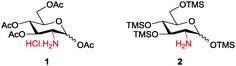Chemoselective per-O-trimethylsilylation and homogeneous N-functionalisation of amino sugars†
A. Abragam
Joseph
ab,
Vijay M.
Dhurandhare
acd,
Chun-Wei
Chang
ac,
Ved Prakash
Verma
a,
Girija Prasad
Mishra
ae,
Chiao-Chu
Ku
a,
Chun-Cheng
Lin
*b and
Cheng-Chung
Wang
*ac
aInstitute of Chemistry, Academia Sinica, Taipei 115, Taiwan. E-mail: wangcc@chem.sinica.edu.tw; Fax: +886-2-2783-1237; Tel: +886-2-2789-8618
bDepartment of Chemistry, National Tsing Hua University, Hsinchu 300, Taiwan. E-mail: cclin66@mx.nthu.edu.tw; Fax: +886-2-2783-1237; Tel: +886-2-2789-8618
cChemical Biology and Molecular Biophysics Program, Taiwan International Graduate Program, Academia Sinica, Taipei 115, Taiwan
dInstitute of Bioinformatics and Structural Biology, National Tsing Hua University, Hsinchu 300, Taiwan
eGenomics Research Center, Academia Sinica, Taipei 115, Taiwan
First published on 4th November 2014
Abstract
A highly efficient CH3CN-promoted hexamethyldisilazane per-O-trimethylsilylation of amino sugars was developed. Its applications in homogenous N-functionalisation and a concise synthesis of glucosamine 6-phosphate are described.
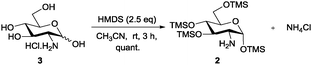
Amino sugars are widely dispersed in nature and have been found in numerous biologically potent polysaccharides such as mucopolysaccharides or mucoproteins, bacterial capsular polysaccharides, lipopolysaccharides, glycolipids, N-glycans, glycosaminoglycans, and numerous antibiotics.1,2 More than 60 amino sugars are known, and amongst them, D-glucosamine and its N-acetylated and -sulfonated derivatives are commonly found in bioactive molecules.2 Therefore, amino group functionalisation is one of the most fundamental modifications when dealing with amino sugars, but the current methods are laborious. Efforts have been made to functionalise the amino groups in the presence of multiple hydroxyl groups of sugar molecules. Amine groups can be chemoselectively functionalised in the presence of multiple hydroxyl groups by using the reactivity differences in nucleophilicity under the Schotten–Baumann conditions (acid chloride and aqueous NaHCO3); however, this method requires an excessive number of reagents. Moreover, isolating products from aqueous media and salts is often difficult and time consuming. In many cases, reverse-phase chromatography or per-O-acetylation of the product after the reactions are occasionally required because of the high polarity of desired molecules, but the yields are usually only moderate. Therefore, upscaling these reactions is often difficult even though it is often the first step in the synthesis process.3 To enable homogeneous N-functionalisation of glucosamine, per-O-acetylated glucosamine hydrochloride 1,4 which requires three steps to prepare, is usually used as an alternative.5 Hence, to produce these functionalised amino sugars on a large scale, an efficient, simple, economic, and reliable method is still required (Scheme 1).
Because solubility is the key concern in the synthesis of amino sugar derivatives, we propose chemoselective masking of all hydroxyl groups over the amine group to enable subsequent amine modification as an efficient solution to the aforementioned problems. Therefore, we expected that per-O-trimethylsilylated glucosamine 2 would satisfy the requirements. Although 26 and its triethylsilyl derivatives have been prepared and Boysen et al. used them to prepare Glucobox derivatives,7 the existing method to prepare them needs a large excess of silylating reagents, including TMSCl (10 eq.), hexamethyldisilazane (HMDS) (10 eq.) and bis-(trimethylsilyl)acetamide (BSA) (1 eq.), and a longer reaction time. Difficult workup and additional purifications are required to remove the extra reagents, the N-trimethylsilylated derivative and salts, giving lower yield.6 The applications of 2 have neither been studied extensively nor extended, probably because it is difficult to prepare.
