A triple-function zwitterion for preparing water compatible diclofenac imprinted polymers†
Feng
Shen
a,
Qingxi
Zhang
b and
Xueqin
Ren
*b
aDepartment of Plant Nutrition, College of Resources and Environmental Sciences, China Agricultural University, Beijing, 100193, P. R. China
bDepartment of Environmental Sciences & Engineering, College of Resources and Environmental Sciences, China Agricultural University, Beijing, 100193, P. R. China. E-mail: renxueqin@cau.edu.cn; Fax: +86-10-62731016; Tel: +86-10-62733407
First published on 17th October 2014
Abstract
A novel zwitterion acting as both a functional monomer and a crosslinker with the protein-resistant ability concomitantly was synthesized for preparing water compatible diclofenac imprinted polymers. This new imprinted polymer showed high imprinting efficiency for template and strong anti-protein adsorption in aqueous medium.
Molecular imprinting technology (MIT) is a versatile and straightforward method to prepare artificial receptors with a predetermined selectivity and specificity for a given target molecule.1–5 During the last few decades, molecularly imprinted polymers (MIPs) have been extensively applied in many fields such as solid-phase extraction,6,7 chromatographic separation,8 catalysis,9,10 drug delivery systems11 and sensors.12
Despite the tremendous progress made in these fields, many challenges still remain to be addressed. Water incompatibility is the primary concern of imprinting technology.13–16 The reported MIPs were normally prepared in organic solvent and showed poor specific binding for the template in pure aqueous media. This is because the presence of polar solvent, especially water, can disturb the hydrogen bond formation between the template and the functional monomer. Although hydrophilic modifications (e.g. HEMA,13 β-CDs17) of MIP surfaces were employed to solve this problem, the processes were complex and time-consuming. Moreover, a large amount of cross-linking agent (around 80–90%) had to be used to preserve the memory of the template in MIPs. As a result, only a small portion of the imprinted sites was available leading to the undesired low adsorption capacities.18–20 In addition to the problems mentioned above, biomacromolecules such as proteins always interfered with the determination of small target compounds in environmental water and biological samples. The nonspecific protein adsorption severely affected the sensitivity and capacity of MIPs for the target template. A layer of porous hydrophilic polymer (e.g. chitosan21) coating on the surface of adsorption materials was explored to solve this problem, and the pore size after coating was appropriate to hold back the macromolecules without influencing the adsorption of target analytes. Poly(ethylene glycol) (PEG) is one of the best synthetic materials being widely used to resist nonspecific protein adsorption.22 However, PEG is susceptible to decomposition in the presence of oxygen and transition metal ions.23,24 Recent studies have demonstrated that zwitterionic groups, including phosphorycholine, sulfobetaine, and carboxybetaine, have been widely used as a new generation of non-biofouling materials which can strongly resist nonspecific protein adsorption via a hydration layer bound through solvation of the charged terminal groups in addition to hydrogen bonding.23,25–27 In 2010, Wang et al. reported that the addition of 2-methacryloyloxyethyl phosphorylcholine, a common zwitterion-type monomer, can potentially suppress the inflammation response of the MIPs biosensors.28 Reports on zwitterions applied in MIPs for the resistance of non-specific protein adsorption are still few.
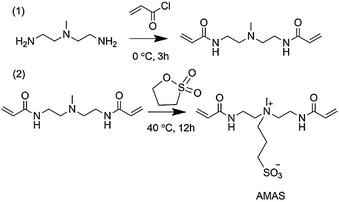
In this study, a novel kind of zwitterionic monomer, 3-(bis(2-acrylamidoethyl) (methyl) ammonio) propane-1-sulfonate (AMAS), was synthesized for preparing water compatible MIPs with strong resistance to non-specific protein adsorption. AMAS was synthesized according to Scheme 1. The newly prepared monomer bears zwitterionic groups in the middle together with two terminal vinyl groups on both sides. Zwitterionic groups provide the monomer with high hydrophilicity and electrostatic interaction with the target template in aqueous media, which could be applied for preparing water compatible MIPs. The as-prepared MIPs can be expected to resist nonspecific protein adsorption due to the zwitterionic groups as well. Moreover, the new functional monomer AMAS could act as a crosslinker simultaneously by virtue of the two terminal vinyl groups. Overall, the featured structure with two double bonds and zwitterionic groups could endow this new monomer with triple functions: as a functional monomer providing electrostatic interaction with the target template, as a water soluble crosslinker, and strong resistance to protein adsorption.
