Simultaneous detection of parathion and imidacloprid using broad-specificity polyclonal antibody in enzyme-linked immunosorbent assay
Lening
Zhang
a,
Zhanfeng
Wang
b,
Yubin
Wen
c,
Jingwei
Shi
*d and
Jincheng
Wang
*e
aDepartment of Thoracic Surgery, China-Japan Union Hospital of Jilin University, Changchun, 130033, China
bThe Second Department of Neurosurgery, China-Japan Union Hospital of Jilin University, Changchun, 130033, China
cDepartment of Radiology, China-Japan Union Hospital of Jilin University, Changchun, 130033, China
dDepartment of Clinical Laboratory, China-Japan Union Hospital of Jilin University, Changchun, 130033, China. E-mail: shi123jingwei@163.com
eDepartment of Orthopedics, The Second Hospital of Jilin University, Changchun, 130000, China. E-mail: jinchengwang@hotmail.com
First published on 29th August 2014
Abstract
A heterologous indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) for the gross detection of parathion and imidacloprid residues is developed, based on a broad-specificity polyclonal antibody (BsPAb). Multi-determinant immunogen was prepared by conjugating parathion haptens and imidacloprid haptens to bovine serum albumin (BSA) in turns. The BsPAb was generated by immunized male New Zealand white rabbits with the multi-determinant immunogen. The effects of coating antigens on the performance of the ELISA were optimized for obtaining satisfactory assay sensitivity. Under the optimized conditions, the 50% inhibition concentration (IC50) value for parathion and imidacloprid mixture was 2.41 mg L−1, with a limit of detection (LOD) of 0.025 mg L−1. There was no obvious cross-reactivity (CR) with most of the neonicotinoids and organophosphorus insecticides, except for parathion–methyl (23.11%) and imidaclothiz (15.96%). The recoveries of parathion and imidacloprid in tap water samples ranged from 80.3% to 121%, and the relative standard deviation (RSD) were lower than 6.99%. The ELISA results were also confirmed by gas chromatography (GC). These results showed that the ic-ELISA can be used as a sensitive tool for detecting the gross (parathion and imidacloprid) in environmental samples.
1. Introduction
Organophosphorus compounds have been widely used in medicine as anthelmintics, in agriculture as pesticides and as chemical warfare agents.1–5 Parathion, an organophosphorus compound, has been used to control pests on numerous crops and its effects on heart, liver, kidney and endocrine activity in humans has been reported.6 Neonicotinoid insecticides have great systemic activity, but they may cause oxidative stress and inflammation of the central nervous system.7 Imidacloprid, a promising neonicotinoid insecticide, acts as an agonist at the nicotinic acetylcholine receptors of insects,8 and has been widely used to control sucking pests.9 Parathion and imidacloprid are used on crops and vegetables for controlling pests. Furthermore, both parathion and imidacloprid could act on the nervous system of mammals.6 Therefore, detecting the parathion and imidacloprid residues have a great significance.Immunoassay, which has been certified as an alternative to traditional analytical methods,10 is known to be a rapid, sensitive and specific technique for analyzing samples. The immunoassay, for routine measurements, has been widely used in medical laboratories.11 Enzyme-linked immunosorbent assay (ELISA) has become a general name for medical laboratories, regulatory bodies, external quality assessment and proficiency-testing organizations.11 However, in general, ELISAs are capable of detecting only single analyte per assay.12 It would be more efficient and economical to develop an ELISA that could directly and simultaneously detect the total quantity of mixed pesticides, instead of one assay for each individual target.13 Liu and Yan developed a novel strategy for direct quantitatively detecting the total quantity of imidaclothiz and thiacloprid.14 This method, using a standard curve, quantitatively analyzed the total quantity of imidaclothiz and thiacloprid for the first time. However, two antibodies with a considerably similar affinity constant should be prepared, and the method has a narrow working range for analyzing the pesticides. Furthermore, hapten density of hapten-HRP conjugates was a prerequisite for the development of this type of an assay. Therefore, these drawbacks have restricted its application. Compared to the abovementioned methods, the heterologous dc-ELISA using the broad-specificity polyclonal antibody (BsPAb) could detect the total quantity of parathion and imidacloprid, and it also has a broader working range for analyzing the pesticides.
The present work aims at developing a novel multi-residue immunosorbent assay for the detection of gross pesticide residues of parathion and imidacloprid, using an identical standard curve. Multi-haptens antigen, which is prepared by conjugating two haptens to bovine serum albumin (BSA) in turns, was used for generating BsPAb. An indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) based on BsPAb was developed and applied to determine the gross pesticide in tap water. The ic-ELISA provides a potential application for the direct determination of the total quantity of mixed pesticides residues in environmental samples.
2. Materials and methods
2.1. Reagents and instruments
All reagents and solvents were of analytical grade. Bovine serum albumin (BSA), ovalbumin (OVA), skim milk, Freund's complete and incomplete adjuvants, goat anti-rabbit immunoglobulin horseradish peroxidase conjugate (GAR-HRP), 3,3′,5,5′-tetramethylbenzidine (TMB), N-hydroxysuccinimide (NHS), dicyclohexylcarbodiimide (DCC), N,N-dimethylformamide (DMF) and polyoxyethylene sorbitan monolaurate (Tween-20) were purchased from Sigma Chemical Co. (St. Louis, USA). Carbonate-buffered saline (CBS, 0.05 mol L−1, pH 9.6), phosphate-buffered saline (PBS, 0.01 mol L−1, pH 7.4), phosphate-buffered saline containing 0.05% Tween-20 (PBST), the substrate solution containing 0.025 mol L−1 of citrate and 0.062 mol L−1 of sodium phosphate (pH 5.4), the TMB solution containing 10 mg mL−1 TMB and 0.75% H2O2 in substrate solution were used. UV-Vis absorption spectra were recorded on a Cintra 10e UV-Vis spectrometer (GBC, Victoria, Australia). 96-well polystyrene microplates (Maxisorp) were purchased from Nunc (Roskilde, Denmark). An Agilent Technology (AT, Palo Alto, CA) 6890 gas chromatograph with a flame photometric detector (FPD) was used to detect parathion. An Agilent Technology (AT, Palo Alto, CA) 7890 gas chromatograph with nitrogen–phosphorus detector (NPD) was used to detect imidaclothiz and imidacloprid.2.2. Hapten synthesis
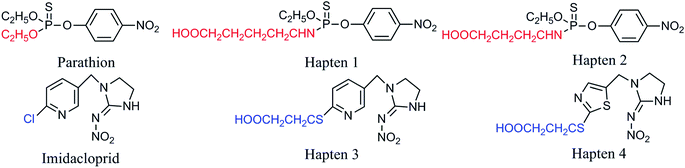
Haptens of parathion and imidacloprid were synthesized according to Liu et al.15 and Fang et al.,16 respectively. The structures of the haptens and pesticides are showed in Fig. 1.2.3. Preparation of multi-determinant immunogen
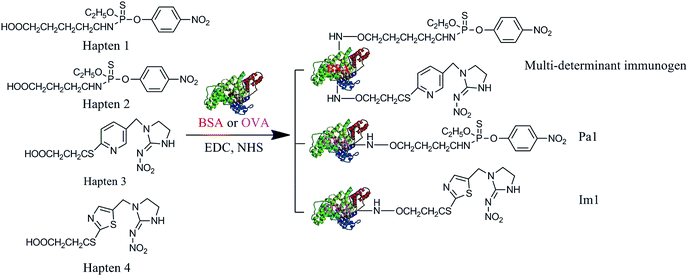
The multi-determinant immunogen was prepared using the active ester method.17 The hapten 1 (96.3 mg) was added to 1.0 mL of DMF containing NHS (28.8 mg) and DCC (51.5 mg). The mixture was stirred for 1 h at room temperature and then centrifuged to obtain a supernatant. The supernatant (400 μL) was slowly added to BSA (500 mg) in 16 mL CBS with stirring. After constantly stirring the reaction for 2.5 h, the content was dialyzed in PBS (containing 5% methanol) for 3 days, and subsequently dialyzed in CBS (containing 5% methanol) for 9 h to obtain BSA-hapten 1.The hapten 3 (81.3 mg) was activated by the above-described method. The half of the supernatant was slowly added to the 1-BSA, which dissolved in CBS. The conjugated mixture was stirred for 5 h and then dialyzed to obtain the multi-determinant immunogen. The characterization of hapten with protein conjugate was confirmed by UV-Vis spectroscopy.
2.4. Preparation of coating antigens
OVA-hapten 2 and OVA-hapten 4 were prepared by mixed anhydride method.18 Haptens (25 mmol) were dissolved in 1.0 mL of DMF and mixed with 60 μL of tributylamine and 30 μL of isobutyl chloroformate. After stirring the mixture for 1 h at room temperature, 300 μL of the solution was added to 5.0 mL of a 10.0 mg mL−1 OVA solution in CBS. The conjugates were sequentially stirred for 2.5 h and then dialyzed to obtain coating antigen. The conjugates were confirmed by UV-Vis spectroscopy.2.5. Preparation of broad-specificity polyclonal antibody (BsPAb)
The polyclonal antibody was obtained according to the works of Yan.6 Two male New Zealand white rabbits weighing approximately 2 kg were immunized with multi-determinant immunogen. Usually, the first immunization was performed by injecting 2 mg of the multi-determinant immunogen, which was dissolved in 0.5 mL of physiological saline solution and emulsified with 0.5 mL of Freund's complete adjuvant, at multiple sites on the back of each rabbit. A second immunization was made using 3 mg of the immunogen emulsified with Freund's incomplete adjuvant and injected as a booster shot after 3 weeks. Booster shots were given 4 times with two-week intervals. Whole blood was obtained from the heart of each rabbit, 8 days after the last injection. BsPAb was prepared by centrifuging and then purified via the caprylic acid–ammonium sulfate precipitation method18 and stored at −20 °C after freeze–drying.2.6. The procedures of indirect competitive ELISA (ic-ELISA)
Polystyrene micro-well plates were coated overnight at 4 °C with 100 μL per well of the coating antigens in CBS. The plates were washed 5 times with PBST and were blocked by incubation with 5% (w/v) skim milk in PBS (200 μL per well) for 50 min at 37 °C. After the plates were washed, 50 μL per well of BsPAb, diluted in PBST, and 50 μL per well of analyte or sample were added, and then incubated for 1 h at 37 °C. Before GAR/HRP (diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000, 100 μL) was added into each well, the plates were washed again. After incubation for 1 h at 37 °C and rinsing the plates for 5 times, 100 μL per well of TMB solution was added and equilibrated for 5 min. The reaction was stopped by adding 50 μL per well of H2SO4 (2 mol L−1), simultaneously absorbance was read in a micro plate reader in a dual wavelength mode (the absorbance at 450 nm as test and the absorbance at 655 nm as reference). The standard curve was constructed in the form of B/B0versus the logarithm of the concentration of analyte (logC). %(B/B0) was calculated using the following equation:
2000, 100 μL) was added into each well, the plates were washed again. After incubation for 1 h at 37 °C and rinsing the plates for 5 times, 100 μL per well of TMB solution was added and equilibrated for 5 min. The reaction was stopped by adding 50 μL per well of H2SO4 (2 mol L−1), simultaneously absorbance was read in a micro plate reader in a dual wavelength mode (the absorbance at 450 nm as test and the absorbance at 655 nm as reference). The standard curve was constructed in the form of B/B0versus the logarithm of the concentration of analyte (logC). %(B/B0) was calculated using the following equation:| %(B/B0) = [(Ax − Amin)/(Amax − Amin)] × 100 |
2.7. Specificity
The specificity of the developed ic-ELISA was evaluated by testing the cross-reactivity (CR) of BsPAb with other analogues of the analyte. The CR values were calculated as follow:| CR (%) = (IC50 of analyte/IC50 of analogue) × 100. |
2.8. Analysis of samples
The optimized ic-ELISA was used for determining parathion and imidacloprid in environmental samples (tap water). The water samples (tap water) were spiked with 0.1, 0.5 and 1 mg L−1 of parathion and imidacloprid. The spiked samples were analyzed by gas chromatography (GC) according to the method described by Yan et al.20 The measured results were compared with the ELISA results. The experiment of each sample was conducted in triplicate. The average recoveries of analyte were calculated.3. Results and discussion
3.1 Synthesis of immunogen and coating antigen
The design and synthesis of hapten, immunogen and coating antigen are critical for immunoassays.19 Because the hapten of pesticide has a low molecular weight, it lacks immunogenicity. To generate a specific antibody, the hapten should be covalently conjugated with carrier proteins to elicit an immune response of experimental animal. As shown in Fig. 2, multi-determinant immunogen and coating antigen were synthesized by conjugating four haptens with BSA or OVA. Hapten heterology may improve ELISA sensitivity.20 For the homologous assay, the same hapten was used to synthesize the coating antigen and immunogen. When the immunizing hapten is different from the coating hapten, it is a heterologous system. These types of heterologous system were used in this study; hapten 1 and 3 were conjugated with BSA as multi-determinant immunogen, hapten 2 and 4 were conjugated with OVA as coating antigens [the coating antigens for parathion (Pa1) and imidacloprid (Im1), respectively]. The hapten density of the immunogen was 7.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for parathion and 11.7
1 for parathion and 11.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for imidacloprid calculated by molar absorbance, and the hapten density of coating antigen was 8.4
1 for imidacloprid calculated by molar absorbance, and the hapten density of coating antigen was 8.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for parathion and 10.5
1 for parathion and 10.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for imidacloprid.
1 for imidacloprid.
3.2 Feasibility of the simultaneous detection of parathion and imidacloprid
The coating antigens Pa1 and Im1 were coated in different wells of ELISA plate, and then parathion and imidacloprid (0.5 mg L−1) were added. When BsPAb was added to the ELISA plate, the wells coated with Im1 and Pa1 had a colour development, the results demonstrated that the developed BsPAb could recognise parathion and imidacloprid. Furthermore, there was little change of colour in wells coated with Im1, when BsPAb with or without parathion was added. Thus, the presence of parathion did not affect the bonding reaction between Im1 and BsPAb. It was concluded that BsPAb could be used to analyse parathion and imidacloprid.3.3 Optimisation of working concentration
To develop an ELISA for simultaneous detecting parathion and imidacloprid residues using the indirect competitive format, the concentrations of coating antigens were optimised. The coating antigen (Pa1) for parathion and the coating antigen (Im1) for imidacloprid were coated in the same wells and parathion and imidacloprid were simultaneously analyzed. As shown in Table 1, the combinations (I–VIII) were separately coated. The IC50 values for the proportion of 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 parathion and imidacloprid were from 1.97 to 17.09 mg L−1, the IC50 values for 1
1 parathion and imidacloprid were from 1.97 to 17.09 mg L−1, the IC50 values for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 proportion were from 0.32 to 2.34 mg L−1, and the IC50 values for 1
1 proportion were from 0.32 to 2.34 mg L−1, and the IC50 values for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 proportion were from 1.83 to 8.79 mg L−1. When combination VII was used in the assay, the IC50 values for analyzing the parathion and imidacloprid mixture were 3.02, 1.80 and 2.31 mg L−1. The results showed that the IC50 values were generally similar, thus combination VII was used as the coating antigens for the simultaneous detection of parathion and imidacloprid.
49 proportion were from 1.83 to 8.79 mg L−1. When combination VII was used in the assay, the IC50 values for analyzing the parathion and imidacloprid mixture were 3.02, 1.80 and 2.31 mg L−1. The results showed that the IC50 values were generally similar, thus combination VII was used as the coating antigens for the simultaneous detection of parathion and imidacloprid.
Combination (Im1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pa1 Pa1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BsPAb, mg L−1) BsPAb, mg L−1) |
Different proportions of parathion and imidacloprid (m/m) | ||||||
|---|---|---|---|---|---|---|---|
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
|||||
| IC50 (mg L−1) | Max | IC50 (mg L−1) | Max | IC50 (mg L−1) | Max | ||
| I | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
17.09 | 1.79 | 1.63 | 2.25 | 2.18 | 1.88 |
| II | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
10.30 | 1.76 | 1.03 | 1.81 | 1.83 | 1.74 |
| III | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3.76 | 2.25 | 1.14 | 2.26 | 8.79 | 2.11 |
| IV | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2.74 | 2.02 | 0.87 | 2.16 | 5.93 | 2.03 |
| V | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2.29 | 1.97 | 0.62 | 1.56 | 7.28 | 1.54 |
| VI | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1.97 | 0.94 | 0.32 | 1.08 | 3.22 | 1.39 |
| VII | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
3.02 | 1.29 | 1.80 | 1.25 | 2.31 | 0.96 |
| VIII | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
4.99 | 1.43 | 2.34 | 1.35 | 2.17 | 1.02 |
3.4 Performance of the ic-ELISA for pesticides measurements
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
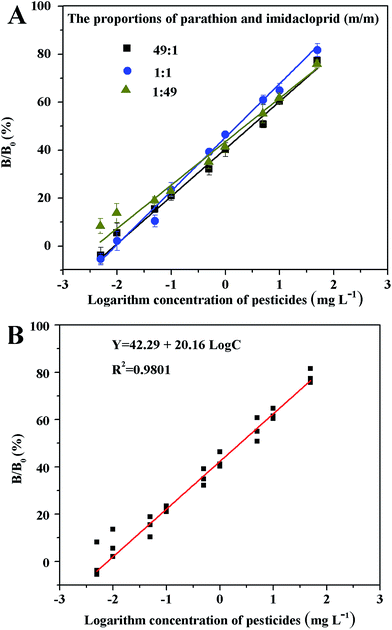
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49, m m−1) was almost the same, when combination VII was used as the coating antigens (Fig. 3A). Based on the similar linear sections of the standard curves, a calibration curve was obtained (Fig. 3B). The competitive curve (Y = 20.16
49, m m−1) was almost the same, when combination VII was used as the coating antigens (Fig. 3A). Based on the similar linear sections of the standard curves, a calibration curve was obtained (Fig. 3B). The competitive curve (Y = 20.16 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[the mixture analyte] + 42.29, mg L−1, R2 = 0.9801) for pesticides showed a linear working range, defined as the concentration range, resulting in 20–70% inhibition, which was 0.078–23.69 mg L−1, with an average IC50 value of 2.41 mg L−1 and a LOD (IC10) value of 0.025 mg L−1. The IC10 represents the 10% inhibitory concentration of pesticide. Compared to the maximum residue limits (MRL) of parathion (0.1 mg L−1) and imidacloprid (1 mg L−1),21 the sensitivity of both the combinations could satisfy the requirements of the detection of parathion and imidacloprid.
log[the mixture analyte] + 42.29, mg L−1, R2 = 0.9801) for pesticides showed a linear working range, defined as the concentration range, resulting in 20–70% inhibition, which was 0.078–23.69 mg L−1, with an average IC50 value of 2.41 mg L−1 and a LOD (IC10) value of 0.025 mg L−1. The IC10 represents the 10% inhibitory concentration of pesticide. Compared to the maximum residue limits (MRL) of parathion (0.1 mg L−1) and imidacloprid (1 mg L−1),21 the sensitivity of both the combinations could satisfy the requirements of the detection of parathion and imidacloprid.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NO2 structure. These results indicated that the developed ic-ELISA was highly specific for the parathion and imidacloprid mixture, and it is possible to detect parathion and imidacloprid using BsPAb in the same analytical conditions.
N–NO2 structure. These results indicated that the developed ic-ELISA was highly specific for the parathion and imidacloprid mixture, and it is possible to detect parathion and imidacloprid using BsPAb in the same analytical conditions.
| Analogues | IC50 (mg L−1) | CR (%) |
|---|---|---|
| Parathion + imidacloprid | 2.41 | 100 |
| Parathion–methyl | 10.43 | 23.11 |
| Fenitrothion | >200 | <1.2 |
| Profenofos | >200 | <1.2 |
| Surecide | >200 | <1.2 |
| Fenthion | >200 | <1.2 |
| Fenamiphos | >200 | <1.2 |
| Phoxim | >200 | <1.2 |
| Isocarbophos | >200 | <1.2 |
| Imidaclothiz | 15.10 | 15.96 |
| Acetamiprid | >200 | <1.2 |
| Thiacloprid | >200 | <1.2 |
| Thiamethoxam | >200 | <1.2 |
| Clothianidin | >200 | <1.2 |
| Dinotefuran | >200 | <1.2 |
| Pymetrozine | >200 | <1.2 |
| Nitenpyram | >200 | <1.2 |
3.5 Sample analysis
To validate and evaluate the applicability of the ic-ELISA for determination of pesticides, the recoveries of the spiked environmental samples with parathion and imidacloprid were monitored by the ic-ELISA and GC methods (Table 3). The recoveries from tap water ranged from 80.3% to 121%, and the RSD were lower than 6.99%. Therefore, the established ic-ELISA could detect the parathion and imidacloprid mixture residues in environmental samples.| Total spiked level (mg L−1) | Proportions of parathion and imidacloprid (m/m) | Detected (mg L−1) | RSD (n = 3, %) | |
|---|---|---|---|---|
Parathion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) imidacloprid imidacloprid |
Ic-ELISA | GC | ||
| 0.1 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.0803 | 0.085 | 3.76 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.116 | 0.109 | 2.77 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
0.106 | 0.090 | 4.86 | |
| 0.5 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.438 | 0.495 | 4.98 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.605 | 0.483 | 5.32 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
0.459 | 0.505 | 3.96 | |
| 1 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.895 | 0.950 | 2.85 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.164 | 0.932 | 5.83 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
0.986 | 0.963 | 6.99 | |
4. Conclusions
A broad-specificity antibody with multi-determinant was prepared to simultaneously detect parathion and imidacloprid. An indirect competitive enzyme-linked immunosorbent assay was optimized for the detection of gross parathion and imidacloprid residues, with an IC50 value of 2.41 mg L−1. The method showed high specificity and was successfully applied to the analysis of spiked samples. Compared with the traditional ELISA method, the ic-ELISA with broad-specificity antibody was simpler and more useful for the detection of gross parathion and imidacloprid residues. Furthermore, using two coating antigens in the same conditions, the proposed method has a big advantage in saving detection time and workload.References
- S. Young, L. Balluz and J. Malilay, Sci. Total Environ., 2004, 322, 3–20 CrossRef CAS.
- R. G. Hendrickson and J. R. Hedges, Crit. Care Clin., 2005, 21, 641–652 CrossRef PubMed.
- B. C. Giordano and G. E. Collins, Curr. Org. Chem., 2007, 11, 255–256 CrossRef CAS.
- B. Piña-Guzmán, M. Sánchez-Gutiérrez, F. Marchetti, I. Hernández-Ochoa, M. J. Solís-Heredia and B. Quintanilla-Vega, Toxicol. Appl. Pharmacol., 2009, 238, 141–149 CrossRef PubMed.
- J. E. Storm, K. R. Karl and J. Doull, Toxicology, 2000, 150, 1–29 CrossRef CAS.
- X. Yan, H. Y. Shi and M. H. Wang, Anal. Methods, 2012, 4, 4053–4057 RSC.
- V. Duzguner and S. Erdogan, Pestic. Biochem. Physiol., 2010, 97, 13–18 CrossRef CAS PubMed.
- F. Z. Zhang, Y. J. Li, C. S. Yu and C. P. Pan, Bull. Environ. Contam. Toxicol., 2012, 88, 885–890 CrossRef CAS PubMed.
- K. Li and Q. X. Li, J. Agric. Food Chem., 2000, 48, 3378–3382 CrossRef CAS PubMed.
- M. C. Hennion and D. Barcelo, Anal. Chim. Acta, 1998, 362, 3–34 CrossRef CAS.
- R. M. Lequin, Clin. Chem., 2005, 51, 2415–2418 CAS.
- W. J. Gui, Y. H. Liu, C. M. Wang, X. Liang and G. N. Zhu, Anal. Biochem., 2009, 393, 88–94 CrossRef CAS PubMed.
- X. Yan, H. X. Li, Y. Yan and X. G. Su, Anal. Methods, 2014, 6, 3543–3554 RSC.
- Z. J. Liu, X. Yan, X. Y. Xu and M. H. Wang, Analyst, 2013, 138, 3280–3286 RSC.
- Y. H. Liu, C. M. Wang, W. J. Gui, J. C. Bi, M. J. Jin and G. N. Zhu, Ecotoxicol. Environ. Saf., 2009, 72, 1673–1679 CrossRef CAS PubMed.
- S. Fang, B. Zhang, K. W. Ren, M. M. Cao, H. Y. Shi and M. H. Wang, J. Agric. Food Chem., 2011, 59, 1594–1597 CrossRef CAS PubMed.
- S. T. Wang, W. J. Gui, Y. R. Guo and G. N. Zhu, Anal. Chim. Acta, 2007, 587, 287–292 CrossRef CAS PubMed.
- Y. Liang, X. J. Liu, Y. Liu, X. Y. Yu and M. T. Fan, Anal. Chim. Acta, 2008, 615, 174–183 CrossRef CAS PubMed.
- W. X. Jiang, H. Y. Zhang, X. M. Li, X. X. Liu, S. X. Zhang, W. M. Shi, J. Z. Shen and Z. H. Wang, J. Agric. Food Chem., 2013, 61, 10925–10931 CrossRef CAS PubMed.
- X. Yan, X. J. Tang, H. X. Li, E. Z. Sheng, D. D. Yang and M. H. Wang, Food Anal. Methods, 2014, 7, 1186–1194 CrossRef.
- http://www.chinapesticide.gov.cn/mprlsv/mprls.html .
| This journal is © The Royal Society of Chemistry 2015 |