A synergistic biocomposite for wound healing and decreasing scar size based on sol–gel alumina
K. V. Volodinaab,
N. L. Solov'evac,
Vasiliy V. Vinogradova,
V. E. Soboleva,
Alexander V. Vinogradova and
Vladimir V. Vinogradov*a
aLaboratory of Solution Chemistry of Advanced Materials and Technologies, ITMO University, St Petersburg, 197101, Russian Federation. E-mail: Vinogradoffs@mail.ru
bIvanovo State University of Chemistry and Technology, Ivanovo, 153000, Russian Federation
cIvanovo State Medical Academy, Ivanovo, 153012, Russian Federation
First published on 17th October 2014
Abstract
The synthesis of new biocomposites exhibiting a synergistic effect is a promising step in the healing of acute and chronic wounds. In the present study we have combined four materials: chlorhexidine digluconate as a antimicrobial agent, lidocaine as a painkiller, chymotrypsin as a necrolytic agent, and sol–gel processed alumina as a carrier for the sustained delivery of drugs and as an established wound healer. Composites were synthesized and characterized for surface morphology, crystalline structure and in vitro drug release. In vivo wound healing efficacy was assessed using a full thickness excision wound model in Wistar rats. The main result, was that a marked decrease in scar size was observed because of the wound healing composite, in fact the area of the scar in the test group of rats was 2.4 times smaller than that in the control group. Wound closure analysis revealed that complete epithelialization was observed after 15 ± 1 days using the biocomposite, whereas this took 17 ± 1 days and 19 ± 1 days using the healing solution alone or pure alumina gel, respectively. It was concluded that the synergistic combination of healing drugs, with sol–gel alumina as dressing material, provides a highly attractive biomaterial for the treatment of surface wounds, burns and foot ulcers.
1. Introduction
It is known that every year one-tenth of the world's population suffers some kind of injury, and the number of victims increases sharply during hostilities, acts of terrorism and natural disasters. The search for new wound healing drugs is therefore a major concern.1,2 Wound healing is a complex process the course of which requires a balance of micro-elements, antioxidants, matrix metallo-proteinases and other factors.3 The modern strategy for the effective management of surface wounds, burns and foot ulcers is to develop biomaterials, which produce a synergistic effect.4 Advanced wound healing biomaterials combine current drug treatments and tissue engineering substitutes to address the pathology of acute and chronic wounds.5 As a general rule, more effective wound healing materials give a decrease in the size of the final scar, which is related to minimal inflammatory response.6Although inflammation is inevitable during normal healing, the presence of a macrophage on a wound bed can inhibit the subsequent proliferative phase.7 Bacterial infections are the main cause of prolonged inflammatory processes. The study of the mechanisms of inflammatory response to wound infection has become the subject of intense research. Many researchers are focusing on developing wound dressing materials with good antimicrobial properties which minimize local contamination and infection from surrounding areas.8 Infectious organisms are often present at the wound site beneath the dressing, resulting in serious infections that require repeated removal of the dressing material and the use of local antimicrobial therapy.9 Modern wound healing materials usually exhibit bactericidal, anaesthetic and necrolytic properties10 and in the present study, chlorhexidine digluconate (CH), lidocaine (LD) and chymotrypsin (CHTR), respectively, were chosen for this purpose.
To prepare an advanced biomaterial with exceptional wound healing properties, it is essential to choose a biocompatible drug carrier, which has been approved for parenteral injection into the human body. Sol–gel alumina is the ideal candidate, because it is the only example of a metal oxide which is approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a common immunologic adjuvant. Since immunologic adjuvants do not themselves exhibit biological activity in wound healing, sol–gel alumina is regarded as an inert matrix.
In our previous studies an original approach to the synthesis of high purity alumina and titania sols in aqueous solution11,12 was developed. The sols obtained were mixed with albumin and subsequently dried and then the thermal stability of the proteins was studied. The results revealed a shift to higher temperatures for a number of systems, amounting to more than 51 °C higher than the native conditions for protein molecules. This observation was subsequently extended using specific enzymes in intractable disease therapy, for example asparaginase, horseradish peroxidase, and acid phosphatase. Samples were studied in vitro using a series of physico-chemical methods. The results showed that enzymes not only retained their activity, but also demonstrated remarkable stability, with no significant change in activity even under prolonged heating up to 60 °C.13
In the present study it has been demonstrated that a group of medical compounds, including LD, CH and CHTR, entrapped in an alumina sol–gel film exhibit exceptional properties during the wound healing process and produced a significant decrease in scar size. For comparison, the wound healing properties of both a matrix and the individual drugs in solution were also studied. To the best of our knowledge this is the first example of a decrease in scar size and the promotion of wound healing given by sol–gel materials. The objective of the present study was to evaluate the wound healing efficacy of a sol–gel alumina biocomposite and to assess its applicability as a wound dressing material.
2. Materials and methods
2.1 Chemicals
Aluminum isopropoxide (Al(C3H7O)3), lidocaine hydrochloride (2% solution), α-chymotrypsin (catalogue no C4129), chlorhexidine digluconate (20% solution) were all obtained from Sigma-Aldrich. Glycine buffer (pH = 7.4) was prepared using glycine solutions (0.05 M; Sigma-Aldrich) and the required volume of 1.0 M NaOH.2.2 Preparation of sol–gel alumina
The alumina sol was prepared using a previously reported method.13 In detail, 2.2 g of Al(C3H7O)3, was added to 50 mL deionized water at 90 °C, and a white precipitate was immediately formed. Prior to ultrasonic mixing the precipitate was held at 90 °C for 15 min under vigorous stirring to allow the production of boehmite nanoparticles and to evaporate the isopropanol formed during hydrolysis. The final suspension was mixed ultrasonically for 2 h, during which a viscous sol formed and which was then cooled to room temperature. For the preparation of wound coverings, 25 mL of the sol was dried to a volume of 5 mL in a vacuum desiccator at room temperature. The final gel readily produced dense boehmite films within 10 minutes after coating open areas of skin.2.3 Preparation of the healing solution
To prepare the healing solution, a mixture of 1 mL of 20% CH solution and 4 mL of LD solution was prepared, and 20 mg of CHTR was subsequently dissolved in the solution. Immediately prior to coating the wound the final solution was held for 30 min at 37 °C under constant stirring.2.4 Preparation of the healing composite based on sol–gel alumina
Prior to coating, 25 mL of freshly synthesized sol was mixed with 5 mL of healing solution. The mixture was dried in a vacuum desiccator at room temperature to give a volume of 5 mL. Similarly to pure alumina gel, the final composite readily produced a film within 10 min of coating on open skin areas. In this case the quantity of medicine and matrix on the wound was equivalent to that used for treating the wound with the individual components.2.5 Assessment of wound healing properties
Male Wistar rats (body weight range, 250–280 g) were used for the study. The animals were acclimatized under standard animal laboratory conditions for seven days before the experiment. All experiments were approved by the institutional animal ethics committee of the Ivanovo State Medical Academy, Russia, and were in agreement with the guidelines for the use of animals in biomedical research. The animals were divided into four groups of five rats:Group 1: medicines loaded in sol–gel alumina (test group).
Group 2: healing solution with test medicines (reference group).
Group 3: sol–gel alumina (reference group).
Group 4: undressed wound (control group).
The animals were anaesthetized with ketamine (60 mg kg−1) by intraperitoneal injection, and the dorsal hair was shaved and the area disinfected. Full thickness wounds measuring 1 × 1 cm2 were created by excising the dorsal skin. The dressings were applied to the excised wounds, covered and held in place with absorbent gauze. The wounds were treated daily with 0.5 mL of either alumina or composite gel. As a reference, 0.1 mL of healing solution was also used. Wound sizes were measured daily until the healing was complete. For this the wound outline was transferred to a transparent film once each day and scanned and then the wound area was calculated using ImageJ 1.30v software, and the percentage wound reduction calculated according to the following formula:14
| Cn = [(S0 − Sn)/S0] × 100, | (1) |
2.6 Characterization
Prior to analysis the wet gels were dried at room temperature and then degassed for 24 h at room temperature. Specific surface area, pore volume and pore size distribution were determined using nitrogen adsorption–desorption at 77 K (Quantachrome Instruments, Nova 1200e). Surface area was calculated using the Brunauer, Emmett and Teller (BET) technique, and pore volume and distribution by the Barrett–Joyner–Halenda (BJH) method. The crystal phase and crystallinity of the samples were determined by X-ray diffraction (XRD; Bruker, D8 Advance) using CuKα irradiation (λ = 1.54 Å), samples being scanned along 2θ within the range 4–75° at a speed of 0.5° per minute.Analysis of the amorphous and crystalline phases was carried out using TOPAS (Bruker) software. The drug release study was carried out using a double beam spectrophotometer (PG Instruments, T80) at a fixed wavelength of the maximum absorbance for the compound released (LD at 264 nm, CH at 300 nm and CHTR at 224 nm). Samples for transmission electron microscopy (TEM; FEI, Tecnai G2 F20, at an operating voltage of 200 kV) were obtained using a small probe in a homogeneous suspension in ethanol, the droplet obtained being coated on a copper mesh covered with carbon.