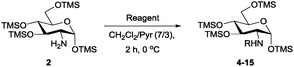
Per-O-trimethylsilyl derivatives of carbohydrates have been applied widely in the preparation of building blocks for oligosaccharide synthesis.8 We recently developed a highly efficient TMSOTf-catalysed HMDS silylation of carbohydrates.9 Although nitromethane-promoted HMDS silylation of alcohols was reported,10 no effective solvent-promoted HMDS trimethylsilylation of amino sugars has been described. Here, we report that glucosamine hydrochloride (3) can be treated with 2.5 equivalents of HMDS in acetonitrile for 3 h at room temperature under catalyst-free conditions to yield 2 as a single α-isomer in a nearly quantitative yield after simply filtering and evaporating acetonitrile (Scheme 2), and the reaction can be easily scaled up to a 25 g scale (see ESI†). Therefore, the hydroxyl groups of an amino sugar can be masked efficiently and easily with the amine groups unaffected by taking advantage of the stronger bond dissociation energy of O–Si compared with that of the N–Si bond.
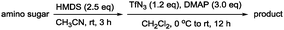
In addition, our method involves using a minimal amount of reagents and enables amine protection or functionalisation reactions after simple filtration and evaporation. Because the amino group remains intact, we screened various N-functionalisation for 2 by using various amine protecting groups in CH2Cl2/Pyr (7/3) at 0 °C to produce compounds 4–15 with 75% to 95% yields as summarised in Table 1.
| Entry | Reagent | R (product) | Yield (%) |
|---|---|---|---|
| 1 | TCACl | TCA (4) | 87 |
| 2 | Ac2O | Ac (5) | 77 |
| 3 | TFAA | TFA (6) | 88 |
| 4 | TrocCl | Troc (7) | 91 |
| 5 | CbzCl | Cbz (8) | 81 |
| 6 | MsCl | Ms (9) | 85 |
| 7 | TsCl | Ts (10) | 75 |
| 8 | DNSCl | DNS (11) | 89 |
| 9 | Benzenesulfonyl chloride | Benzenesulfonyl (12) | 78 |
| 10 | p-Nitro-benzenesulfonyl chloride | p-Nitrobenzenesulfonyl (13) | 77 |
| 11 | 2,4-Dinitrobenzenesulfonyl chloride | 2,4-Dinitrobenzenesulfonyl (14) | 78 |
| 12 | Lauoryl chloride | Lauoryl (15) | 86 |
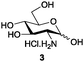
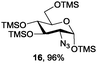
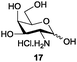
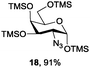
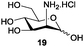
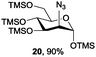
Azide is amongst the few neighbouring nonparticipating amine protecting groups and is essential for 1,2-cis glycosylation reactions of 2-amino glycosides.3b,11 The diazotransfer reactions of amino glycosides traditionally begin with direct amine functionalisation of amino sugars under heterogeneous H2O/CH2Cl2 biphase conditions, and, thus, vigorous stirring and a long reaction time are required. Furthermore, the arduous workup and purification processes require large quantities of solvents and eluents. Although further acetylation11a,c or silylation11d of the crude products can facilitate purification, these treatments are also inefficient and require an excessive number of reagents. By contrast, we per-O-trimethylsilylated the amino sugars first, thus, the diazotransfer reactions could be conducted under homogeneous conditions with TfN3 and DMAP in CH2Cl2 (Table 2).12 Considering D-glucosamine (3) as an example (entry 1), the reaction was completed within 12 h, and, without workup and extraction, product 16 was obtained easily by evaporation and simple filtration through a short pad of silica gel to give 96% yield of 16 as a single α-anomer. Similarly, by using the same reaction conditions and starting from D-galacto- (17) and D-mannosamine (19) (entries 2 and 3), the corresponding azide products 18 and 20 were obtained in 91% and 90% yields, respectively, as single α-anomers. With the amino group functionalised, the O-trimethylsilyl groups can be easily removed or further utilized for other functional group modifications.8,9
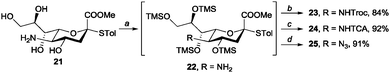
Based on these results, we extended our method to sialic acid. Specifically, we applied our method to the sialic acid derivative 2113 to prepare sialic acid donors with various N-functional groups, which play crucial roles in the stereoselectivity of sialylation reactions.14 The chemoselective HMDS silylation of 21 yielded 22 quantitatively, and then amine functionalisation was performed in the same pot. Sialic acid derivatives with C5 NHTroc (23), NHTCA (24), and N3 (25) were easily obtained in 84%, 92%, and 91% yields, respectively (Scheme 3).
