Co-solvent exfoliation and suspension of hexagonal boron nitride†
K. L.
Marsh
a,
M.
Souliman
a and
R. B.
Kaner
*ab
aDepartment of Chemistry and Biochemistry and California NanoSystems Institute, University of California, Los Angeles (UCLA), Los Angeles, CA 90095, USA. E-mail: kaner@chem.ucla.edu
bDepartment of Materials Science and Engineering, UCLA, Los Angeles, CA 90095, USA
First published on 4th November 2014
Abstract
A simple method is presented for exfoliating and suspending hexagonal boron nitride using a co-solvent approach. A 60 w/w% concentration of tert-butanol in water is very effective at exfoliating boron nitride especially when compared to the individual components alone as indicated by UV-vis and transmission electron microscopy. Molecular weight and surface tension are found to play inverse roles in the exfoliation.
Two-dimensional materials have exploded in popularity following the advent of graphene. These materials include hexagonal boron nitride (h-BN), transition metal chalcogenides, and metal halides, among many others.1 Particular interest has been focused toward the exfoliation of these materials into single- or few-layered (<20) atomic sheets. The nanosheet form of these materials allows access to exaggerated versions of their characteristics, such as high surface area and structural, electronic, and thermal properties.1–3
Often referred to as “white graphite,” h-BN is isoelectronic with its carbon counterpart, with covalently bound interplanar B and N atoms replacing C atoms to form the sp2 “honeycomb” network. Hexagonal boron nitride is known for its lack of electrical conductivity, high thermal conductivity, excellent mechanical strength, and chemical stability.3,4 Boron nitride nanosheets (BNNS) have also been confirmed to outperform their bulk counterpart in the areas of composite fillers, solid lubrication, and transistors. Traditional methods used for graphite exfoliation, such as ion intercalation, mechanical delamination, or chemical reduction (e.g. reduction of graphite oxide to form graphene), do not transfer to h-BN, despite the two having almost identical interlayer spacing (3.33–3.35 Å for graphite, vs. 3.30–3.33 Å for h-BN). The electronegativity differences between B and N atoms cause π electrons to localize around N atomic centers, and it is this polarity that causes interlayer electrostatic interactions between the partially positive B and partially negative N atoms.5 This results in a complex mix of multipole and dispersion interactions as well as Pauli repulsions that result in a similar interlayer distance to graphite, despite having radically different electronic properties.
Currently, the most popular routes for producing BNNS are through chemical vapor deposition (CVD) and liquid exfoliation.1,6–10 CVD allows for control of the growth process, and almost guarantees a low-defect, single atomic sheet of h-BN. However, CVD is a high-temperature process that is difficult to scale up. Sinitskii et al. recently showed that BN nanoribbons can be obtained by the splitting of BN nanotubes in the presence of potassium vapor;11 however, this process deals with the handling of highly reactive potassium metal, which makes it unattractive for high throughput synthesis.
Liquid exfoliation is a simple method to produce BNNS from bulk h-BN powder. Generally, h-BN powder is mixed with a solvent, and energy, usually ultrasonic energy, is introduced into the system. Studies have shown that h-BN disperses reasonably well in isopropyl alcohol (IPA),12N,N-dimethylformamide (DMF),3,4 dimethyl sulfoxide (DMSO),4,9 and N-methylpyrrolidone (NMP).1,13–15 However, many of these solvents are harmful and/or dangerous to work with. There have also been a few studies describing the exfoliation of h-BN using water as a solvent. Connell et al. recently showed that water serves a critical role in h-BN exfoliation, hydrolyzing planar defects to introduce hydroxyl groups that help to stabilize the suspended BNNS.16
Here we describe a refined method of producing BNNS from bulk h-BN powders using a simple co-solvent approach. This system combines common organic solvents with water to create a mixture that exfoliates and suspends h-BN much more efficiently than the individual components. In this way, we reap the benefits of two solvent types that have each been shown to successfully exfoliate h-BN on their own. This co-solvent system is inexpensive, safe to work with, and completely scalable. Although a single co-solvent system (IPA in water) has been reported,12 by studying the trends associated with different co-solvent combinations we have developed a much more comprehensive understanding of their effects on h-BN suspension and exfoliation. This understanding has led us to discover an optimized co-solvent system for producing and suspending BNNS. Recent literature reports have suggested that there exists a small window of surface tension values for which co-solvent exfoliation is optimal.2,9,17,18 Here we discuss the roles of solvent surface tension and molecular weight (M.W.) in the exfoliation of h-BN, and propose a mechanism for co-solvent exfoliation.