3. Results and discussion
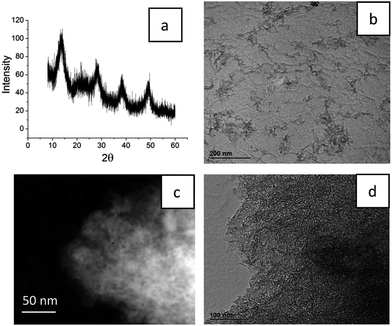
Development of new wound healing materials is not only accompanied by new architecture for the biocomposites and their composition, but must also meet the strict requirements of the world agencies, the FDA and EMA, and the regulators of pharmaceutical applications. Because penetration of particles and medical compounds of the wound healing materials into blood is often inevitable, all the components must be approved by the FDA for parenteral injection into the human body. From this point of view, the use of sol–gel alumina is perfectly reasonable because boehmite–aluminum oxyhydroxide is already widely applied as an adjuvant.15,16To identify the crystallinity of the structure, the composite synthesized using sol–gel alumina and containing medical compounds was studied using XRD (Fig. 1(a)). The position of the maxima in the XRD pattern was typical of the boehmite structure. Analysis of the size of the crystallites in the material using the Scherer equation indicated an average of 3–4 nm. A characteristic halo in the area 2θ = 20–25 indicated the presence of the amorphous organic phase. Quantitative analysis of the amorphous and crystalline phases gave ratios of 12% and 88%, respectively.
To produce composites providing slow release of medicines, a drug carrier was used which has a well developed structure of micro- and meso-pores.17 In this case the pore size was probably the result of interparticle spacing in the alumina sol–gel matrix. An alumina hydrosol synthesized using ultrasound presented a set of branched inorganic polymer chains consisting of boehmite nanorods (Fig. 1(b)). During the sol condensation, a strong compression of the structure of the alumina scaffold was observed, resulting in a uniform distribution of drugs along the internal surface of the matrix. As seen in Fig. 1(c) and (d), the composite presents a homogeneous structure with uniform pore size distribution. Because it is the porous structure that determines the further release of medicines, nitrogen adsorption–desorption was used to provide a more detailed and thorough analysis.
Surface area and porosity analysis (using nitrogen adsorption, results analyzed using the BET and BJH equations) were in agreement with typical micro-mesoporosity (Fig. 2(a) and (b)). For the composite the values were: surface area: 114 m2 g−1, pore volume: 0.105 cm3 g−1 and pore size < 3 nm, with a maximum at 2.8 nm (BJH method). Similar results were obtained for pure sol–gel alumina: surface area: 134 m2 g−1, pore volume: 0.112 cm3 g−1 and pore size: 2.7 nm. It is believed that this size is optimal, because on the one hand the release of drugs should proceed relatively slowly to give a prolonged effect and, on the other hand, rapid and unimpeded removal of excess moisture formed at the wound may take place because of high affinity of the ceramic alumina matrix for water molecules. To confirm this, we studied the release of medicines from a porous composite at pH 7.4.