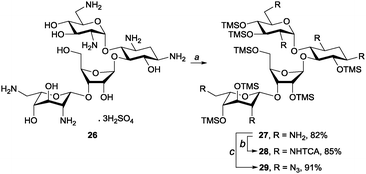
To test the applicability of our method to molecules containing multiple amine groups, we applied our method to neomycin sulphate (26). Because of the poor solubility of 26, the chemoselective per-O-silylation required a longer reaction time (36 h) and more HMDS (7.0 eq.) as compared to the previous examples. However, the pure product 27 was obtained after simple filtration and solvent evaporation in an 82% yield. Compound 27 was subjected to amine functionalisation reactions, which yielded per-N-trichloroacetylated 28 and per-N-azidated 29 in 85% and 91% yield, respectively (Scheme 4).
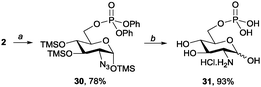
Glucosamine 6-phosphate (31) has recently received substantial attention because of its potent biological activity in ribosomal cleavage.6c,15 Although some chemical16a,b and enzymatic syntheses have been reported in recent years,6c,16c there is still room for an efficient chemical synthesis. The concise and efficient synthesis of 31 from 2 was achieved using our method, producing an overall yield of 73%. As shown in Scheme 5, per-O-trimethylsilylated glucosamine (2) was first treated with TfN3 and DMAP to conduct a diazotransfer reaction. The homogeneous conditions then enabled a C6 phosphorylation reaction to be conducted in a one-pot manner. Thus, pyridine and (PhO)2POCl were added subsequently. The glucosamine 6-phosphate derivative 30 was isolated in a 78% yield by using our recently reported method.9b Reducing the azide group of 30, neutralising the amine group with HCl(aq.), and hydrogenolysing the diphenyphosphate group yielded glucosamine 6-phosphate 30 in a 93% yield.
In conclusion, we reported a simple, efficient and consistent method for preparation of per-O-trimethylsilylated amino sugars with unprotected amines. In addition, various homogeneous chemoselective N-functionalisations, which have much improved procedures as compared to the traditional methods, especially the N-azidation, were achieved. Our method was effective for both mono and multiple amine substrates. Moreover, we synthesised glucosamine 6-phosphate efficiently. We believe that this method has simplified and advanced the preparation of carbohydrate building blocks containing amino groups, especially on a large scale, and will facilitate the synthesis of carbohydrate molecules containing amino sugars.
This work was supported by the Ministry of Science and Technology of Taiwan (NSC 101-2113-M-001-011-MY2), (MOST 103-2113-M-001-022) and Academia Sinica.
Notes and references
- Essentials of Glycobiology, ed. A. Varki, D. R. Cummings, J. D. Esko, H. H. Freeze, P. Stanley, C. R. Bertozzi, G. W. Hart and M. E. Etzler, Cold Spring Harbor, New York, 2nd edn, 2008 Search PubMed.
- (a) C. E. Bryant, D. R. Spring, M. Gangloff and N. Gay, Nat. Rev. Microbiol., 2010, 8, 8 CAS; (b) M. G. Paulick and C. R. Bertozzi, Biochemistry, 2008, 47, 6991 CrossRef CAS PubMed; (c) N. S. Gandhi and R. L. Mancera, Chem. Biol. Drug Des., 2008, 72, 455 CrossRef CAS PubMed; (d) L.-X. Wang and W. Huang, Curr. Opin. Chem. Biol., 2009, 13, 592 CrossRef CAS PubMed; (e) S. Taube, M. Jiang and C. E. Wobus, Viruses, 2010, 2, 1011 CrossRef CAS PubMed; (f) M. R. J. Salton, Annu. Rev. Biochem., 1965, 34, 143 CrossRef CAS PubMed; (g) M. Emmadi and S. S. Kulkarni, Nat. Protoc., 2013, 8, 1870 CrossRef CAS PubMed.
