A bioinspired light induced avenue for the design of patterned functional interfaces†
Corinna M.
Preuss
ab,
Thomas
Tischer
ab,
Cesar
Rodriguez-Emmenegger
abc,
Markus M.
Zieger
ab,
Michael
Bruns
d,
Anja S.
Goldmann
ab and
Christopher
Barner-Kowollik
*ab
aPreparative Macromolecular Chemistry, Institut für Technische Chemie und Polymerchemie, Karlsruhe Institute of Technology (KIT), Engesserstr. 18, 76131 Karlsruhe, Germany. E-mail: christopher.barner-kowollik@kit.edu; Web: http://www.macroarc.de
bInstitut für Biologische Grenzflächen (IBG), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
cCell- and Neurobiology, Zoologisches Institut, Karlsruhe Institute of Technology (KIT), Haid-und-Neu-Str. 9, Karlsruhe, Germany
dInstitut für Angewandte Materialien (IAM), Karlsruhe Nano Micro Facility (KNMF), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 22nd October 2013
Abstract
An avenue for the development of spatially resolved functional interfaces is presented. By introducing a novel, photo-reactive molecule – carrying a DOPA functionality and a photo-reactive group – we merge the ability of mussels to adhere to any surface with the spatial and temporal control of photo-click reactions, opening a plethora of applications in the biomedical and materials fields.
Over the last decade, bioinspired mussel adhesives have been extensively studied by many researchers in various fields, such as polymer chemistry, materials science, biochemistry and medicine.1–3 The inimitable ability of marine organisms such as mussels and other bivalves to adhere to virtually any material, such as metal, rocks, wooden piers or ships even under aqueous maritime conditions as well as their remarkable ability to resist turbulent tidal environments inspired scientists to develop a plethora of novel applications.1,2,4–7 Maritime mussels exhibit an exceptional adhesion system – the mussel byssus – which is composed of a bundle of extracellularly secreted collagenous threads and adhesive plaque at their distal ends. The latter allow the bivalve to glue to virtually any surface.1,2,8 The much-acclaimed strong adhesion originates from the composition of proteins that were found near the plaque–substrate interface, i.e. Mytilus edulis foot protein 5 (Mefp-5).1,9 Within these protein sequences, L-lysine and 3,4-dihydroxy-L-phenylalanine (L-DOPA) were identified to be responsible for the strong adhesion properties.1 Inspired by the composition of mussel adhesive proteins, DOPA and dopamine, the decarboxylated derivative of DOPA, were utilized as precursor molecules for biomimetic mussel adhesives. Swan et al. and later Messersmith et al. proposed a method to form multifunctional polymer coatings by simple dip-coating of different materials in an aqueous solution of dopamine.1,10 Under aqueous alkaline conditions (pH = 7.4–8.5), dopamine spontaneously oxidizes and forms highly reactive products such as 5,6-dihydroxyindole, 5,6-indole-semiquinone and -quinone,1 forming water-insoluble polymers through polycondensation between the indole units.11,12 These self-assemble and produce colloidal particles that further aggregate, resulting in a thin homogeneous layer at the surfaces in contact with the solution.1,13,14 This process is also observed for L-DOPA15 and for the natural generation of eumelanine, a bio-polymer formed in vivo by enzymatic (tyrosinase catalyzed) metabolism of L-tyrosine in a process designated as melangonesis.16 Importantly, contrary to catechol containing molecules which can only self-assemble on metal oxides, poly(DOPA) and poly(dopamine) form highly adhesive films even on low surface energy materials.17
Polymerization of various catecholamines can be induced by employing a Tris-buffer solution of pH = 8.5.18 Lee et al. showed the attachment of these polydopamine glues onto a wide variety of materials, even those with very low surface energy such as poly(tetrafluoroethylene).1 Kim et al. presented the attachment on biological surfaces.1,19 Based on these findings, several biomimetic mussel adhesive materials have been developed for biomedical applications such as tissue engineering, silver-releasing antibacterial hydrogels, biointerfaces and sealants for fetal membrane repair.7,20–23 Several research groups have employed dopamine and DOPA in numerous surface modification and functionalization studies.1,2,17,23–27 The ability to pattern cell adhesive moieties and growth factors as well as other bio-/artificial molecules is of central importance to modify the surface of implants in order to increase compatibility, induce the adhesion of specific cells and promote the integration with surrounding tissues.28 In principle various techniques such as μ-contact printing and lithography have been used to pattern dopamine-containing molecules onto various surfaces, and yet are not the subject of the present study. These approaches are often of limited applicability as the complex shape and topography of implants as well as the need for precise temporal and spatial control of the patterned molecules pose an insurmountable challenge. This is a critical problem for translating these technologies to medical practice.