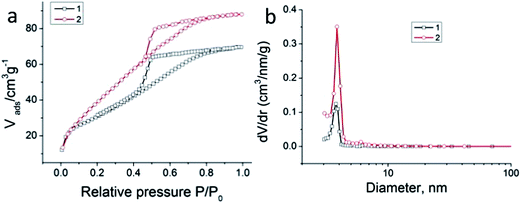 | ||
| Fig. 2 (a) Nitrogen adsorption–desorption isotherm, and (b) BJH mesopore size distribution of pure sol–gel alumina (1) and a composite containing medicines (2). | ||
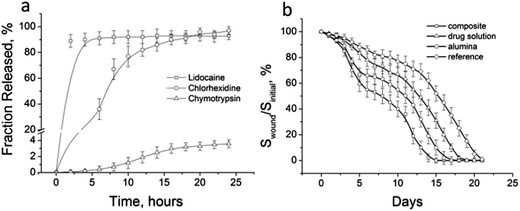
Although the three medicines were simultaneously entrapped in the boehmite matrix, the rate of their release may differ significantly. In an ideal situation the maximum release of LD would be desirable during the hours soon after the damage has been caused, because at this stage the sensation of pain is most intense and may have to be treated with anaesthetics. The release of an antibacterial agent should proceed uniformly to prevent the formation of colonies of bacteria, both directly at the wound and also in the dressing material. Release of a necrolytic drug should proceed at the final stage, essentially after the formation of a scab and once the process of tissue necrosis is completed.
Release curves for composite drugs are shown in Fig. 3(a). It is worth noting that the gel was applied to the wound just once per day, and the release test therefore lasted 24 h. The medicines were released in a manner close to the ideal. A 91% release of the LD had already occurred during the first 3 h. At the same time, CH was released gradually and the curve flattened only after 20 h, corresponding to a release of 90% of the drug. CHTR is a small size (25 kDa–1.5 nm (ref. 18)) enzyme, nevertheless its release is very strongly limited to diffusion over the micro/mesoporous network of the matrix. As expected, release of CHTR during the first 5 h did not occur, and over 24 h it amounted to only 3.9%.
These contrasting release profiles can be fitted remarkably well (Table 1), giving high correlation coefficients, R2, using the empirical Weibull model adapted to heterogeneous systems.19 Within this model, the ratio Q(t)/Q0 between the cumulative percentage of drug released at time t and at infinite time was:
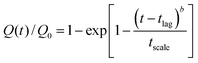 | (2) |
| LD | CH | CHTR | |
|---|---|---|---|
| Q0 (%) | 91 | 90 | 3.9 |
| b | 0.82 | 1.74 | 2.69 |
| tlag (h) | 0.02 | 0.07 | 5.1 |
| tscale (h) | 3.2 | 16.7 | 22.2 |
| R2 | 0.995 | 0.993 | 0.991 |
The parameter b can thus provide an indication of the degree of homogeneity of the extractable population:20 a value close to 1 implying a relatively homogeneous extractable population, while a value far from 1 implies sample heterogeneity. The high b value for the curve of CHTR release is thus indicative of slow release. This heterogeneity of the release of CHTR is a consequence of strong diffusion limitations, the pore size being complementary to the quantity of product released.
Further experiments were concerned with in vivo tests. A group of rats without a wound healing coating were used as a control group. Groups of rats treated with either sol–gel alumina or healing solution were used as reference, and the composite sol–gel alumina containing healing solution was used as the test sample. The final estimates included the wound healing time, T, and the size of the post-operative scar, L are given in Table 2.
| T (d) | L (mm2) | |
|---|---|---|
| Medicine loaded sol–gel alumina (test group) | 15 ± 1 | 41 ± 7 |
| Healing solution with medicines (reference group) | 17 ± 1 | 55 ± 10 |
| Sol–gel alumina (reference group) | 19 ± 1 | 76 ± 5 |
| Undressed wound (control group) | 21 ± 1 | 93 ± 12 |
Fig. 3(b) shows the kinetic curves for the change in the wound area during healing. One can see that experimental full thickness wounds treated with the composite were completely healed after 15 days, but wound healing for the control group occurred only after 21 days. Treating with the healing solution promoted a four day decrease in time for complete wound healing. Coating with the alumina gel alone reduced complete healing by only two days.