Solvents were chosen based on size (M.W.), boiling point, conformation, and safety considerations. Methanol (MeOH), ethanol (EtOH), 1-propanol (1-prop), 2-propanol (IPA), acetone, and tert-butanol (tBA) were chosen under these criteria. BNNS were prepared by way of ultrasonication. Briefly, bulk h-BN powder (average particle size of 1–13 μm, Momentive Performance Materials) was added to a co-solvent mixture at a loading of 2 mg mL−1. The suspension was then sonicated for 3 h in a bath sonicator, rotating the sample vials every 30 minutes to ensure the most homogeneous mixing possible. Samples were then centrifuged at 3200 rpm for 20 minutes, and the supernatant was collected for analysis. Great care was taken throughout all steps to ensure minimal evaporation of the solvent mixture2 (see ESI† for additional experimental details).
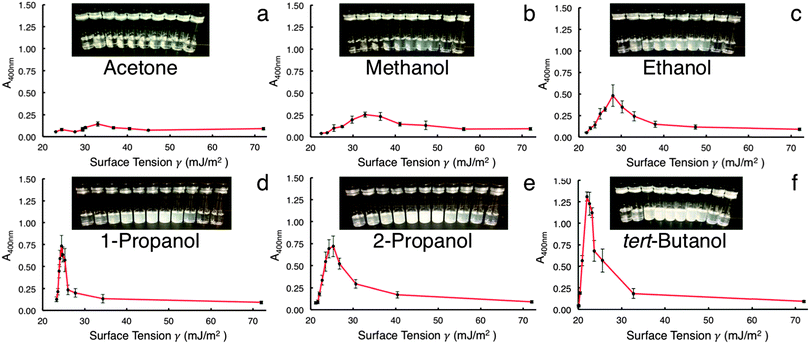
Fig. 1 shows the UV-vis data collected for the different co-solvent mixtures. Because h-BN does not exhibit any prominent absorption peaks, a wavelength of 400 nm was used to compare the relative absorbances between samples.12 All data are the averaged results of five trials for each co-solvent system. The surface tension of pure water (0 w/w% solvent) at standard conditions is 72.0 mJ m−2; this value decreases relative to which solvent is mixed in and at what concentration.19,20 This is represented on the X-axis in Fig. 1a–f: the surface tension increases from left to right corresponding to an increase in solvent w/w%. While other studies have shown evidence for both pure water16 and pure solvent9 successfully exfoliating h-BN, it is clear that a mixture of solvent and water trumps the results of the two liquids by themselves. The UV-vis data indicate that 60 w/w% tBA is superior at dispersing and retaining h-BN, with IPA and 1-prop being second best (Fig. 1f, e, and d, respectively). Co-solvent mixtures were stable after 2 months of sitting on the lab bench. Maximum absorbance (Amax) values for each solvent occur around 40–60 w/w% and increase in the following order: acetone < MeOH < EtOH < 1-prop < IPA < tBA. These data parallel results from other studies involving other inorganic graphene analogues.2,12
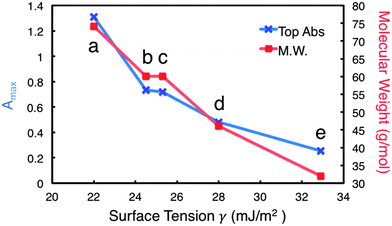
It is interesting to note that the increase in absorbance is directly proportional to increasing M.W. Fig. 2 illustrates this relationship for the solvents with similar chemical structure, tBA having the highest M.W. (74.12 g mol−1) and MeOH having the lowest (32.04 g mol−1). These data also agree with previous studies involving other inorganic layered materials.2,12 Perhaps more revealing is the relationship between M.W. and surface tension at the Amax for each solvent. As M.W. increases linearly with absorbance, the matching surface tension decreases. Thus, the relationship of surface tension is inversely proportional to both increasing M.W. and increasing Amax. Moreover, the range of surface tensions involved in Amax for each solvent is much wider than what the literature values suggest,2,9,17,18 with the maximums for tBA, IPA, 1-prop, EtOH, and MeOH corresponding to 21.3, 24.5, 25.3, 28, and 32.9 mJ m−2, respectively (Fig. S3, ESI†). These values represent a difference of approximately 11.5 mJ m−2.