If the technology is to be translated into practice, the unmatched adhesion properties of poly(dopamine) or DOPA–melanine films need to be combined with modular ligation protocols allowing facile control of the patterning. Recently, we introduced a post-polymerization modification of poly(dopamine) films with a tetrazole acid chloride, which allowed us to spatially control the adhesion of fibroblasts.26 However, avoidance of harsh conditions such as the use of an acid chloride is prerequisite when labile or biological substrates are modified. This can be achieved utilizing a DOPA analogue bearing a photo-clickable moiety. Under mild alkaline conditions, it could generate thin highly adherent films offering the ability to photo-pattern substrates regardless of their surface energy.
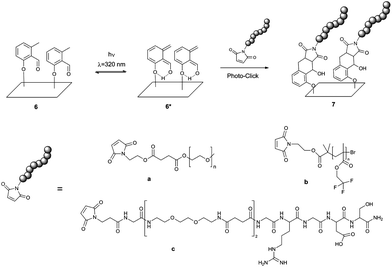
The present work merges the biomimicry and extraordinary ability of DOPA–melanine films to attach to a wide variety of materials with the exquisite bio-orthogonality of photo-triggered photoenol ligations. We synthesized a new molecule, photo-DOPA (5), bearing a DOPA moiety, which under alkaline conditions can polymerize in a similar fashion as polydopamine and DOPA–melanine10,12,16,29,30 forming ultra-thin films on virtually any surface. Photo-DOPA possesses an o-methylphenyl aldehyde moiety (photoenol (PE)), which is covalently bonded to DOPA and operates as a diene in a Diels–Alder (DA) reaction after irradiation in the UVA region (λ = 320 nm).31,32 The highly reactive diene can react in a DA reaction with suitable electron-deficient dienophiles, such as maleimides, or in a hetero-DA (HDA) reaction with the dithioester moiety of a polymer prepared by reversible addition fragmentation chain transfer (RAFT) polymerization.31,33 This type of photo-ligation reaction is commonly referred to as a photoenol reaction. Our team first applied this system to macromolecular ligation processes.31 Additionally, the concept of photo-triggered conjugation reactions on surfaces, such as photoenol reactions, oxime ligations, phencyclone-amine reactions and trapping of photo-generated thioaldehydes with nucleophiles for spatially controlled surface grafting, were further investigated by our group.32,34–36 Compared to previously reported photo-conjugation methods, the photoenol ligation fulfills the stringent conditions of a click reaction as well as being markedly more efficient, not generating by-products nor fluorescent products.
In the current study, we merge the remarkable adhesion properties of mimicking mussel adhesives based on L-DOPA with the concept of photoenol-ligation to form a surface grafting system, which (i) allows modification of a great number of materials with different topographies and surface energy, (ii) can selectively and bio-orthogonally attach different polymers and peptides and, most importantly (iii) allows for spatially and temporal control over the ligation. In addition, the utilization of light induced ligation for the design of functional biointerfaces enables spatial and temporal control of the functionalities ligated to the interface, a feature not provided by thermally driven reactions. It is noteworthy that, having the photoenol (PE) functionality covalently bound to every DOPA unit assures that PE is available on the entire surface, regardless of the topography or shape of the substrate. Thus, the approach presented here can be readily extended to other systems.