Despite the fact that the alumina matrix was inert and did not participate in the biological process of wound healing, it accelerated the process because of its mechanical features. Because of the capillary effect of the drying process, contraction of the wound area occurred, resulting, as a consequence, in more rapid healing. The effect of the contraction on the acceleration of wound healing has been observed previously.21 The application of a biocomposite is characterized by simultaneous wound contraction and biological recovery of the skin. Thus, there was a benefit from use of the composite, the substantial synergistic effect being particularly pronounced during the first day following skin damage.
It is known that wound healing is a complex set of biological processes and that they can be divided into three stages (inflammation, regeneration, and formation and reorganization of the scar), characterized by the occurrence of a number of specific reactions.22 A two-fold decrease in the area of the wounds in the first days compared to the control group because of the action of the composite indicated a substantial influence of the film on the inflammation stage. This was characterized by the cleaning of the wound and the migration of macrophages to the site of injury, activating the formation of collagen by fibroblasts. In addition, the prolonged antibacterial effect of CH inhibited the secondary infection of the wounds and wound dressings. This course of wound healing is the main priority and is considered to be the most economical and functionally favorable, in other words the best way to heal wounds.23
In addition to objective healing factors, the general condition of the rats was assessed. After wounding, the control group was characterized by a general emotional disorder accompanied by constant tail twitching. On the other hand, the behavior of the test group was normal, clearly affected by the release of LD, which has an analgesic effect.
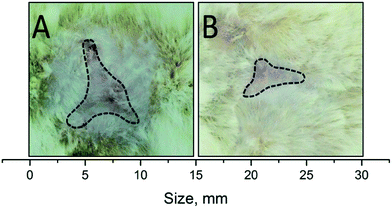
Apart from quicker wound healing, the main result observed was the strong decrease in scar size because of the effect of the sol–gel alumina biocomposite (Fig. 4). As seen in Table 2, the area of the scar in the test group was almost 2.4 times smaller than that in the control group. This effect is particularly relevant in the case of large wounds, where patients may suffer from motor dysfunction because of the tightening effect.
The decrease in scarring is associated with the minimal inflammatory response of the body when the composite is used. As seen in Fig. 3(b), the highest rate of wound healing on the first day was typical in the test group of rats. It is known that wound healing is a multi-component process, characterized by a series of stages reflecting the dominant biological mechanisms and ending with scar formation and epithelialization of the wound.24 Damage arising at one stage has a negative impact on the whole process of wound healing. Inflammatory response, triggering the mechanism of wound cleaning from necrosis, may in certain situations be itself a source of tissue alteration. This is most clearly manifested when septic inflammation provokes secondary necrosis, involving intact tissue. This appears to have been observed in the control and test groups of rats. Occurrence of necrotic tissue is an additional factor in the progression of wound infection, lengthening wound healing and forming rough scar tissue. These facts indicate minimal inflammatory response in the wound because of the action of the composite and provision of conditions that eliminated, or at least minimized, the development of septic inflammation.
4. Conclusions
The present study for the first time has considered wound healing materials based on sol–gel alumina. The alumina gel was used to carry medicines (CH, LD and CHTR). The final composite was coated on the wound and produced xerogel films within 10 minutes with medicines entrapped. A morphological study showed a uniform pore size distribution in the alumina matrix, allowing slow release of the medicines. The sustained delivery of medicines from the alumina matrix certainly helped to combat infection throughout the application period. An in vivo study revealed that the matrix developed showed an excellent rate of wound healing and an exceptional decrease in scarring – the scar area for the test group of rats turned out to be almost 2.4 times smaller than that in the control group. In addition, the wound healing composite showed a 30% increase in the rate of healing compared to the control group. It is concluded that the biocomposite based on sol–gel alumina has high potential as a dressing material effective for the treatment of either infectious or chronic wounds.Acknowledgements
We appreciate greatly, the fruitful discussions with Professor David Avnir of the Hebrew University, who pioneered investigations into injectable sol–gel alumina. The authors are also grateful to the Center for Nanoscience and Nanotechnology at the Hebrew University for assistance with the TEM and scanning electron microscopy experiments.References
- Center for Disease Control and Prevention, 2011.