Obviously surface tension is not the only factor at play in the exfoliation of h-BN, as M.W. appears to have a great impact, even if the surface tension changes drastically. This may support the importance of considering the Lennard-Jones potential between the surface of h-BN and the co-solvent system, suggesting that larger solvent molecules serve to stabilize the individually dispersed sheets more effectively than smaller solvent molecules.2 This is likely due to the larger molecules' ability to sterically separate the nanosheets, preventing their recombination in suspension.
Also notable is the effect chemical structure plays on liquid exfoliation. 1-prop and IPA result in essentially identical BNNS dispersions, despite being isomers of each other. This raises questions on the potential effect other less common solvents could have on h-BN exfoliation, specifically isomers.
Furthermore, acetone performed the worst of all the solvents, despite having a higher M.W. (58.08 g mol−1) than both MeOH and EtOH, likely due to the absence of a hydroxyl group to stabilize the BNNS in the presence of water. Future studies will further probe the relationship between surface tension, M.W., and structural dependencies in the exfoliation of h-BN.
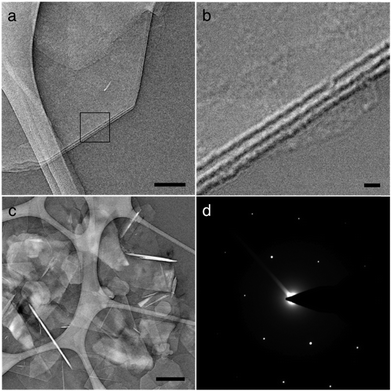
Fig. 3 shows transmission electron microscope (TEM) images of BNNS after sonication. TEM is ideal for successful analysis of BNNS, as the nature of sample preparation allows for the resolution of atomically thin layers of material while avoiding restacking of the sheets (Fig. S1, ESI†). Fig. 3b (inset of Fig. 3a) reveals the thickness of a few-layered BNNS to be approximately 7–9 nm, which corresponds to roughly 14–19 atomic layers. This agrees with a semi-quantitative assessment of BNNS thickness through TEM analyses, which suggests an average thickness range of 6–10 nm for exfoliated BNNS. Fig. 3c illustrates the scrolling effect seen in BNNS, a result of sonication and the presence of extremely thin sheets.15 The authors have experienced this same phenomenon before with few-layer graphene.21Fig. 3c also shows the plethora of single- and few-layered sheets present on the TEM sample. Fig. 3d shows a diffraction pattern for a few-layered BNNS.10,14,22,23 Fig. S2 (ESI†) also shows partial exfoliation of h-BN, an intermediate step to successful exfoliation.
In conclusion, we have implemented a co-solvent approach to exfoliate and suspend h-BN into BNNS. This method is completely scalable, inexpensive, and extremely safe to handle. We have found that a 60 w/w% mixture of tBA in water is most effective at creating a stable suspension of BNNS, with TEM confirming exfoliation into few-layered sheets. This is in contrast to studies that suggest pure water or pure solvent is effective at exfoliating h-BN. We have shown that Amax and M.W. are inversely proportional to co-solvent surface tension, and that the range of surface tension values corresponding to Amax is approximately 11.5 mJ m−2, a much broader spread than what is suggested in the literature. The critical factor for choosing a solvent system to exfoliate h-BN is not solely surface tension; rather, it should be a combinatory consideration of surface tension, M.W., and chemical structure. This lends itself to the importance of phenomena such as the Lennard-Jones potential in the exfoliation of layered materials. The authors are currently pursuing the use of more exotic solvents in the exfoliation and suspension of h-BN in order to further clarify the roles of M.W. and chemical structure.
The authors would like to thank Momentive Performance Materials, Inc. for providing the boron nitride powders and Dr Anand Muragaiah and Dr Hao Qu for many helpful discussions.
Notes and references
- V. Nicolosi, M. Chhowalla, M. G. Kanatzidis, M. S. Strano and J. N. Coleman, Science, 2013, 340, 1226419 CrossRef.
- U. Halim, C. R. Zheng, Y. Chen, Z. Lin, S. Jiang, R. Cheng, Y. Huang and X. Duan, Nat. Commun., 2013, 4, 2213 Search PubMed.
- C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889–2893 CrossRef CAS.
- G. Lian, X. Zhang, M. Tan, S. Zhang, D. Cui and Q. Wang, J. Mater. Chem., 2011, 21, 9201 RSC.
- O. Hod, J. Chem. Theory Comput., 2012, 8, 1360–1369 CrossRef CAS.
- G. Kim, A.-R. Jang, H. Y. Jeong, Z. Lee, D. J. Kang and H. S. Shin, Nano Lett., 2013, 13, 1834–1839 CAS.
- K. K. Kim, A. Hsu, X. Jia, S. M. Kim, Y. Shi, M. Hofmann, D. Nezich, J. F. Rodriguez-Nieva, M. Dresselhaus, T. Palacios and J. Kong, Nano Lett., 2012, 12, 161–166 CrossRef PubMed.
- M. Seth, S. G. Hatzikiriakos and T. M. Clere, Polym. Eng. Sci., 2002, 42, 743–752 CAS.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist and V. Nicolosi, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- Y. Shi, C. Hamsen, X. Jia, K. K. Kim, A. Reina, M. Hofmann, A. L. Hsu, K. Zhang, H. Li, Z.-Y. Juang, M. S. Dresselhaus, L.-J. Li and J. Kong, Nano Lett., 2010, 10, 4134–4139 CrossRef CAS PubMed.
- A. Sinitskii, K. J. Erickson, W. Lu, A. L. Gibb, C. Zhi, Y. Bando, D. Golberg, A. Zettl and J. M. Tour, ACS Nano, 2014, 8, 9867–9873 CrossRef CAS PubMed.
- K.-G. Zhou, N.-N. Mao, H.-X. Wang, Y. Peng and H.-L. Zhang, Angew. Chem., Int. Ed., 2011, 50, 10839–10842 CrossRef CAS PubMed.
- J. M. Hughes, D. Aherne and J. N. Coleman, J. Appl. Polym. Sci., 2013, 127, 4483–4491 CrossRef CAS.
- X. Chen, J. F. Dobson and C. L. Raston, Chem. Commun., 2012, 48, 3703–3705 RSC.
- X. Chen, R. A. Boulos, J. F. Dobson and C. L. Raston, Nanoscale, 2013, 5, 498–502 RSC.
- Y. Lin, T. V. Williams, T.-B. Xu, W. Cao, H. E. Elsayed-Ali and J. W. Connell, J. Phys. Chem. C, 2011, 115, 2679–2685 CAS.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'Ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- L. Cao, S. Emami and K. Lafdi, Mater. Express, 2014, 4, 165–171 CrossRef CAS PubMed.
- Handbook of Chemistry and Physics, CRC Press, 84th edn, 2003, pp. 6–186 Search PubMed.
- J. Gliński, G. Chavepeyer and J.-K. Platten, J. Chem. Phys., 1995, 102, 2113 CrossRef PubMed.
- L. M. Viculis, J. J. Mack and R. B. Kaner, Science, 2003, 299, 1361 CrossRef CAS PubMed.
- M. Du, X. Li, A. Wang, Y. Wu, X. Hao and M. Zhao, Angew. Chem., Int. Ed., 2014, 53, 3645–3649 CrossRef CAS PubMed.
- M. L. Odlyzko and K. A. Mkhoyan, Microsc. Microanal., 2012, 18, 558–567 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental and characterization information, additional electron microscopy images and photographs, and CRC handbook values for surface tension. See DOI: 10.1039/c4cc07324j |
| This journal is © The Royal Society of Chemistry 2015 |