- For examples: (a) N. S. Simpkins and S. Stokes, Tetrahedron Lett., 1992, 33, 793 CrossRef CAS; (b) P. B. Alper, S.-C. Hung and C.-H. Wong, Tetrahedron Lett., 1996, 37, 6029 CrossRef CAS; (c) Y. Jia, N. Ma, Z. Liu, M. Bois-Choussy, E. Gonzalez-Zamora, A. Malabarba, C. Brunati and J. Zhu, Chem. – Eur. J., 2006, 12, 5334 CrossRef CAS PubMed; (d) M. E. Jung, T. A. Dong and X. Cai, Tetrahedron Lett., 2011, 52, 2533 CrossRef CAS PubMed.
- D. J. Silva, H. Wang, N. M. Allanson, R. K. Jane and M. J. Sofia, J. Org. Chem., 1999, 64, 5926 CrossRef CAS.
- (a) V. R. Krishnamurthy, A. Dougherty, M. Kamat, X. Song, R. D. Cummings and E. L. Chaikof, Carbohydr. Res., 2010, 345, 1541 CrossRef CAS PubMed; (b) R. Roychoudhury and N. L. B. Pohl, Org. Lett., 2014, 16, 1156 CrossRef CAS PubMed.
- (a) C. C. Sweeley, R. Bentley, M. Makita and W. W. Wells, J. Am. Chem. Soc., 1963, 85, 2497 CrossRef CAS; (b) J. Karkkainen and R. Vihko, Carbohydr. Res., 1969, 10, 113 CrossRef CAS; (c) J. Lim, B. C. Grove, A. Roth and R. R. Breaker, Angew. Chem., Int. Ed., 2006, 45, 6689 CrossRef CAS PubMed; (d) S. Pothukanuri and N. Winssinger, Org. Lett., 2007, 9, 2223 CrossRef CAS PubMed; (e) L. Yin, M. C. Dalsin, A. Sizovs, T. M. Reineke and M. A. Hillmyer, Macromolecules, 2012, 45, 4322 CrossRef CAS.
- (a) T. Minuth, M. Irmak, A. Groschner, T. Lehnert and M. M. K. Boysen, Eur. J. Org. Chem., 2009, 997 CrossRef CAS; (b) M. Irmak, T. Lehnert and M. M. K. Boysen, Tetrahedron Lett., 2007, 48, 7890 CrossRef CAS PubMed; (c) M. Irmak, A. Groschner and M. M. K. Boysen, Chem. Commun., 2007, 177 RSC; (d) M. Irmak and M. M. K. Boysen, Adv. Synth. Catal., 2008, 350, 403 CrossRef CAS; (e) T. Minuth and M. M. K. Boysen, Synthesis, 2010, 2799 CAS.
- (a) C.-C. Wang, J.-C. Lee, S.-Y. Luo, S. S. Kulkarni, Y.-W. Huang, C.-C. Lee, K.-L. Chang and S.-C. Hung, Nature, 2007, 446, 896 CrossRef CAS PubMed; (b) C.-C. Wang, S.-S. Kulkarni, J.-C. Lee, S.-Y. Luo and S.-C. Hung, Nat. Protoc., 2008, 3, 97 CrossRef CAS PubMed; (c) C.-C. Wang, M. M. L. Zulueta and S.-C. Hung, Chimia, 2011, 65, 54 CrossRef CAS; (d) K.-L. Chang, M. M. L. Zulueta, X.-A. Liu, Y.-Q. Zhong and S.-C. Hung, J. Org. Chem., 2010, 75, 7424 CrossRef CAS PubMed; (e) M. A. Witschi and J. Gervay-Hague, Org. Lett., 2010, 12, 4312 CrossRef CAS PubMed; (f) H.-W. Hsieh, M. W. Schombs, M. A. Witschi and J. Gervay-Hague, J. Org. Chem., 2013, 78, 9677 CrossRef CAS PubMed; (g) G. Despras, D. Urban, B. Vauzeilles and J.-M. Beau, Chem. Commun., 2014, 50, 1067 RSC; (h) Y.-C. Ko, C.-F. Tsai, C.-C. Wang, V. M. Dhurandhare, P.-L. Hu, T.-Y. Su, L. S. Lico, M. M. L. Zulueta and S.-C. Hung, J. Am. Chem. Soc., 2014, 136, 14425 CrossRef CAS PubMed.
- (a) A. A. Joseph, V. P. Verma, X.-Y. Liu, C.-H. Wu, V. M. Dhurandhare and C.-C. Wang, Eur. J. Org. Chem., 2012, 744 CrossRef CAS; (b) A. A. Joseph, C.-W. Chang and C.-C. Wang, Chem. Commun., 2013, 49, 11497 RSC.
- T. Kadam and S. S. Kim, Green Chem., 2010, 12, 94 RSC.
- (a) R.-B. Yan, F. Yang, Y. Wu, L.-H. Zhang and X.-S. Ye, Tetrahedron Lett., 2005, 46, 8993 CrossRef CAS PubMed; (b) H. Ye, R. Liu, D. Li, Y. Liu, H. Yuan, W. Guo, L. Zhou, X. Cao, H. Tian, J. Shen and P. G. Wang, Org. Lett., 2013, 15, 18 CrossRef CAS PubMed; (c) S. Masuko, S. Bera, D. E. Green, M. Weïwer, J. Liu, P. L. De Angelis and R. J. Linhardt, J. Org. Chem., 2012, 77, 1449 CrossRef CAS PubMed; (d) K.-L. Chang, M. M. L. Zulueta, X.-A. Lu, Y.-Q. Zhong and S.-C. Hung, J. Org. Chem., 2010, 75, 7424 CrossRef CAS PubMed.
- A. Vasella, C. Witzig, J.-L. Chiara and M. Martin-Lomas, Helv. Chim. Acta, 1991, 74, 2073 CrossRef CAS.
- K.-C. Lu, S.-Y. Tseng and C.-C. Lin, Carbohydr. Res., 2002, 337, 755 CrossRef CAS.
- (a) C.-S. Yu, K. Niikura, C.-C. Lin and C.-H. Wong, Angew. Chem., Int. Ed., 2001, 40, 2900 CrossRef CAS; (b) C.-C. Lin, N.-P. Lin, L. Sk. Sahabuddin, V. R. Reddy, L.-D. Huang, K. C. Hwang and C.-C. Lin, J. Org. Chem., 2010, 75, 4921 CrossRef CAS PubMed.
- (a) D. J. Klein and A. R. Ferré-D'Amare, Science, 2006, 313, 1752 CrossRef CAS PubMed; (b) J. H. Davis, B. F. Dunican and S. A. Strobel, Biochemistry, 2011, 50, 7236 CrossRef CAS PubMed; (c) B. Gong, D. J. Klein, A. R. Ferre-D'Amare and P. R. Carey, J. Am. Chem. Soc., 2011, 133, 14188 CrossRef CAS PubMed; (d) J. Viladoms and M. J. Fedor, J. Am. Chem. Soc., 2012, 134, 19043 CrossRef CAS PubMed.
- (a) J. J. Posakony and A. R. Ferré-D'Amare, J. Org. Chem., 2013, 78, 4730 CrossRef CAS PubMed; (b) F. Maley and H. A. Lardy, J. Am. Chem. Soc., 1956, 78, 1393 CrossRef CAS; (c) H. K. Chenault, R. F. Mandes and K. R. Hornberger, J. Org. Chem., 1997, 62, 331 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data for new compounds, and copies of NMR spectra. See DOI: 10.1039/c4cc06645f |
| This journal is © The Royal Society of Chemistry 2015 |