- G. A. James, E. Swogger, R. Wolcott, E. de Lancey Pulcini, P. Secor, J. Sestrich, J. W. Costerton and P. S. Stewart, Wound Repair Regen., 2007, 16, 37–44 CrossRef PubMed
.
- G. E. J. Poinern, D. Fawcett, Y. J. Ng, N. Ali, R. K. Brundavanam and Z.-T. Jiang, J. Biomed. Nanotechnol., 2010, 6, 497 CrossRef CAS PubMed
.
- S. Perumal, S. K. Ramadass and B. Madhan, Eur. J. Pharm. Sci., 2014, 52, 26 CrossRef CAS PubMed
.
- K. S. Midwood, L. V. Williams and J. E. Schwarzbauer, Int. J. Biochem. Cell Biol., 2004, 36, 1031 CrossRef CAS PubMed
.
- G. G. Gauglitz, H. C. Korting, T. Pavicic, T. Ruzicka and M. G. Jeschke, J. Mol. Med., 2011, 17, 113 CAS
.
- P. M. Newton, J. A. Watson, R. G. Wolowacz and E. J. Wood, J. Inflammation, 2004, 28, 207 CrossRef CAS
.
- M. D. Kerstein, Am. J. Surg., 1994, 167, 2 CrossRef
.
- F. L. Mi, Y. B. Wu, S. S. Shyu, J. Y. Schoung, Y. B. Huang, Y. H. Tsai and J. Y. Hao, J. Biomed. Mater. Res., 2002, 59, 438 CrossRef CAS PubMed
.
- J. S. Boateng, K. H. Matthews, H. N. E. Stevens and G. M. Eccleston, J. Pharm. Sci., 2008, 97, 2892 CrossRef CAS PubMed
.
- A. Rutenberg, V. V. Vinogradov and D. Avnir, Chem. Commun., 2013, 49, 5636 RSC
.
- A. V. Vinogradov, A. V. Ermakova, M. F. Butman, E. Hey-Hawkins and V. V. Vinogradov, Phys. Chem. Chem. Phys., 2014, 16, 10614 RSC
.
- V. Vinogradov and D. Avnir, J. Mater. Chem. B, 2014, 2, 2868 RSC
.
- S. N. Park, H. J. Lee, K. H. Lee and H. Suh, Biomaterials, 2003, 24, 1631 CrossRef CAS
.
- E. B. Lindbland, Immunol. Cell Biol., 2004, 82, 497 CrossRef PubMed
.
- H. Li, Y. Li, J. Jiao and H.-M. Hu, Nat. Nanotechnol., 2011, 6, 645 CrossRef CAS PubMed
.
- C. Barbe, J. Bartlett, L. G. Kong, K. Finnie, H. Q. Lin, M. Larkin, S. Calleja, A. Bush and G. Calleja, Adv. Mater., 2004, 16, 1959 CrossRef CAS
.
- H. P. Erickson, Biol. Proced. Online, 2009, 11, 32 CrossRef CAS PubMed
.
- P. Macheras and A. Iliadis, Modeling in Biopharmaceutics, Pharmacokinetics, and Pharmacodynamics, Springer, New York, 2006 Search PubMed
.
- R. Ben-Knaz, R. Pedahzur and D. Avnir, Adv. Funct. Mater., 2010, 20, 2324 CrossRef CAS
.
- Y.-H. Lee, J.-J. Chang, C.-T. Chien, M.-C. Yang and H.-F. Chien, Exp. Diabetes Res., 2012, 504693, DOI:10.1155/2012/504693
.
- J. H. Musset and A. J. Winfield 1998, Wound management, stoma and incontinence products, in Pharmacy practice, ed. A. J. Winfield and R. M. E. Richards, Churchill Livingstone, UK, 2nd edn, pp. 176–187 Search PubMed
.
- A. Agarwal, T. B. Nelson, P. R. Kierski, M. J. Schurr, C. J. Murphy, C. J. Czuprynski, J. F. McAnulty and N. L. Abbott, Biomaterials, 2012, 33, 6783 CrossRef CAS PubMed
.
- F. García-Esteo, G. Pascual, N. García-Honduvilla, A. Gallardo, J. San Román, J. M. Bellón and J. Buján, Histol. Histopathol., 2005, 20, 53 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2014 |