AMAS was synthesized via a facile two-step method as shown in Scheme 1. First, amidation of 2,2′-diamio-N-methyldiethylamine was conducted using acryloyl chloride in dichloromethane at 0 °C. Then, 1,3-propane sultone was added and the final product was obtained through a ring-opening reaction. The 1H NMR (300 MHz, D2O) spectrum of AMAS is shown in Fig. 1(a): 6.16 (2 H, m), 5.71 (1 H, m), 4.70 (3 H, s), 3.59 (5 H, m), 3.12 (1 H, s), 2.89 (1 H, dd, J 12.8, 5.6), 2.16 (1 H, m), 1.97 (1 H, s). In addition, 13C NMR, MS spectra of the AMAS, and 1H, 13C-NMR and MS spectra of the first step product are shown in Fig. S1 (ESI†). The synthesis method of AMAS is simple, has a fast reaction rate, employs mild reaction conditions, and provides high product yield (80%). Fig. 1(b) shows the water solubility of AMAS compared with three commonly used crosslinkers. Unlike ethylene glycol dimethacrylate (EDMA), poly(ethylene glycol)diacrylate (PEGDA, Mw 258), and N,N′-methylenebisacrylamide (MBA), which showed poor solubility in water, AMAS possessed extremely high solubility in water. 300 mg of AMAS was completely dissolved in 1 mL of water at 20 °C. To the best of our knowledge, as-prepared AMAS showed superior water solubility compared to the known crosslinkers by far. The highly solubility of AMAS is attributed to the dissociation of zwitterionic groups. Thus, instead of using aprotic organic solvents such as toluene, acetonitrile and chloroform as porogens, MIP was readily made in pure aqueous solution using AMAS as a crosslinker and a functional monomer.
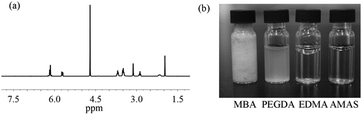 | ||
| Fig. 1 (a) 1H NMR spectrum of AMAS and (b) water-solubility of different crosslinkers (300 mg of each monomer dissolved in 1 mL of water). | ||
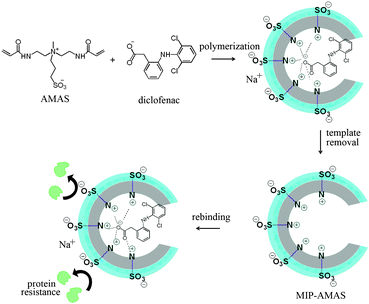
To validate this, diclofenac (DFC), an emerging drug pollutant in wastewater, was employed as a model template. DFC imprinted polymers were prepared in water with the new monomer by precipitation polymerization as illustrated in Scheme 2. First, DFC and AMAS were dissolved in 40 mL of distilled water. After the mixture was stirred for 30 min, polymerization was initiated at 60 °C for 3 h. The template was removed by washing with methanol/acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) three times. During preparation, the content of AMAS as well as MBA in the MIPs was optimized and the results are shown in Fig. S2 (ESI†). SEM images (Fig. S3, ESI†) and specific surface areas and pore volumes of the resultant MIPs and NIPs are listed and discussed in ESI.† Adsorption capacity, selectivity, and resistance to nonspecific protein adsorption of the resulting MIPs were evaluated in aqueous medium.
1, v/v) three times. During preparation, the content of AMAS as well as MBA in the MIPs was optimized and the results are shown in Fig. S2 (ESI†). SEM images (Fig. S3, ESI†) and specific surface areas and pore volumes of the resultant MIPs and NIPs are listed and discussed in ESI.† Adsorption capacity, selectivity, and resistance to nonspecific protein adsorption of the resulting MIPs were evaluated in aqueous medium.
To demonstrate the superior water compatibility of the AMAS based MIPs (MIP-AMAS), 2-vinylpyridine (2-VP), a commonly used component for the synthesis of DFC imprinted polymers in organic solvent, was employed for preparation of MIPs in water by the same procedure for comparison. Fig. 2(a) shows the amount of DFC bound to the MIPs and non-imprinted polymers (NIPs) at various initial concentrations. The adsorption capacities (Q, the calculation method is shown in ESI†) of the MIPs (MIP-AMAS, MIP-VP) were much higher than that of corresponding NIPs (NIP-AMAS, NIP-VP) for each of the different individual concentration values of DFC. Clearly, the MIP-AMAS revealed a considerably higher maximum binding capacity (207 mg g−1) for DFC in water than MIP-VP (68 mg g−1). Scatchard analysis indicated that a low dissociation constant of 2.16 mg L−1 for MIP-AMAS was obtained (ESI,† Section 6). One of the reasons is that the AMAS based MIPs were more water compatible due to the hydrophilic zwitterionic monomer. Another important reason was that the newly synthesized monomer AMAS could act as a crosslinker as well as functional monomer simultaneously. Therefore, the resulting MIPs had more imprinting sites at the equal cross-linking degree (1.20 mmol crosslinker). Furthermore, the kinetic uptake of DFC by the MIPs and NIPs was investigated, and experimental results showed that the adsorption equilibrium was reached after 10 min for both MIPs and NIPs (Fig. S4, ESI†), which was much faster than that of typical imprinted materials. Therefore, this material can be efficiently applied in practice.
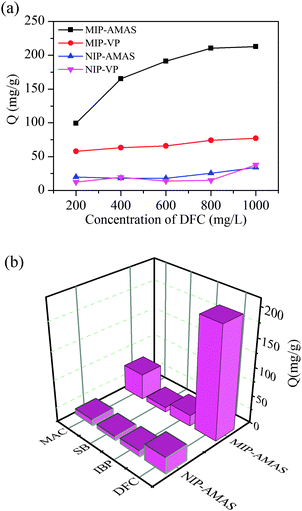 | ||
| Fig. 2 (a) Static adsorption curves of the MIPs and NIPs for DFC. (b) Binding selectivity of MIP-AMAS and NIP-AMAS. | ||
Selectivity is an important factor to evaluate the imprinting efficiency of MIPs. In this study, mefenamic acid (MAC), ibuprofen (IBP) and sodium benzoate (SB) were employed as the competitive analogues of DFC. Molecular structures of the four analytes are listed in Fig. S5 (ESI†) and the concentration of analytes was measured by HPLC. As shown in Fig. 2(b). The amount of template DFC adsorbed by MIP-AMAS (197.4 mg g−1) was found to be significantly higher than that of MAC (46.0 mg g−1), IBP (19.5 mg g−1) and SB (8.7 mg g−1). While NIPs showed unbiased enrichment towards DFC, MAC, IBP and SB. The imprinting factor (IF) calculated using the equation IF = QMIP/QNIP was used to evaluate the selectivity of the MIPs. For the template DFC the IF was 6.9, which was much higher than the IF of MAC (4.4), IBP (1.9), and SB (1.4). The IF of MAC was higher than other two compounds, indicating that the MIP could recognize its structural analogues of DFC over other compounds tested. This is because the structure and functional groups of MAC were very close to the template. These results indicated that the MIPs possess a high level of selectivity for the template molecule DFC. The selectivity was promoted not only by the binding sites created by the template molecule effectively leaving a memory of its size and shape, but also by the strong electrostatic interactions that occurred between the target molecules and the AMAS. In contrast, only chemical interaction was retained on the NIPs, and the selectivity was therefore comparable for DFC, MAC, IBP, and SB.
Bovine serum albumin (BSA) was employed as a model protein interferent to examine the high protein resistance of MIP-AMAS. To visually reveal the function of AMAS resisting protein adsorption, BSA was labelled with fluorescein isothiocyanate (FITC). After FITC-BSA was adsorbed by MIP-AMAS or MIP-VP, the fluorescent residue on MIPs was observed under a fluorescence microscope (Olympus FV-1000) and the concentration of BSA was measured by HPLC. As shown in Fig. 3, the intensity of fluorescence of MIP-VP was much stronger after incubating with FITC-BSA. The adsorption amounts of FITC-BSA reached 58.2 mg g−1. However, after the MIP was modified with AMAS (MIP-AMAS), the fluorescence residue was almost invisible and the corresponding FITC-BSA adsorption amount was significantly decreased to 3 mg g−1. To further demonstrate the low non-specific protein binding properties of MIP-AMAS, a recovery study was carried out on the BSA samples (10 mg mL−1) spiked with 10–60 mg L−1 of DFC. Experiment results (Table S1, ESI†) showed that good recoveries of 92–95% were obtained. Generally, the proteins always interfere with the determination of small target compounds and the recovery would be very low. The high recovery in this work should be attributed to the protein-resisting ability of AMAS. These results strongly indicate that the zwitterion monomer AMAS embedded in the MIPs showed high specificity and strong resistance to protein adsorption.
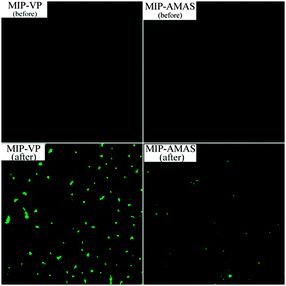 | ||
| Fig. 3 Fluorescent images of MIP-VP and MIP-AMAS before (top) and after (bottom) rebinding with FITC-BSA. | ||
In summary, we have synthesized a triple-function (a crosslinker, a functional monomer and a protein resistant monomer) zwitterion AMAS for preparing water compatible DFC imprinted polymers. MIP-AMAS exhibited high imprinting efficiency for the template in water due to the hydrophilicity and multi-functionalities of AMAS. Moreover, the resulting MIP-AMAS demonstrated strong resistance to non-specific protein adsorption in the water phase owing to the zwitterionic groups of AMAS.
This work was supported by the Chinese National Scientific Foundation (21375146).
Notes and references
- B. Sellergren, Angew. Chem., Int. Ed., 2000, 39, 1031–1037 CrossRef CAS.
- G. Wulff, Chem. Rev., 2002, 102, 1–28 CrossRef CAS PubMed.
- C. Alexander, H. S. Andersson, L. I. Andersson, R. J. Ansell, N. Kirsch, I. A. Nicholls, J. O'Mahony and M. J. Whitcombe, J. Mol. Recognit., 2006, 19, 106–180 CrossRef CAS PubMed.
- L. Ye and K. Mosbach, Chem. Mater., 2008, 20, 859–868 CrossRef CAS.
- K. Haupt, Chem. Commun., 2003, 171–178 RSC.
- M. L. Mena, P. Martínez-Ruiz, A. J. Reviejo and J. M. Pingarrón, Anal. Chim. Acta, 2002, 451, 297–304 CrossRef CAS.
- X. Cai, J. Li, Z. Zhang, F. Yang, R. Dong and L. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 305–313 CAS.
- J. Wang, P. A. Cormack, D. C. Sherrington and E. Khoshdel, Angew. Chem., Int. Ed., 2003, 42, 5336–5338 CrossRef CAS PubMed.
- N. Kirsch, J. Hedin-Dahlström, H. Henschel, M. J. Whitcombe, S. Wikman and I. A. Nicholls, J. Mol. Catal., 2009, 58, 110–117 CrossRef CAS PubMed.
- V. Abbate, A. R. Bassindale, K. F. Brandstadt and P. G. Taylor, J. Catal., 2011, 284, 68–76 CrossRef CAS PubMed.
- C. Alvarez-Lorenzo and A. Concheiro, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 804, 231–245 CrossRef CAS PubMed.
- K. Haupt and K. Mosbach, Chem. Rev., 2000, 100, 2495–2504 CrossRef CAS PubMed.
- E. Benito-Pena, S. Martins, G. Orellana and M. C. Moreno-Bondi, Anal. Bioanal. Chem., 2009, 393, 235–245 CrossRef CAS PubMed.
- F. Horemans, A. Weustenraed, D. Spivak and T. J. Cleij, J. Mol. Recognit., 2012, 25, 344–351 CrossRef CAS PubMed.
- Y. Ma, Y. Zhang, M. Zhao, X. Guo and H. Zhang, Chem. Commun., 2012, 48, 6217–6219 RSC.
- M. Singh, A. Kumar and N. Tarannum, Anal. Bioanal. Chem., 2013, 405, 4245–4252 CrossRef CAS PubMed.
- Y. Yang, Y. Long, Q. Cao, K. Li and F. Liu, Anal. Chim. Acta, 2008, 606, 92–97 CrossRef CAS PubMed.
- L. N. Mu, X. H. Wang, L. Zhao, Y. P. Huang and Z. S. Liu, J. Chromatogr. A, 2011, 1218, 9236–9243 CrossRef CAS PubMed.
- Z. H. Wei, L. N. Mu, Y. P. Huang and Z. S. Liu, J. Chromatogr. A, 2012, 1237, 115–121 CrossRef CAS PubMed.
- X. Liu, H. Y. Zong, Y. P. Huang and Z. S. Liu, J. Chromatogr. A, 2013, 1309, 84–89 CrossRef CAS PubMed.
- X. Zhang, H. Niu, Y. Pan, Y. Shi and Y. Cai, Anal. Chem., 2010, 82, 2363 CrossRef CAS PubMed.
- S. Jo and K. Park, Biomaterials, 2000, 21, 605–616 CrossRef CAS.
- R. E. Holmlin, X. Chen, R. G. Chapman, S. Takayama and G. M. Whitesides, Langmuir, 2001, 17, 2841–2850 CrossRef CAS.
- E. Ostuni, R. G. Chapman, R. E. Holmlin, S. Takayama and G. M. Whitesides, Langmuir, 2001, 17, 5605–5620 CrossRef CAS.
- J. Ladd, Z. Zhang, S. Chen, J. C. Hower and S. Jiang, Biomacromolecules, 2008, 9, 1357–1361 CrossRef CAS PubMed.
- Q. Shi, Y. Su, W. Zhao, C. Li, Y. Hu, Z. Jiang and S. Zhu, J. Membr. Sci., 2008, 319, 271–278 CrossRef CAS PubMed.
- S. Chen, J. Zheng, L. Li and S. Jiang, J. Am. Chem. Soc., 2005, 127, 14473–14478 CrossRef CAS PubMed.
- C. Wang, A. Javadi, M. Ghaffari and S. Gong, Biomaterials, 2010, 31, 4944–4951 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analysis, NMR data, SEM data, etc. See DOI: 10.1039/c4cc04739g |
| This journal is © The Royal Society of Chemistry 2015 |