Facile colorimetric detection of human chorionic gonadotropin based on the peptide-induced aggregation of gold nanoparticles†
Chia-Chen
Chang‡
 a,
Chen-Yu
Chen‡
bcd,
Chie-Pein
Chen
b and
Chii-Wann
Lin
*aef
a,
Chen-Yu
Chen‡
bcd,
Chie-Pein
Chen
b and
Chii-Wann
Lin
*aef
aInstitute of Biomedical Engineering, National Taiwan University, Taipei 106, Taiwan
bDepartment of Obstetrics and Gynecology, Mackay Memorial Hospital, Taipei 104, Taiwan
cMackay Medical College, Taipei 252, Taiwan
dMackay Junior College of Medicine, Nursing and Management, Taipei 112, Taiwan
eInstitute of Biomedical Electronic and Bioinformatics, National Taiwan University, Taipei 106, Taiwan
fCenter for Emerging Material and Advanced Devices, National Taiwan University, Taipei 106, Taiwan
First published on 1st October 2014
Abstract
The human chorionic gonadotropin (hCG) assay for ectopic pregnancy is typically conducted using an enzyme immunoassay (EIA); however, EIA is complicated and time-consuming. Herein, we developed a simple, rapid and sensitive colorimetric assay for hCG based on the anti-aggregation of unmodified gold nanoparticles (AuNPs). The introduction of positively charged hCG-specific peptides into an AuNP solution causes the aggregation of the AuNPs, resulting in a color change from red to blue. When hCG is present, peptide/hCG complexes are formed, thus inhibiting the peptide-induced AuNP aggregation. The dynamic range for hCG detection ranged from 50 to 1000 mIU mL−1, with a detection limit of 25 mIU mL−1. Additionally, this colorimetric sensor was successfully evaluated with serum samples spiked with hCG, demonstrating its ability to be used for the onsite monitoring of hCG.
Ectopic pregnancy, a potentially life-threatening negative pregnancy outcome, refers to implantation of the fertilized ovum outside the endometrial cavity. Although only an estimated 1–2% of all reported pregnancies are ectopic, this high-risk condition has significant health consequences and represents a common cause of morbidity in early pregnancy.1 The diagnosis of ectopic pregnancy is achieved based on transvaginal ultrasound observations and measurements of human chorionic gonadotropin (hCG) values.2 Although ultrasound scanning can aid in the identification of this pregnancy condition, it is unable to determine the location of a pregnancy in a significant number of women. Furthermore, the diagnosis of ectopic pregnancy was not able to be carried out by ultrasound at hCG concentrations below 1000 mIU mL−1.3 Additionally, serial hCG measurements in women with a non-diagnostic ultrasound scan showed that hCG increases of less than 66% in 48 hours indicate an increased likelihood of ectopic pregnancy.4 Thus, the level of hCG is considered an important clinical parameter for ectopic pregnancy. In clinical diagnosis, enzyme-linked immunosorbent assay (ELISA) is the most popular and powerful method for hCG detection at very low concentrations. However, this method has limitations; it requires additional enzyme-labeled antibodies and multiple steps for analysis. Therefore, the development of a sensitive, selective, and simple assay for hCG has driven substantial research efforts in recent years.
To date, various antibody-based methods have been applied to the detection of hCG, including fluorescence, electrochemistry, photoluminescence, and immunochromatographic assays. Liu's group developed an immunogold chromatographic strip test with a detection limit of 5 ng mL−1 (50 mIU mL−1),5 but it required the immunogold labeling technique. Although Gu's group provided a photoluminescence sensor based on antibody-conjugated ZnO arrays that had a detection sensitivity for hCG of as low as 2 ng mL−1 (20 mIU mL−1),6 it needed multistep operation and fails to achieve the rapid analysis. Serrate's group reported a lateral flow immunoassay for hCG based on a giant magnetoresistance sensor,7 but it requires expensive equipment. In addition to antibody-based assays, it has been demonstrated that the short peptide motif PPLRINRHILTR has a high affinity for hCG, with a binding constant stronger than that of native hCG-binding antibodies.8 This peptide probe has been used in a liquid-crystal (LC)-based optical sensor by Yang's group; however, the LC-based method suffers from poor sensitivity. Thus, it is desirable to design a simple and rapid detection for label-free and sensitive hCG detection.
Colorimetric methods are extremely attractive for biological and chemical sensing applications9,10 because they can be clearly read out by the naked eye. In particular, metal (typically gold and silver) nanoparticle (NP)-based colorimetric assays have received considerable attention due to the straightforward synthesis and interesting optoelectronic properties of metal NPs.11 For example, the aggregation of gold nanoparticles (AuNPs) leads to a large absorption band shift and a color change from red to blue as a result of interparticle plasmon coupling.12 This color change has been used as the basis for colorimetric detection based on directly or indirectly induced AuNP aggregation. Various analytes have been detected using this approach.13–20
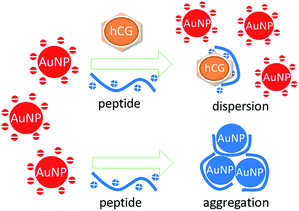
Building on these insights, we propose a facile but sensitive method for rapid hCG detection based on the anti-aggregation of AuNPs (Fig. 1). The peptide probe PPLRINRHILTR (Pro-Pro-Leu-Arg-Ile-Asn-Arg-His-Ile-Leu-Thr-Arg) is employed here for the assay of hCG.8 The total charge of the peptide is positive at pH 7.4 because the Arg residues in the sequence are positively charged, while no residue is negatively charged. In the absence of hCG, the positively charged peptide probe can easily adsorb to the citrate-capped AuNP surfaces through electrostatic attraction. The binding of peptides diminishes the negative charge density on the AuNP surfaces, resulting in the instability of the AuNPs. Subsequently, this change in stability leads to the aggregation of the AuNPs and a corresponding red-to-blue color change. When the target (hCG) is present in solution, the peptide probe selectively recognizes and tightly binds to hCG, preventing the probes from attaching to the surfaces of the AuNPs. Consequently, the citrate-capped AuNPs remain highly stable and the color of the solution remains red. A color change reflects the absence of the target, which can be used as a colorimetric indicator for hCG detection.
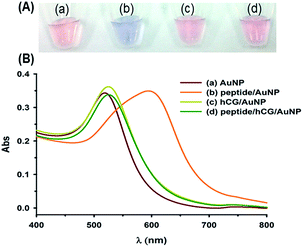
To verify the feasibility of the method, the UV-vis spectra and color changes in the AuNPs in different solutions are shown in Fig. 2. The absorption peak of the as-prepared citrate-capped AuNP solution was observed at approximately 520 nm, whereas upon addition of 500 nM peptide to the AuNPs, the solution turned blue within 1 min due to peptide-induced aggregation, accompanied by the broadening and red-shifting of the spectral peak to 600 nm. On the other hand, when only hCG proteins were added to the AuNP solution, the peak slightly shifted to approximately 525 nm, indicating no significant effect on the stability of the AuNPs. In the target sensing case, hCG (5 IU mL−1) was added along with the peptide (500 nM), and then the AuNP solutions were added. In the presence of the mixture of hCG/peptide/AuNPs, a single peak was observed at 525 nm, and the solution remained red in color, suggesting that there was a strong interaction between the peptide and hCG and that the AuNPs remained dispersed well. Hence, based on changes in the absorption spectra and color of the AuNPs, it is convenient to detect hCG based on the target-induced inhibition of AuNP aggregation.
To achieve better analytical performance, the effects of various assay parameters, including the concentration of the peptide, the incubation time, and the reaction time, were investigated. The effects of these parameters are shown in Fig. S1.† The incubation time between hCG and the peptide probe was selected to be 10 min. Moreover, the optimal condition involved the use of 500 nM peptide with a reaction time of 16 min.
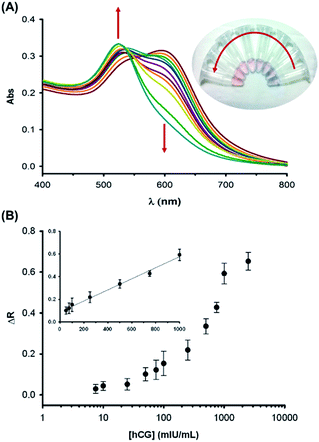
The linear range of the assay was measured under the optimal conditions, as shown in Fig. 3. The absorption spectrum of the colorimetric system was sensitive to hCG. Namely, an increase in the hCG concentration induced a gradual increase in the absorbance at 525 nm (A525) and a continuous decrease in the UV-vis absorption at 600 nm (A600) (Fig. 3A). Furthermore, the color of the solution gradually changed from blue to violet and finally to red when the hCG level was increased. When the hCG concentration was below 25 mIU mL−1, no significant changes in the absorption peaks were detected. When the concentration was increased beyond 1000 mIU mL−1, no further obvious changes were observed, and the spectrum response completely recovered to that of the plain AuNP suspension. The change in the absorbance ratio [ΔR = (R0 − R)/R0] is shown in Fig. 3B, where R0 is the absorbance ratio (A600/A525) without hCG, and R represents the ratio after incubation with different concentrations of hCG. A higher ΔR is associated with dispersed AuNPs that are red in color, while a lower ΔR corresponds to aggregated AuNPs that are blue in color. There was a linear change in ΔR as a function of hCG concentration in the hCG concentration range from 50 to 1000 mIU mL−1, with a detection limit (LOD) of 25 mIU mL−1. The sensing performance (including the LOD and linear range) of the proposed colorimetric assay for hCG was compared with that of other reported analytical methods (Table S1 in the ESI†). Although the LOD was not as good as that achieved by electrochemical sensors,21,22 it was comparable to that of methods based on immunochromatography, photoluminescence, fluorescence, and surface plasmon resonance.5,6,23–25 Importantly, this limit is 40-fold higher than that of liquid crystal (LC)-based immunoassays using the same anti-hCG peptide.8 Our colorimetric assay could be performed more rapidly than could the LC assay, including a 1 h incubation time. From a diagnostic or prognostic perspective, the LOD value of our assay is within the generally accepted threshold range used in the clinical diagnosis of pregnancy (25–50 mIU mL−1).26,27 As mentioned before, an increase of 1000 mIU mL−1 within 48 h is useful in differentiating a normal pregnancy from an ectopic pregnancy in serial quantitative hCG measurements.28,29 Moreover, a high hCG level (>1000 mIU mL−1) links to a poor prognosis and disease progression for germ cell tumor patients.30 Therefore, our approach not only provides other attractive advantages such as simple and easy operation, but also has potential in the diagnosis and management of hCG-secreting diseases with desirable sensitivity.
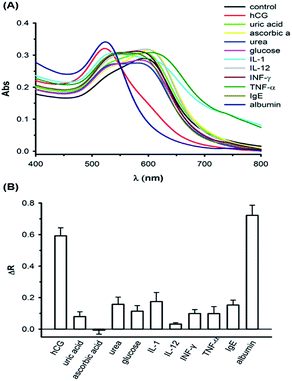
Specificity is one of the most basic properties required in an analytical assay; we therefore examined the response of the AuNP probes to the chemical interferents (including ascorbic acid, glucose, uric acid, and urea) and protein interferents (including IL-1, IL-12, INF-γ, TNF-α, and serum albumin). As shown in Fig. 4, in solutions containing 2000 ng mL−1 chemical interferents, 500 ng mL−1 protein interferents, 40 mg mL−1 albumin, and 1000 mIU mL−1 (equal to 100 ng mL−1) hCG, different spectral responses were observed. When the concentrations of chemical interferents were 20-fold higher than that of hCG, the ΔR for hCG was at least 3-fold higher than that of the chemical interfering agents. Additionally, we were concerned with false-positive response if a positively charged anti-hCG peptide would be interacted with other biomolecules mostly showing different isoelectric potentials at pH 7.4. Among these non-specific proteins, IL-1 (pI = 6.2), IL-12 (pI = 5.9), TNF-α (pI = 6.4), IgE (pI = 5.8), and albumin (pI = 5.3) are negatively charged while INF-γ (pI = 8.6) is positively charged. Based on the observation of our results, no false-positive responses were monitored from other proteins, IL-1, IL-12, TNF-α, IgE, and INF-γ, except serum albumin. The noticeable spectral response with albumin was presumably resulted from the aggregation resistance of AuNPs by serum albumin proteins. High concentrations of albumin proteins may be adsorbed on negatively charged citrate-stabilized AuNPs via an electrostatic attraction31 or a ligand exchange reaction,32 thus leading to the anti-aggregation of the colloidal particles.33 If this is the case, AuNPs should be coated with polymers, such as thiolated oligoethylene glycol (OEG), increasing the charge stabilization in the future.34
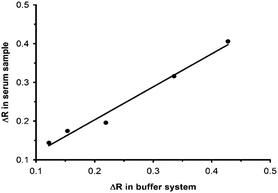
The colorimetric immunoassay was further applied for the detection of hCG in biological fluids, such as human serum samples. The spiked samples were prepared using an appropriate dilution of serum and the addition of an hCG standard solution (Fig. S2A†). As shown in Fig. 5, the responses to hCG in the buffer solutions were well correlated with the responses from the spiked serum samples, showing a good linear relationship with a correlation coefficient (R2) of 0.98. Therefore, the as-prepared assay enabled the rapid detection of hCG in biological fluids. Interestingly, compared to the detection of hCG in 50-fold diluted serum, a lower response was measured in the presence of 0.8 mg mL−1 albumin (50-fold dilution of 40 mg mL−1 albumin) in the buffer system (Fig. S2B†). The difference of sensing response intensities between these two tests may be due to the different compositions of proteins in the buffer and diluted serum solution. Namely, albumin may have interactions with various substances in 50-fold diluted serum,35 thus hindering the attachment of albumin to AuNPs; however, there are no interfering substances present in the standard buffer so most albumin proteins (0.8 mg mL−1) can easily adsorb on AuNPs, resulting in a reduced response. In this study, although the LOD was reported to be 25 mIU mL−1, it was calculated with the standard. In practical applications, samples were subjected to a 50-fold dilution before analysis, and therefore, the LOD for samples ought to be multiplied by this number, which means 1250 mIU mL−1. As a result, further effort is required to improve the LOD of this assay for practical applications by using more sensitive detectors (SPR sensors).36
In summary, we described the colorimetric detection of hCG based on the inhibition of peptide-induced AuNP aggregation by hCG without any additives, such as metal salts or ions. Although this colorimetric assay did not have the highest sensitivity for the detection of hCG, the sensitivity might have potential clinical applications for hCG in relation to diagnosis and prognosis. The advantage of such a method depends on its speed, convenience, and simplicity. On the other hand, the disadvantage is the limited ability to reduce the interfering substances to the AuNP's surface. This drawback might be further eliminated through the potential surface ligand functionalization upon the AuNP. In addition, further practical applications in human blood serum by this colorimetric assay approach can be envisioned. The sensitivity was sufficient without requiring costly and complicated readout equipment. In addition, high selectivity was confirmed by the detection of possible interferents. Therefore, the proposed method may present potential practical applications in human blood serum.
Acknowledgements
The authors gratefully acknowledge the financial support of the Ministry of Science and Technology of Taiwan (102-2218-E-002-014-MY3 and 103-2811-E-002-008), and the Mackay Memorial Hospital (MMH-103-46).Notes and references
- N. M. van Mello, F. Mol, W. M. Ankum, B. W. Mol, F. van der Veen and P. J. Hajenius, Fertil. Steril., 2012, 98, 1066–1073 CrossRef PubMed.
- C. R. Gracia and K. T. Barnhart, Obstet. Gynecol., 2001, 97, 464–470 CrossRef CAS.
- A. M. Lozeau and B. Potter, Am. Fam. Physician, 2005, 72, 1707–1714 Search PubMed.
- K. Visconti and N. Zite, Clin. Obstet. Gynecol., 2012, 55, 410–417 CrossRef PubMed.
- J. Su, Z. Zhou, H. Li and S. Liu, Anal. Methods, 2014, 6, 450–455 RSC.
- X. Yan, Z. Huang, M. He, X. Liao, C. Zhang, G. Yin and J. Gu, Colloids Surf., B, 2012, 89, 86–92 CrossRef CAS PubMed.
- D. Serrate, J. M. De Teresa, C. Marquina, J. Marzo, D. Saurel, F. A. Cardoso, S. Cardoso, P. P. Freitas and M. R. Ibarra, Biosens. Bioelectron., 2012, 35, 206–212 CrossRef CAS PubMed.
- X. Ding and K. L. Yang, Anal. Chem., 2013, 85, 10710–10716 CrossRef CAS PubMed.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- H. Jans and Q. Huo, Chem. Soc. Rev., 2012, 41, 2849–2866 RSC.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- C. C. Chang, S. C. Wei, T. H. Wu, C. H. Lee and C. W. Lin, Biosens. Bioelectron., 2013, 42, 119–123 CrossRef CAS PubMed.
- J. Xin, L. Miao, S. Chen and A. Wu, Anal. Methods, 2012, 4, 1259–1264 RSC.
- X. Xie, R. Deng, F. Liu, W. Xu, S. F. Yau Li and X. Liu, Anal. Methods, 2013, 5, 1116–1119 RSC.
- J. Y. Byun, Y. B. Shin, D. M. Kim and M. G. Kim, Analyst, 2013, 138, 1538–1543 RSC.
- C. C. Chang, C. Y. Chen, X. Zhao, T. H. Wu, S. C. Wei and C. W. Lin, Analyst, 2014, 139, 3347–3351 RSC.
- Y. Xing, P. Wang, Y. Zang, Y. Ge, Q. Jin, J. Zhao, X. Xu, G. Zhao and H. Mao, Analyst, 2013, 138, 3457–3462 RSC.
- R. Chandrawati and M. M. Stevens, Chem. Commun., 2014, 50, 5431–5434 RSC.
- P. Chen, R. Selegard, D. Aili and B. Liedberg, Nanoscale, 2013, 5, 8973–8976 RSC.
- N. Xuan Viet, M. Chikae, Y. Ukita, K. Maehashi, K. Matsumoto, E. Tamiya, P. Hung Viet and Y. Takamura, Biosens. Bioelectron., 2013, 42, 592–597 CrossRef PubMed.
- J. Lu, S. Liu, S. Ge, M. Yan, J. Yu and X. Hu, Biosens. Bioelectron., 2012, 33, 29–35 CrossRef CAS PubMed.
- C. Zhou, H. Yuan, H. Shen, Y. Guo, X. Li, D. Liu, L. Xu, L. Ma and L. S. Li, J. Mater. Chem., 2011, 21, 7393–7400 RSC.
- R. Nooney, E. McCormack and C. McDonagh, Anal. Bioanal. Chem., 2012, 404, 2807–2818 CrossRef CAS PubMed.
- M. Piliarik, M. Bocková and J. Homola, Biosens. Bioelectron., 2010, 26, 1656–1661 CrossRef CAS PubMed.
- A. D. Pronovost and T. T. Lee, US Pat., 5786220 A, 1998.
- L. A. Cole and D. G. Ladner, Clin. Biochem., 2009, 42, 168–175 CrossRef CAS PubMed.
- B. Grønlund and A. Marushak, Aust. N. Z. J. Obstet. Gynaecol., 1993, 33, 312–314 CrossRef PubMed.
- B. Cacciatore, U. L. F. H. Stenman and P. YlÖStalo, Br. J. Obstet. Gynaecol., 1990, 97, 904–908 CrossRef CAS PubMed.
- S. B. Saxman, D. Finch, R. Gonin and L. H. Einhorn, J. Clin. Oncol., 1998, 16, 702–706 CAS.
- S. H. Brewer, W. R. Glomm, M. C. Johnson, M. K. Knag and S. Franzen, Langmuir, 2005, 21, 9303–9307 CrossRef CAS PubMed.
- D. H. Tsai, F. W. DelRio, A. M. Keene, K. M. Tyner, R. I. MacCuspie, T. J. Cho, M. R. Zachariah and V. A. Hackley, Langmuir, 2011, 27, 2464–2477 CrossRef CAS PubMed.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. Puntes, ACS Nano, 2010, 4, 3623–3632 CrossRef CAS PubMed.
- M. Schollbach, F. Zhang, F. Roosen-Runge, M. W. A. Skoda, R. M. J. Jacobs and F. Schreiber, J. Colloid Interface Sci., 2014, 426, 31–38 CrossRef CAS PubMed.
- N. Hassler, D. Baurecht, G. Reiter and U. P. Fringeli, J. Phys. Chem. C, 2011, 115, 1064–1072 CAS.
- T. L. Chuang, C. C. Chang, Y. Chu-Su, S. C. Wei, X. H. Zhao, P. R. Hsueh and C. W. Lin, Lab Chip, 2014, 14, 2968–2977 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ay01429d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |