A. Fujishima, Langmuir, 2002, 18, 7777 CrossRef CAS.
- K. Woan, G. Pyrgiotakis and W. Sigmund, Adv. Mater., 2009, 21, 2233 CrossRef CAS.
- S. Guo and S. Dong, Chem. Soc. Rev., 2011, 40, 2644 RSC.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS PubMed.
- L. Han, P. Wang and S. Dong, Nanoscale, 2012, 4, 5814 RSC.
- S. Q. Liu, Z. Chen, N. Zhang, Z. R. Tang and Y. J. Xu, J. Phys. Chem. C, 2013, 117, 8251 CAS.
- Q. Xiang and J. Yu, J. Phys. Chem. Lett., 2013, 4, 753 CrossRef CAS.
- M. Q. Yang and Y. J. Xu, Phys. Chem. Chem. Phys., 2013, 15, 19102 RSC.
- Y. H. Zhang, Z. R. Tang, X. Z. Fu and Y. J. Xu, ACS Nano, 2011, 5, 7426 CrossRef CAS PubMed.
- C. Han, M. Q. Yang, B. Weng and Y. J. Xu, Phys. Chem. Chem. Phys., 2014, 16, 16891 RSC.
- M. Q. Yang, N. Zhang, M. Pagliaro and Y. J. Xu, Chem. Soc. Rev. 10.1039/c4cs00213j.
- J. Wang, C. An, J. Liu, G. Xi, W. Jiang, S. Wang and Q. Zhang, J. Mater. Chem. A, 2013, 1, 2827 CAS.
- P. Wang, Y. Zhai, D. Wang and S. Dong, Nanoscale, 2011, 3, 1640 RSC.
- D. W. Hwang, H. G. Kim, J. Kim, K. Y. Cha, Y. G. Kim and J. S. Lee, J. Catal., 2000, 193, 40 CrossRef CAS.
- H. Kim, D. Hwang, Y. Kim and J. Lee, Chem. Commun., 1999, 1077 RSC.
- M. M. Milanova, M. Kakihana, M. Arima, M. Yashima and M. Yoshimura, J. Alloys Compd., 1996, 242, 6 CrossRef CAS.
- K. Onozuka, Y. Kawakami, H. Imai, T. Yokoi, T. Tatsumi and J. N. Kondo, J. Solid State Chem., 2012, 192, 87 CrossRef CAS PubMed.
- K. W. Li, Y. Wang, H. Wang, M. Zhu and H. Yan, Nanotechnology, 2006, 17, 4863 CrossRef CAS.
- C. H. Wu, Y. Z. Zhang, S. Li, H. J. Zheng, H. Wang, J. B. Liu, K. W. Li and H. Yan, Chem. Eng. J., 2011, 178, 468 CrossRef CAS PubMed.
- M. Q. Yang, B. Weng and Y. J. Xu, Langmuir, 2013, 29, 10549 CrossRef CAS PubMed.
- J. S. Lee, K. H. You and C. B. Park, Adv. Mater., 2012, 24, 1084 CrossRef CAS PubMed.
- X. J. Bai, L. Wang, R. L. Zong, Y. H. Lv, Y. Q. Sun and Y. F. Zhu, Langmuir, 2013, 29, 3097 CrossRef CAS PubMed.
- X. Yan, Y. J. Li, F. Du, K. Zhu, Y. Q. Zhang, A. Y. Su, G. Chen and Y. J. Wei, Nanoscale, 2014, 6, 4108 RSC.
- O. Akhavan, ACS Nano, 2010, 4, 4174 CrossRef CAS PubMed.
- Y. Y. Bu, Z. Y. Chen, W. B. Li and B. R. Hou, ACS Appl. Mater. Interfaces, 2013, 5, 12361 CAS.
- C. H. Wu, Y. Z. Zhang, S. Lia, H. J. Zheng, H. Wang, J. B. Liu, K. W. Li and H. Yan, Chem. Eng. J., 2011, 178, 468 CrossRef CAS PubMed.
- S. J. Hu, L. C. Jia, B. Chi, J. Pu and J. Li, J. Power Sources, 2014, 6, 304 CrossRef PubMed.
- S. J. Hu, B. Chi, J. Pu and J. Li, J. Mater. Chem. A, 2014, 2, 19260 CAS.
- D. W. Hwang, H. G. Kim, J. S. Jang, S. W. Bae, S. M. Ji and J. S. Lee, Catal. Today, 2004, 93–95, 845 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994 CrossRef CAS PubMed.
- L. Yuan, M. Q. Yang and Y. J. Xu, Nanoscale, 2014, 6, 6335 RSC.
- A. A. Ismail and D. W. Bahnemann, J. Phys. Chem. C, 2011, 115, 5784 CAS.
- M. S. Zhu, Z. Li, B. Xiao, Y. T. Lu, Y. K. Du, P. Yang and X. M. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 1732 CAS.
- Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575 CrossRef CAS PubMed.
- N. Jiang, Z. L. Xiu, Z. Xie, H. Y. Li, G. Zhao, W. P. Wang, Y. Z. Wu and X. P. Hao, New J. Chem., 2014, 38, 4312 RSC.
- V. Bessergenev, R. Pereira, M. Mateus, I. Khmelinskii, D. Vasconcelos, R. Nicula, E. Burkel, A. Botelho do Rego and A. Saprykin, Thin Solid Films, 2006, 503, 29 CrossRef CAS PubMed.
- M. Sunding, K. Hadidi, S. Diplas, O. Løvvik, T. Norby and A. Gunnæs, J. Electron Spectrosc. Relat. Phenom., 2011, 184, 399 CrossRef CAS PubMed.
- S. Bouattour, A. M. Botelho do Rego and L. F. Vieira Ferreira, Mater. Res. Bull., 2010, 45, 818 CrossRef CAS PubMed.
- H. He, T. Riedl, A. Lerf and J. Klinowski, J. Phys. Chem., 1996, 100, 19954 CrossRef CAS.
- C. Gómez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499 CrossRef PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS PubMed.
- S. Chen, J. Zhu and X. Wang, J. Phys. Chem. C, 2010, 114, 11829 CAS.
- T. Nakajima, A. Mabuchi and R. Hagiwara, Carbon, 1988, 26, 357 CrossRef CAS.
- H. L. Guo, X. F. Wang, Q. Y. Qian, F. B. Wang and X. H. Xia, ACS Nano, 2009, 3, 2653 CrossRef CAS PubMed.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994 CrossRef CAS PubMed.
- M. S. Zhu, Y. K. Du, P. Yang and X. Wang, Catal. Sci. Technol., 2013, 3, 2295 CAS.
- C. Y. Zhai, M. S. Zhu, Y. T. Lu, F. F. Ren, C. Q. Wang, Y. K. Du and P. Yang, Phys. Chem. Chem. Phys., 2014, 16, 14800 RSC.
- N. Zhang, M. Q. Yang, Z. R. Tang and Y. J. Xu, ACS Nano, 2014, 8, 623 CrossRef CAS PubMed.
- S. Q. Liu, M. Q. Yang and Y. J. Xu, J. Mater. Chem. A, 2014, 2, 430 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10522b |
| This journal is © The Royal Society of Chemistry 2014 |