Induction of mild therapeutic hypothermia in treatment of aluminium phosphide poisoning; an experimental study
Amir Mohammad
Kazemifar
a,
Esmail
Abbasi
*b,
Parisa
Bazahang
b,
Mina
lotfizadeh
b,
Seyyed Mohammad
Mehdi Mirjalili
c and
Hassan
Solhi
d
aDepartment of Internal Medicine, Qazvin's University of Medical Sciences, Qazvin, Iran. E-mail: dr.houshmand@yahoo.com; Tel: +989198487965
bDepartment of Pharmacology, Qazvin's University of Medical Sciences, Qazvin, Iran. E-mail: abbasiesmail2002@yahoo.com
cDepartment of Pathology, Qazvin's University of Medical Sciences, Qazvin, Iran
dDepartment of Toxicology, Arak's University of Medical Sciences, Arak, Iran
First published on 2nd September 2013
Abstract
Background: Exposure to aluminium phosphide (ALP) is a common cause of poisoning from agricultural pesticide exposures in some parts of the world. The absence of a specific antidote results in very high mortality due to acute ALP poisoning. The effect of induction of mild therapeutic hypothermia (MTH) on survival during poisoning from a lethal dose of ALP has been evaluated in the present study. Materials and methods: Male Sprague-Dawley rats were randomly divided into 4 groups. The first group received only the vehicle of ALP. The second group (SHAM group) received no drug. The third and the fourth group were given ALP in LD50 dose by gavage with an orogastric cannula. The core body temperature of animals in the fourth group was kept in the 32–35 degree centigrade range during the experiment (24 hours). The survival rate and histopathology of liver and kidney were used to evaluate outcomes. Findings: Mean survival time in group 3 was 9.143 hours (3.527–14.759; 95% CI), while it was 18.182 (14.730–21.634; 95% CI) in group 4. A Kaplan–Meier test showed that the difference between the groups was statistically significant (chi-square 15.408, p-value = 0.0001). The treatment group also experienced less histopathologic change attributed to ALP poisoning in their kidney and liver compared to the control group. However, only the difference in kidney histopathology was statistically significant (p-value = 0.016). Conclusion: Results of the current study showed that MTH may be beneficial in the treatment of acute ALP poisoning in rats in terms of survival rate and histopathologic changes in kidney and liver. Conducting experiments in humans is suggested in order to determine its true therapeutic value.
1. Introduction
Aluminium phosphide (ALP) has been reported to be severely toxic to both humans and animals, by virtue of its ability to liberate a highly poisonous gas, phosphine (PH3), under moist conditions.1 ALP is the most common agent of poisoning in the rural or sub-urban zones of some countries such as India, where it is usually ingested for suicide. It is also used as a suicide agent in Iran, but ALP poisoning in other countries may be due to occupational exposure.2–4Phosphine blocks the enzyme cytochrome-C oxidase as a result of which mitochondrial oxidative phosphorylation is inhibited. It also causes a severe drop in mitochondrial membrane potential, causing, in turn, the cells to die rapidly.8 Moreover, PH3 induces oxidative stress in many species. In insects, it inhibits the activity of catalase and peroxidase, and stimulates superoxide dismutase (SOD). In ALP-poisoned rats, elevated malondialdehyde (MDA) levels are observed in cardiac tissue.5,6
Acute ALP poisoning brings multi-organ involvement and variable clinical features. The most common signs and symptoms of ALP poisoning are severe nausea and vomiting, restlessness, abdominal pain, palpitation, resistant hypotension, metabolic acidosis, myocardial suppression, pulmonary edema, cyanosis, hypotension, shock and cardiac arrhythmias.7–10 Other rare effects include hepatitis, acute tubular necrosis, disseminated intravascular coagulation and respiratory alkalosis.8,11
The absence of a specific antidote results in very high mortality (60–80%) from acute ALP poisoning.12 The fast progression to life-threatening symptoms, ineffective therapeutic ways to solve its intoxication and limited data on the efficacy of therapeutic interventions pose challenges to the clinicians and emergency staff.3 There are some theoretical suggestions for the treatment of ALP poisoning such as hydroxyethyl starch,13N-acetyl cysteine and L-NAME,14 sodium selenite,3 and digoxin,12 though their efficacy is observed only in case reports.
Therapeutic hypothermia (TH) has principally been used as a protective therapy following various brain insults; however, there is emerging evidence that it may also be useful for the protection of other organs when at risk of injury.15,16 The use of therapeutic hypothermia has increased worldwide over the past few years.17 This technique has proven advantageous in a variety of other clinical settings including post-cardiac arrest18 and cerebral ischemia.17 It has also been suggested in intoxications.19,20
TH has been claimed to have some effects which may be advantageous in the management of acute ALP poisoning; for instance, decrease in the CNS metabolic rate and oxygen consumption, decrease in the release of free radicals, improvement in brain glucose metabolism, decrease in intracellular acidosis, improvement in tolerance for ischemia, decrease in risk of cardiac arrhythmia due to cell membrane stabilization, reduction of the oxygen demand in low-flow regions, decrease in heart rate, increases in systemic vascular resistance, increase in myocardial contractility, and maintenance of stroke volume and mean arterial blood pressure.16,17
So, the effect of induction of mild therapeutic hypothermia (MTH) on survival during poisoning from a lethal dose of ALP has been evaluated in the present study.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats, weighing 230 ± 10 g, were obtained from Razi Research Institute, Karaj City, Iran. The animals were kept under standard conditions (22 ± 2 °C, 45 ± 5% humidity and 12 h light/dark cycle). They were supplied with standard laboratory diet and water ad libitum, and left to acclimatize for 2 weeks before the experiments. Rats were allowed free access to water but not food for 6 h before the experiment.Rats were randomly divided into 4 groups (7 animals in each group). The first group received a single oral dose of groundnut oil (vehicle of ALP). They served as control. The second group (SHAM group) received no drugs. They also served as control. The third group was given ALP by gavage with an orogastric cannula. Gavage was performed by one of the authors who had previous experience with the procedure. Fresh ALP tablets with unopened package were obtained from the local office of pesticides control. The tablets weighing 3 g were powdered and suspended in groundnut oil just before use, as described in a previous study.1 The strength of ALP suspension was such that requisite doses could be administered in 0.5 ml of oil per 100 g of body weight. They served as positive control. The fourth group received ALP similar to the third group. They served as the study group.
2.2. Experimental design
Rats in the third group were kept in animal cages (4 in each cage) during the experiment. Rats in the first, second and fourth groups were held in the freezer part of a refrigerator periodically (every 30 minutes), while its internal temperature was monitored and changed continuously to keep the core body temperature of the animals at 32–35 degree centigrade during the experiment. Rats in groups one and four also received meperidine 1 mg kg−1 and acetaminophen 10 mg kg−1 intraperitoneally to interfere with their shivering during induction of hypothermia, as suggested in the literature.21 The core body temperature of the animals was checked hourly by a digital rectal thermometer.The experiment was continued for 24 hours. If any animal died during this period, the time of its death would be documented. Mortality rate in 6, 12, and 24 hours after the experiment was calculated for each group.
After the experiment, all live animals were anesthetized with an intramuscular injection of ketamine (80 mg kg−1) and xylazine (15 mg kg−1). After anesthesia, a laparotomy was performed and livers and right kidneys of animals were sent for histopathologic examination; this is also true for animals that had died during the experiment.
The experimental protocol was approved by the Local Animal Care Committee at Qazvin's University of Medical Sciences, Qazvin, Iran. The experimental procedures were carried out in accordance with international guidelines for the care and use of laboratory animals. The authors who were handling animals wore personal protective equipments that included eyeglasses, gowns and gloves during the experiments.
2.3. Histopathologic examination
For histopathology analysis, we processed the midsections of the left lobes of the liver and upper poles of the kidney for light microscopy. The specimen processing consisted of fixation in 4% buffered neutral formalin solution for 24 h, embedding in paraffin wax, slicing sections of 5 micrometers thickness, and staining with hematoxylin–eosin (H&E). The prepared slides were independently examined and scored while the examiner was unaware of the group to which the specimen belonged.Fine isometric cytoplasm vacuolization, centri-lobular congestion, sinusoidal congestion, mono-nuclear infiltration, and patchy necrosis in liver and tubular degeneration, glomerular congestion, parenchymal congestion, and mono-nuclear infiltration in kidney were chosen for histopathologic assessment of the severity of impact of the poisoning on the samples, as reported by previous studies.22,23
The degree of the changes was expressed as the mean of 10 high power fields (HPFs), chosen at random and classified on a scale of 0–4 (no change, 0; changes in few cells, 1; changes in more than 10% but less than 39% of the cells, 2; changes in more than 40% but less than 79% of the cells, 3; changes in more than 80% of the cells, 4).
2.4. Statistical analysis
We used SPSS 16.0 statistical package to perform all statistical analyses. Kaplan–Meier, one-way ANOVA, and chi-square tests were used to test differences between the groups.The results were expressed as mean ± standard deviation (SD). A probability level of <0.05 was considered statistically significant.
3. Results
Mean core body temperature of studied animals is demonstrated in Table 1.| Start of the study | 2 hours after the study | 4 hours after the study | 6 hours after the study | 12 hours after the study | 24 hours after the study | |
|---|---|---|---|---|---|---|
| Group 1 | 37.73 | 35.35 | 35.13 | 35.18 | 34.08 | 35.15 |
| Group 2 | 37.28 | 36.73 | 35.43 | 35.13 | 35.63 | 35.75 |
| Group 3 | 37.51 | 37.45 | 37.53 | 37.44 | 37.45 | 37.47 |
| Group 4 | 37.35 | 32.81 | 30.43 | 33.01 | 33.93 | 33.48 |
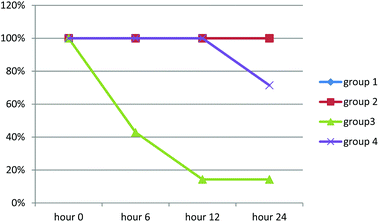
Rats in groups one and two survived during the experiment. Mean survival time in group 3 was 9.143 hours (3.527–14.759; 95% CI), while it was 18.182 (14.730–21.634; 95% CI) in group 4. Kaplan–Meier test showed that the difference between groups was statistically significant (chi-square 15.408, p-value = 0.0001). Survival rate of rats is demonstrated in Chart 1.
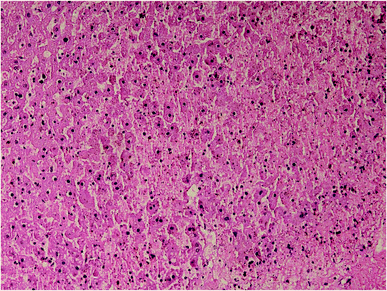
Results of histopathologic examination of liver and kidney of the rats are demonstrated in Fig. 1–6, and Tables 2 and 3. If sum of the scores of the assessed pathologic changes in liver and kidney is considered, their mean and standard deviation are shown in Table 4. The difference between groups was statistically significant by a one-way ANOVA test (p-value = 0.000). Post hoc test (Tukey's HSD) showed statistically significant difference between groups 3 and 4 in the total score of the kidney histopathologic examination (0.20–2.08, 95% CI; p-value = 0.016), but this is not true for liver (p-value = 0.866).
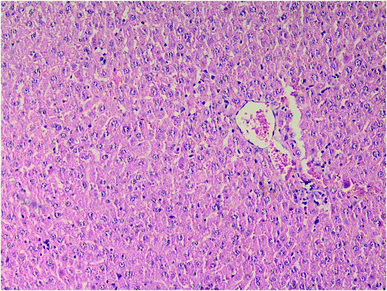 | ||
| Fig. 3 Liver histopathology in group 4 after induction of hypothermia for treatment of ALP poisoning (mid-power). | ||
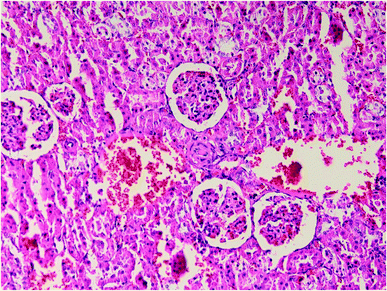 | ||
| Fig. 4 Kidney histopathology in group 1 after induction of hypothermia for treatment of ALP poisoning (mid-power). | ||
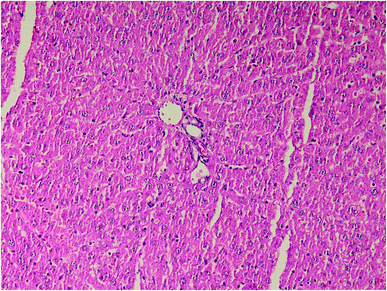 | ||
| Fig. 5 Liver histopathology in group 1 after induction of hypothermia without exposure to ALP (mid power). | ||
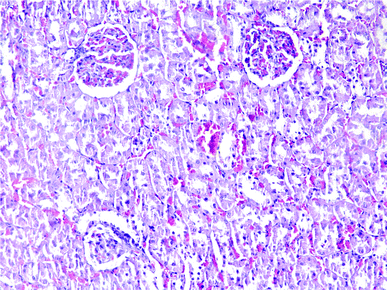 | ||
| Fig. 6 Kidney histopathology in group 1 after induction of hypothermia without exposure to ALP (mid power). | ||
| Group 1 | Group 3 | Group 4 | ||||
|---|---|---|---|---|---|---|
| Grade | Number of cases | Grade | Number of cases | Grade | Number of cases | |
| Fine isometric cytoplasm vacuolization | All were 1 | 1 | 0 | 1 | 0 | |
| 2 | 0 | 2 | 0 | |||
| 3 | 7 | 3 | 5 | |||
| 4 | 0 | 4 | 2 | |||
| Centrilobular congestion | All were 1 | 1 | 0 | 1 | 0 | |
| 2 | 0 | 2 | 5 | |||
| 3 | 7 | 3 | 0 | |||
| 4 | 0 | 4 | 2 | |||
| Sinusoidal congestion | All were 1 | 1 | 1 | 1 | 0 | |
| 2 | 6 | 2 | 5 | |||
| 3 | 0 | 3 | 0 | |||
| 4 | 0 | 4 | 2 | |||