For this purpose, a new molecule, namely 2-((2-((tert-butoxycarbonyl)amino)-3-(3,4-dihydroxyphenyl) propan-oyl)oxy) ethyl 4-((2-formyl-3-methylphenoxy)methyl) benzoate (photo-DOPA) (5) (Fig. 1) was synthesized. Subsequently, 5 was attached to gold (Au), PET (poly(ethyleneterephthalate)) and graphite substrates in a Tris-buffer–ethanol mixture, followed by photoenol-ligation with maleimide-carrying polymers and peptides on selected surfaces (Fig. 2). Surface characterization was performed via X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS).
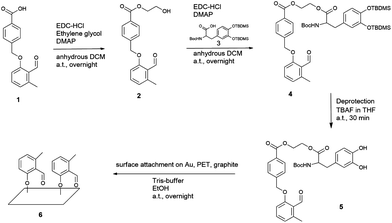 | ||
Fig. 1 Synthesis of photo-DOPA (5) and the following surface attachment to Au, PET and graphite substrates are depicted. Photo-COOH (1) was modified in two esterification steps with, firstly, ethylene glycol and, secondly, DOPA–TBDMS2 (3) to give photo-DOPA–TBDMS2 (4). The catechol groups were deprotected in one step (5) and the surface attachment on various substrates was immediately conducted in a Tris-buffer–ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to form the photo-reactive surface (6). For experimental details see ESI.† 1) to form the photo-reactive surface (6). For experimental details see ESI.† | ||
The novel photoenol–DOPA compound photo-DOPA (PE–DOPA on the surfaces) (5) was prepared in a 3 step synthesis. Firstly, photo-COOH (1) was esterified in a Steglich-type reaction, employing ethylene glycol as the corresponding alcohol to generate the linker molecule photo-Gly (2), which connects the photoenol with the DOPA moiety (Fig. 1). In a second Steglich-type esterification step, TBDMS (tert-butyldimethylsilyl) and Boc (tert-butyloxycarbonyl) protected DOPA (DOPA–TBDMS2) (3) were reacted with the remaining OH moiety on photo-Gly (2) to form the protected species photo-DOPA–TBDMS2 (4) of the target molecule 5. The following deprotection reaction of the catechol group was performed in a 1 M tetra-n-butyl ammonium fluoride (TBAF) solution to yield photo-DOPA (5). For all experimental and analytical details refer to the ESI.† The facile substrate modification was carried out by immersing them in a photoenol–DOPA molecule 5 solution in a Tris-buffer (0.3 M, pH = 8.5)–ethanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (as 5 is soluble in ethanol) onto various substrates (Au, PET, graphite). After 12 h of incubation in the aqueous solution, the substrates exhibited a visible change in colour ascribed to the oxidation of catechol followed by polymerization. Importantly, we found that after coating the contact angle of surfaces with initially very different surface energy (advancing water contact angle of Au 53° and PET 79°) become the same (∼87°), thus demonstrating that the current approach shares the substrate-independent nature film-forming of poly(dopamine) and DOPA–melanine.
1) (as 5 is soluble in ethanol) onto various substrates (Au, PET, graphite). After 12 h of incubation in the aqueous solution, the substrates exhibited a visible change in colour ascribed to the oxidation of catechol followed by polymerization. Importantly, we found that after coating the contact angle of surfaces with initially very different surface energy (advancing water contact angle of Au 53° and PET 79°) become the same (∼87°), thus demonstrating that the current approach shares the substrate-independent nature film-forming of poly(dopamine) and DOPA–melanine.
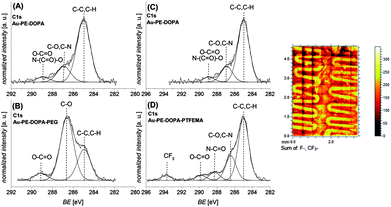
The chemical composition, assessed by XPS, further supports the adhesion properties of 5 on miscellaneous materials that possess different surface energy and abilities to form bonds (6) (for XPS data on graphite substrates, refer to the ESI†). Gold substrates (Au) were chosen as the basic system as they do not possess organic groups interfering with characterization and the relevance of Au surfaces for biomedical and biosensing applications. PET was especially employed to demonstrate photo-ligation of peptide sequences on a flexible substrate which is commonly employed in biomedical devices.
The photoenol reaction on the surface is depicted in Fig. 2. The photo-reactive surface 6 was irradiated with an Arimed B6 UVA lamp (λ ≈ 320 nm) to form an o-xylylene intermediate (6*), which further reacts in a DA reaction with maleimide functionalized (a) poly(ethylene glycol) (Mal-PEG), (b) poly(2,2,2-trifluoroethyl methacrylate) (Mal-PTFEMA) or (c) peptide sequence (Mal-PEP) that contains the amino acid sequence Gly-Arg-Gly-Asp-Ser. All surfaces were characterized via XPS analysis (see ESI† and Fig. 3 and 4). In order to evidence the excellent spatial control of the novel photo-clickable biomimetic system, we studied the ligation of Mal-PTFEMA by employing a shadow mask during irradiation (Scheme 1).
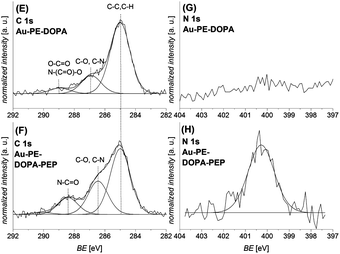 | ||
| Fig. 4 C 1s ((E) and (F)) and N 1s ((G) and (H)) XPS spectra of the photo-ligation reaction of the peptide PEP-maleimide on the photo-reactive gold surface Au–PE–DOPA before ((E) and (G)) and after the photo-reaction ((F) and (H)) are depicted. After the conjugation, the signals assigned to the amide and C–N bonds increase in the C 1s and the N1s spectrum, which confirms the successful reaction. A similar ligation was performed on a PET–PE–DOPA substrate, which can be found in the ESI.† | ||
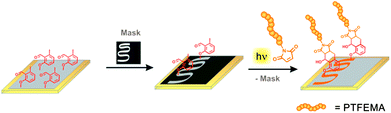 | ||
| Scheme 1 Surface patterning of the F-containing polymer PTFEMA onto a gold surface that was functionalized with photo-DOPA (Au–PE–DOPA) is depicted. A photo-reactive surface was partly covered with a shadow mask, fixed in a holder and irradiated in a PTFEMA-maleimide acetonitrile solution. Subsequently, the mask was removed and PTFEMA was identified only in the meander structured area of the surface via ToF-SIMS analysis (Fig. 3). | ||
Mal-PTFEMA provides fragments (F−, CF3−) which unambiguously evidence the patterning structure by the highly sensitive ToF-SIMS technique.
The PEG-maleimide a and PTFEMA-maleimide b were grafted onto the photo-reactive gold surface (Au–PE–DOPA) under irradiation in an acetonitrile solution for 60 minutes. Fig. 3 depicts the corresponding C 1s XPS spectra. The photo-ligation of PEG-maleimide a (B) onto the Au–PE–DOPA surface (A) was confirmed by the strong increase of the signal assigned to C–O bonds, stemming from the PEG chain, at 286.6 eV.37 The amount of C–O bonds compared to the total number of C–C and C–O bonds increases from 20% before to 66% after the photo-ligation. Sections (C) and (D) of Fig. 3 exhibit the C 1s spectra of the photoenol-surface before (C) and after (D) the photo-conjugation reaction with PTFEMA-maleimide b. The successful grafting of PTFEMA is demonstrated by the formation of the signal at 293.7 eV, which is characteristic of –CF3 groups and the appearance of a signal in the F 1s spectrum at 689.3 eV (refer to the ESI†).38,39 On the right-hand side of Fig. 3, the ToF-SIMS image of the overlay of the F− and CF3− fragments is depicted. The image unambiguously proves the ability of the presented system to be patterned. It is worth noting that – although in the current work we utilize the ‘grafting-to’ of polymers – higher thicknesses and grafting densities can be accessed by ligating maleimide-functional initiators or chain transfer agents enabling a ‘grafting-from’ approach. The approach presented in the current work paves the way for the development of smart systems with spatially resolved functions for a wide range of biomedical applications.
In the second approach, the maleimide-peptide sequence Mal-Gly-Arg-Gly-Asp-Ser (Mal-PEP) was grafted onto PET and Au photo-reactive substrates (PET–PE–DOPA and Au–PE–DOPA) in an acetonitrile–water mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 60 minutes. Fig. 4 depicts the corresponding C 1s and N 1s spectra before ((E) and (G)) and after ((F) and (H)) the photo-ligation. The C 1s spectrum after the ligation (F) exhibits an increase in the signals assigned to C–N and amide bonds (peptide) at 286.5 eV and 288.4 eV, indicating the presence of the peptide on the surface. Additionally, the N 1s spectrum (H) depicts a strong increase of the nitrogen signal at 400.3 eV, which further underlines the peptide attachment.37 Similar results were found for the same reaction sequence on a PET substrate (refer to the ESI†).
1) in 60 minutes. Fig. 4 depicts the corresponding C 1s and N 1s spectra before ((E) and (G)) and after ((F) and (H)) the photo-ligation. The C 1s spectrum after the ligation (F) exhibits an increase in the signals assigned to C–N and amide bonds (peptide) at 286.5 eV and 288.4 eV, indicating the presence of the peptide on the surface. Additionally, the N 1s spectrum (H) depicts a strong increase of the nitrogen signal at 400.3 eV, which further underlines the peptide attachment.37 Similar results were found for the same reaction sequence on a PET substrate (refer to the ESI†).
Conclusions
In summary, we present a novel photochemically driven avenue for the development of spatially and temporally resolved functional interfaces. We introduced a new, photo-reactive molecule that possesses a DOPA functionality and a photoenol. The former endows the molecule with the substrate-independent ability to form highly adherent films, while the latter allows photo-patterning of the surface with different moieties (synthetic polymers, peptides, etc.) that possess an electron-deficient dienophile. Our strategy allows generating surfaces able to perform variable functions in different regions and paves the way for the development of novel surface structured materials, biosensors and biomedical devices where the control of surfaces properties and functions is essential.Acknowledgements
C.B.-K. acknowledges continued funding from the Karlsruhe Institute of Technology (KIT) and the Helmholtz association via the BioInterfaces program, the German Research Council (DFG), and the Ministry of Science and Arts of the state of Baden-Württemberg supporting the current project. C. M. P.'s PhD studies are funded by the Deutsche Telekom Stiftung. C. R.-E. thanks the Alexander von Humboldt Foundation for financial support an Humboldt Research Fellowship for postdoctoral researchers and to the Grant Agency of the Czech Republic under Contract No P205121702. The authors acknowledge Prof. Hans Börner and Katharina Linkert (HU Berlin, Germany) for supplying a short chain maleimide-functionalized peptide. We additionally thank Dr. Alexander Welle (KIT) for his support in performing the contact angle measurements.Notes and references
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426 CrossRef CAS PubMed.
- K. J. Coyne, X.-X. Qin and J. H. Waite, Science, 1997, 277, 1830 CrossRef CAS.
- J. Saiz-Poseu, J. Sedó, B. García, C. Benaiges, T. Parella, R. Alibés, J. Hernando, F. Busqué and D. Ruiz-Molina, Adv. Mater., 2013, 25, 2066 CrossRef CAS PubMed.
- H. Silverman and F. Roberto, Mar. Biotechnol., 2007, 9, 661 CrossRef CAS PubMed.
- S. C. Nicklisch and J. H. Waite, Biofouling, 2012, 28, 865 CrossRef CAS PubMed.
- S. M. Kang, N. S. Hwang, J. Yeom, S. Y. Park, P. B. Messersmith, I. S. Choi, R. Langer, D. G. Anderson and H. Lee, Adv. Funct. Mater., 2012, 22, 2949 CrossRef CAS PubMed.
- C. M. Haller, W. Buerzle, C. E. Brubaker, P. B. Messersmith, E. Mazza, N. Ochsenbein-Koelble, R. Zimmermann and M. Ehrbar, Prenatal Diagn., 2011, 31, 654 CrossRef CAS PubMed.
- J. H. Waite, N. H. Andersen, S. Jewhurst and C. Sun, J. Adhes., 2005, 81, 297 CrossRef CAS.
- J. H. Waite and X. Qin, Biochemistry, 2001, 40, 2887 CrossRef CAS PubMed.
- J. A. G. King, A. Percival, N. C. Robson and G. A. Swan, J. Chem. Soc. C, 1970, 1418 RSC.
- M. D'Ischia, A. Napolitano, A. Pezzella, P. Meredith and T. Sarna, Angew. Chem., Int. Ed., 2009, 48, 3914 CrossRef CAS PubMed.
- J. Liebscher, R. Mrówczyński, H. A. Scheidt, C. Filip, N. D. Hădade, R. Turcu, A. Bende and S. Beck, Langmuir, 2013, 29, 10539 CrossRef CAS PubMed.
- O. Pop-Georgievski, N. Neykova, V. Proks, J. Houdkova, E. Ukraintsev, J. Zemek, A. Kromka and F. Rypaček, Thin Solid Films, 2013, 543, 180 CrossRef CAS PubMed.
- O. Pop-Georgievski, Š. Popelka, M. Houska, D. Chvostová, V. Proks and F. Rypáček, Biomacromolecules, 2011, 12, 3232 CrossRef CAS PubMed.
- M. Yu, J. Hwang and T. J. Deming, J. Am. Chem. Soc., 1999, 121, 5825 CrossRef CAS.
- M. D'Ischia, A. Napolitano, A. Pezzella, P. Meredith and T. Sarna, Angew. Chem., Int. Ed., 2009, 48, 3914 CrossRef CAS PubMed.
- O. Pop-Georgievski, S. T. P. N. Popelka, M. Houska, D. Chvostová, V. R. Proks and F. E. Rypáček, Biomacromolecules, 2011, 12, 3232 CrossRef CAS PubMed.
- M.-H. Ryou, Y. M. Lee, J.-K. Park and J. W. Choi, Adv. Mater., 2011, 23, 3066 CrossRef CAS PubMed.
- B. H. Kim, D. H. Lee, J. Y. Kim, D. O. Shin, H. Y. Jeong, S. Hong, J. M. Yun, C. M. Koo, H. Lee and S. O. Kim, Adv. Mater., 2011, 23, 5618 CrossRef CAS PubMed.
- G. Bilic, C. Brubaker, P. B. Messersmith, A. S. Mallik, T. M. Quinn, C. Haller, E. Done, L. Gucciardo, S. M. Zeisberger, R. Zimmermann, J. Deprest and A. H. Zisch, Am. J. Obstet. Gynecol., 2010, 202, 85 CrossRef PubMed.
- K. C. L. Black, J. Yi, J. G. Rivera, D. C. Zelasko-Leon and P. B. Messersmith, Nanomedicine, 2012, 8, 17 CrossRef PubMed.
- D. E. Fullenkamp, J. G. Rivera, Y.-K. Gong, K. H. A. Lau, L. He, R. Varshney and P. B. Messersmith, Biomaterials, 2012, 33, 3783 CrossRef CAS PubMed.
- O. Pop-Georgievski, C. Rodriguez-Emmenegger, A. d. l. S. Pereira, V. Proks, E. Brynda and F. Rypacek, J. Mater. Chem. B, 2013, 1, 2859 RSC.
- A. S. Goldmann, C. Schödel, A. Walther, J. Yuan, K. Loos and A. H. E. Müller, Macromol. Rapid Commun., 2010, 31, 1608 CrossRef CAS PubMed.
- J. Wang, M. N. Tahir, M. Kappl, W. Tremel, N. Metz, M. Barz, P. Theato and H.-J. Butt, Adv. Mater., 2008, 20, 3872 CrossRef CAS.
- C. Rodriguez-Emmenegger, C. M. Preuss, B. Yameen, O. Pop-Georgievski, M. Bachmann, J. O. Mueller, M. Bruns, A. S. Goldmann, M. Bastmeyer and C. Barner-Kowollik, Adv. Mater. DOI:10.1002/adma.201302492.
- V. Proks, J. Jaroš, O. Pop-Georgievski, J. Kučka, Š. Popelka, P. Dvořák, A. Hampl and F. Rypáček, Macromol. Biosci., 2012, 12, 1232 CrossRef CAS PubMed.
- B. Ratner and A. S. Hoffman, Biomaterial Science, 2nd edn, 2004 Search PubMed.
- P. A. Baldry and G. A. Swan, J. Chem. Soc., Perkin Trans. 2, 1977, 1346 RSC.
- G. A. Swan and A. Waggott, J. Chem. Soc. C, 1970, 1409 RSC.
- T. Gruendling, K. K. Oehlenschlaeger, E. Frick, M. Glassner, C. Schmid and C. Barner-Kowollik, Macromol. Rapid Commun., 2011, 32, 807 CrossRef CAS PubMed.
- T. Pauloehrl, G. Delaittre, V. Winkler, A. Welle, M. Bruns, H. G. Börner, A. M. Greiner, M. Bastmeyer and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2012, 51, 1071 CrossRef CAS PubMed.
- K. K. Oehlenschlaeger, J. O. Mueller, N. B. Heine, M. Glassner, N. K. Guimard, G. Delaittre, F. G. Schmidt and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2013, 52, 762 CrossRef CAS PubMed.
- T. Pauloehrl, G. Delaittre, M. Bruns, M. Meißler, H. G. Börner, M. Bastmeyer and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2012, 51, 9181 CrossRef CAS PubMed.
- T. Pauloehrl, A. Welle, M. Bruns, K. Linkert, H. G. Börner, M. Bastmeyer, G. Delaittre and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2013, 52, 9714 CrossRef CAS PubMed.
- T. Pauloehrl, A. Welle, K. K. Oehlenschlaeger and C. Barner-Kowollik, Chem. Sci., 2013, 4, 3503 RSC.
- M. Bruns, C. Barth, P. Brüner, S. Engin, T. Grehl, C. Howell, P. Koelsch, P. Mack, P. Nagel, V. Trouillet, D. Wedlich and R. G. White, Surf. Interface Anal., 2012, 44, 909 CrossRef CAS.
- R. Chebbi, A. Beicha, W. R. W. Daud and R. Zaamouche, Appl. Surf. Sci., 2009, 255, 6367 CrossRef CAS PubMed.
- S. Agraharam, D. W. Hess, P. A. Kohl and S. A. Bidstrup-Allen, J. Electrochem. Soc., 1999, 146, 1960 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3tb21317j |
| This journal is © The Royal Society of Chemistry 2014 |