| Mono-nuclear infiltration | All were 0 | 1 | 1 | 1 | 2 | |
| 2 | 6 | 2 | 5 | |||
| 3 | 0 | 3 | 0 | |||
| 4 | 0 | 4 | 0 | |||
| Patchy necrosis | All were 0 | 1 | 0 | 1 | 0 | |
| 2 | 1 | 2 | 2 | |||
| 3 | 6 | 3 | 5 | |||
| 4 | 0 | 4 | 0 | |||
| Group 1 | Group 3 | Group 4 | ||||
|---|---|---|---|---|---|---|
| Grade | Number of cases | Grade | Number of cases | Grade | Number of cases | |
| Tubular degeneration | All were 0 | 1 | 0 | 1 | 0 | |
| 2 | 7 | 2 | 5 | |||
| 3 | 0 | 3 | 2 | |||
| 4 | 0 | 4 | 0 | |||
| Glomerular congestion | All were 1 | 1 | 0 | 1 | 0 | |
| 2 | 0 | 2 | 0 | |||
| 3 | 1 | 3 | 5 | |||
| 4 | 6 | 4 | 2 | |||
| Mono-nuclear infiltration | All were 0 | 1 | 1 | 1 | 7 | |
| 2 | 6 | 2 | 0 | |||
| 3 | 0 | 3 | 0 | |||
| 4 | 0 | 4 | 0 | |||
| Parenchymal congestion | All were 1 | 1 | 0 | 1 | 0 | |
| 2 | 0 | 2 | 0 | |||
| 3 | 0 | 3 | 0 | |||
| 4 | 7 | 4 | 7 | |||
| Total score in liver | Total score in kidney | |
|---|---|---|
| Histopathologic examination | Histopathologic examination | |
| Group 1 | 3.00 ± 0.000 | 2.00 ± 0.000 |
| Group 3 | 12.57 ± 1.134 | 11.71 ± 0.756 |
| Group 4 | 12.86 ± 1.464 | 10.57 ± 0.976 |
4. Discussion
The present study suggests that induction of mild therapeutic hypothermia (MTH) may help in the treatment of acute ALP poisoning. In our experiment, rats were treated with MTH after exposure to ALP in LD50 dose. Survival time in the group treated with MTH prolonged about two-fold compared to the group that received only the poison. The difference was statistically significant. The treatment group also experienced less histopathologic changes attributed to ALP poisoning in their kidney and liver compared to the control group. However, only the difference in kidney histopathology was statistically significant.Exposure to ALP is a common cause of poisoning from agricultural pesticide exposures in some parts of the world.3 Acute ALP poisoning is highly lethal. After ingestion, phosphine gas produced from acid hydrolysis of ALP is rapidly absorbed throughout the gastrointestinal tract and it is partly carried to the liver by the portal vein.23 Then, it affects the heart, lungs, kidneys and gastrointestinal tract.8 Currently, no unquestionably effective treatment is available for the victims except decontamination and supportive measures.3
The present study was designed to evaluate mild therapeutic hypothermia in decreasing mortality due to acute ALP poisoning. The use of therapeutic hypothermia over the past few years has increased worldwide. This technique has proven advantageous in a variety of clinical settings.17 Some effects of MTH may be helpful in the treatment of acute ALP poisoning. It may counteract some effects of ALP such as release of free radicals, decrease in systemic vascular resistance, myocardial contractility and stroke volume, and mean arterial blood pressure.16 Moreover, MTH decreases CNS metabolic rate and oxygen consumption, improves brain glucose metabolism, improves tolerance for ischemia, and reduces the oxygen demand in low-flow regions of the brain. These effects may help patients to combat the toxic effects of ALP.17 Induction of hypothermia may be associated with some side effects and complications, particularly during re-warming time.15 However, we did not face any side effect during the experiment, possibly because the study had been designed in a way that there was no time for the appearance of the side effects.
Therapeutic hypothermia had been used in the field of toxicology. Marik and colleagues have used therapeutic hypothermia in a patient who had developed acute ischemic–hypoxic brain injury after suicidal poisoning with diazepam and ethylene glycol.20 They prevented brain death in their patient with management. Jo and coworkers have reported that therapeutic hypothermia prolongs 12 hours survival rate in rats intoxicated with paraquat; but not 24 hours survival rate.19 A search in popular scientific databases by the authors did not find a related study on ALP.
There were some limitations in the present study. We tried to maintain the core body temperature of animals in the range of 32–35 degree centigrade during the experiment in all studied groups according to study design. However, the mean core body temperature of group 4 (treatment group) was lower; as it can be seen in Table 1. Furthermore, we considered 6 hours, 12 hours, and 24 hours survival rate as the measuring outcome of ALP poisoning and the studied treatment. It seems reasonable that other laboratory parameters of ALP poisoning would be measured in the animals too. However, we only evaluated liver and kidney histopathology due to resource limitations.
5. Conclusion
The current study showed that MTH may be beneficial for the treatment of acute ALP poisoning in rats. Conducting experiments in humans is suggested in order to determine its true therapeutic value.References
- R. Dua and K. D. Gill, Effect of aluminium phosphide exposure on kinetic properties of cytochrome oxidase and mitochondrial energy metabolism in rat brain, Biochim. Biophys. Acta, 2004, 1674, 4–11 CrossRef CAS PubMed.
- E. Behravan, M. Ghorbani and R. Afshari, Acute aluminum phosphide poisoning: 5 years experience, Toxicol. Lett., 2010, 196S, S37–S351 Search PubMed.
- A. A. Moghadamnia, An update on toxicology of aluminum phosphide, DARU J. Pharm. Sci., 2012, 20, 25 CrossRef CAS PubMed.
- G. Sasanian, S. Shadnia, M. Abdollahi, P. Allami, A. Hosseini, A. Ranjbar and N. Amini-Shirazi, A retrospective 7-years study of aluminum phosphide poisoning in Tehran, Toxicol. Lett., 2010, 196S, S37–S351 Search PubMed.
- C. H. Hsu, B. C. Chi, M. Y. Liu, J. H. Li, C. J. Chen and R. Y. Chen, Phosphine-induced oxidative damage in rats: role of glutathione, Toxicology, 2002, 179, 1–8 CrossRef CAS.
- C. H. Hsu, B. C. Han, M. Y. Liu, C. Y. Yeh and J. E. Casida, Phosphine-induced oxidative damage in rats: attenuation by melatonin, Free Radicals Biol. Med., 2000, 28(4), 636–642 CrossRef CAS.
- M. Arefi, B. Behnoush, M. Lalezari and N. Zamani, The Frequency of the causes of acid base disturbances in patients hospitalized in the toxicology ward of Baharloo hospital in 2009, Iran. J. Toxicol., 2011, 5(1), 410–414 Search PubMed.
- G. S. Bumbrah, K. Krishan, T. Kanchan, M. Sharma and G. S. Sodhi, Phosphide poisoning: A review of literature, Forensic Sci. Int., 2012, 214, 1–6 CrossRef CAS PubMed.
- M. Rahbar Taromsari, B. Shad, D. Aghajani Nargesi, N. Akhoundzadeh and M. Fallah Karkan, The study of various cardiac arrhythmias in patients poisoned with aluminum phosphide (rice tablet), Iran. J. Toxicol., 2011, 5(1), 448–453 Search PubMed.
- F. Taghaddosi Nejad, A. Banagozar Mohammadi, B. Behnoush, A. M. Kazemifar, M. Zaare Nahandi, S. Dabiran, M. Jamalian and A. Bani sheikholeslami, Predictors of poor prognosis in aluminum phosphide intoxication, Iran. J. Toxicol., 2012, 6(16), 610–614 Search PubMed.
- P. Aggarwal, R. Handa, N. Wig, A. Biswas, R. Saxena and J. P. Wal, Am. J. Emerg. Med., 1999, 17, 488–489 CrossRef CAS.
- H. Sanaei-Zadeh, Is there a role for digoxin in the management of acute aluminum phosphide poisoning?, Med. Hypotheses, 2011, 76, 761–767 CrossRef PubMed.
- S. M. Marashi, M. Arefi, B. Behnoush, M. Ghazanfari Nasrabad and Z. Nasri Nasrabadi, Could hydroxyethyl starch be a therapeutic option in management of acute aluminum phosphide toxicity?, Med. Hypotheses, 2011, 76, 596–598 CrossRef CAS PubMed.
- A. Azad, S. B. Lall and S. Mittra, effect of N-acetylcysteine and L-NAME on alliminium phosphide induced cardiovascular toxicity in rat, Acta Pharmacol. Sin., 2001, 22(4), 298–304 CAS.
- B. Kabone, A. Bacher and C. K. Spiss, Therapeutic hypothermia, Best Pract. Res. Clin. Anaesthesiol., 2003, 17(4), 551–568 CrossRef.
- E. M. Moore, A. D. Nichol, S. A. Bernard and R. Bellomo, Therapeutic hypothermia: Benefits, mechanisms and potential clinical applications in neurological, cardiac and kidney injury, Int. J. Care Injured, 2011, 42, 843–854 CrossRef PubMed.
- A. G. Alzaga, M. Cerdan and J. Varon, Therapeutic hypothermia, Resuscitation, 2006, 70, 369–380 CrossRef PubMed.
- P. Kory, J. Weiner, J. P. Mathew, M. Fukunaga, V. Palmero, B. Singh, S. Haimowitz, E. T. Clark, A. Fischer and P. H. Mayo, A rapid, safe, and low-cost technique for the induction of mild therapeutic hypothermia in post-cardiac arrest patients, Resuscitation, 2011, 82, 15–20 CrossRef PubMed.
- Y. H. Jo, K. Kim, J. E. Rhee, G. J. Suh, W. Y. Kwon, S. H. Na and H. B. Alam, Therapeutic hypothermia attenuates acute lung injury in paraquat intoxication in rats, Resuscitation, 2011, 82, 487–491 CrossRef CAS PubMed.
- P. E. Marik and J. Varon, Prolonged and profound therapeutic hypothermia for the treatment of “brain death” after a suicidal intoxication, Am. J. Emerg. Med., 2010, 28, 258e1–258e4 Search PubMed.
- O. Kimberger and A. Kurz, Thermoregulatory management for mild therapeutic hypothermia, Best Pract. Res. Clin. Anaesthesiol., 2008, 22(4), 729–744 CrossRef PubMed.
- O. Mehrpour, M. Dolati, K. Soltaninejad, s. Shadnia and B. Nazparvar, Evaluation of histopathological changes in fatal aluminum phosphide poisoning, Indian J. Forensic Medi. Toxicol., 2008, 2(2), 7–12 Search PubMed.
- s. Saleki, F. Azmoudeh Ardalan and A. Javidan-Nejad, Liver histopathology of fatal phosphine poisoning, Forensic Sci. Int., 2007, 166, 190–193 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |