Tunable photoluminescence and direct white-light emission in Mg-based coordination networks†
Zhao-Feng
Wu
ab,
Bin
Tan
ab,
Jin-Yun
Wang
a,
Cheng-Feng
Du
ab,
Zhong-Hua
Deng
ab and
Xiao-Ying
Huang
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, the Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: xyhuang@fjirsm.ac.cn; Fax: +86 591-83793727; Tel: +86 591-83793727
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 5th November 2014
Abstract
The bpy, dpe and dppe were introduced as auxiliary ligands, respectively, to construct three magnesium-1,4-NDC coordination polymers (Mg-CPs) that exhibited tunable photoluminescence (PL) and direct white-light emission upon varying the excitation light.
White light emitting materials have received continuous attention for their applications in light-emitting devices (LEDs), chemical sensing and so on.1 As a new type of single-component materials, luminescent coordination polymers (L-CPs) have recently undergone tremendous development.2 There are various ways towards white light emission in L-CPs. For instance, it can be realized by appropriately incorporating various lanthanide ions based on primary colors of red–green–blue (RGB) in the 4f and 3d–4f metal-L-CPs arising from lanthanide-centred emission,3 constructing Ag+/Pb2+-L-CPs based on the MLCT or LMCT mechanism4 or changing the guest molecules of L-CPs.5
On the other hand, the L-CPs with tunable photoluminescence (PL) are desirable for some applications.4b,5c An effective method for PL tuning is to introduce different organic chromophores in one structure so that the PL can simply be tuned by exciting different luminophores at different excitation energies.6 This way has rarely been utilized to explore tunable L-CPs, exemplified only by some Zn-CPs in which the emissions could be tuned from blue to yellow gradually.7 To the best of our knowledge, however, direct white emission has never been realized in L-CPs via this method to date.
As a low-cost, non-toxic and abundant metal ion, Mg2+ ion has been rarely exploited compared to the 3d and 4f-metal ions for constructing L-CPs.2c Recently, it has been found that some Mg-CPs exhibited benign luminescent properties.8 Furthermore, due to the electronic configuration of Mg2+, it shall be favorable to build the Mg-L-CPs with tunable PL via the above-mentioned method.
Herein, the pyridine derivatives, namely 4,4′-dipyridyl (bpy), 1,2-di-4-pyridylethene (dpe) and 1,3-di(4-pyridyl)propane (dppe), together with 1,4-naphthalene dicarboxylic acid (1,4-NDCH2), were chosen to construct three Mg-CPs, namely [Mg3(OH)2(1,4-NDC)2(bpy)(H2O)]·0.5H2O (1), [Mg3(OH)2(1,4-NDC)2(dpe)(CH3OH)2]·H2O (2) and [Mg3(OH)2(1,4-NDC)2(dppe)(H2O)] (3). The title compounds exhibited finely tunable PL properties upon simply varying the excitation light, and white light emission could be achieved in compounds 1 and 3. Of particular interest is the unusual persistence of white light emission in 3 through the whole irradiation wavelength varying process.
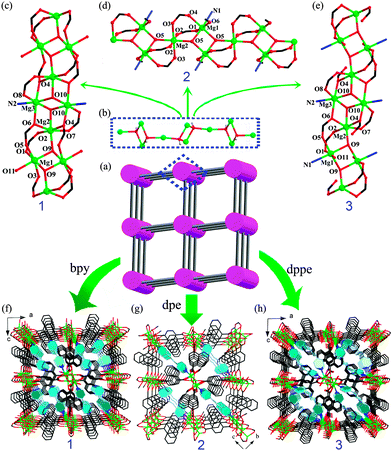
The title compounds were obtained by heating a closed bomb containing a mixture of 1,4-NDCH2, Mg(NO3)2·6H2O, NaOH, the auxiliary ligand (bpy for 1, dpe for 2 and dppe for 3), H2O and CH3OH at 433 K for 6 days, as detailed in the ESI.† The structures of the title compounds were determined via single-crystal X-ray diffraction analyses. Compounds 1, 2 and 3 feature a similar neutral three-dimensional (3D) network (Fig. 1a) that is constructed by interlinking the same parallel rod-like [Mg3(OH)2]n chains9 (Fig. 1b) through the 1,4-NDC ligands. The detailed descriptions for the crystallographically asymmetric units, metal ion coordination modes, and connecting modes of the ligands are given in the ESI.† As depicted in Fig. 1b, the OH− groups act as tridentate ligands to bridge the Mg2+ ions to form a similar 1D chain of [–Mg2–OH–(Mg1)2–OH–Mg2–OH–(Mg3)2–OH–Mg2–] in 1 and 3, and a 1D chain of [–Mg2–OH–(Mg1)2–OH–Mg2–] in 2. Then the 1D chains were further connected to the same four neighboring chains through the COO− groups of the bridging 1,4-NDC ligands with different coordination modes (Fig. S3, ESI†) to give rise to a 3D framework. It is interesting to note that there exist parallel [Mg–OH/COO]n chains in compounds 1–3 which are similar but somewhat different from each other, Fig. 1c–e. For the [Mg–OH/COO]n chains in 1 and 3, the only difference lies in the coordination environment of the Mg1 atom, that is, in 3 the N donor from a dppe ligand replaces the O donor of a COO− group (O3) in 1 that coordinates to the Mg1, so that the O3 in 3 becomes dangling without connecting to any Mg2+ ion. While the difference of the [Mg–OH/COO]n chains between compounds 2 and 3 is that one of the four coplanar COO− groups that is mono-coordinated to Mg2 atom in 2 further connects the neighboring Mg3 atom at the same chain in 3. The auxiliary N-containing ligands take a different part in constructing the structures due to their different molecular length and steric configuration, that is, the bpy acts as a terminal ligand in 1 while the dpe and dppe act as bridging ligands in 2 and 3, Fig. 1f–h.
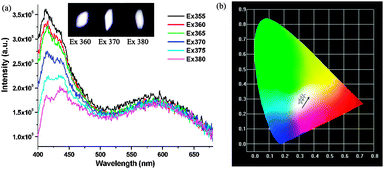
The photoluminescence of the compounds in the solid state was studied in detail. As shown in Fig. 2a, compound 1 exhibited two emission bands when varying the excitation wavelengths from 350 to 410 nm. Upon changing the excitation wavelengths from 350 to 380 nm, 1 showed a maximum emission at ∼525 nm with a broad shoulder at 600 nm, and the overall emission was always in the yellow region, as illustrated by the CIE chromaticity coordinates of the emission spectra in Fig. 2b. When the excitation wavelengths were varied from 390 nm to 410 nm, the green emission band at around 525 nm blue-shifted to 475 nm with enhanced intensities, while the emission band at 600 nm within the yellow region was almost unchanged but the emission intensities reduced. As a result, the overall emissions evolved from the yellow to white-light region little by little. Upon being excited by 410 nm, the optimized chromaticity coordinate was tuned to (0.28, 0.28) that was well located at the value of the white-light region, Fig. 2b.
 | ||
| Fig. 2 (a) Solid-state PL spectra of 1 by varying excitation lights; (b) the photograph of the CIE chromaticity diagram for 1. | ||
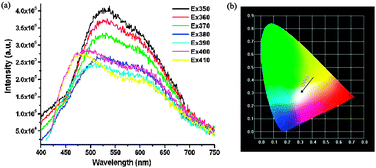
As depicted in Fig. 3a, compound 2 featured a dominant emission band at 475 nm with a shoulder emission at 560 nm. Similar to that of compound 1, compound 2 also featured tunable emission properties. Upon changing the excitation wavelengths from 400 to 425 nm, the intensities of 475 nm emission decreased while the 560 nm emission increased relatively. From the chromaticity diagram (Fig. 3b), the overall emissions of 2 began to change from green light to fall within the yellowish green region gradually. The chromaticity coordinates for 2 were (0.30, 0.40), (0.31, 0.40), (0.31, 0.41), (0.32, 0.41) and (0.33, 0.41) corresponding to the different excitation lights, respectively.
 | ||
| Fig. 3 (a) Solid-state PL spectra of 2 by varying excitation lights; (b) the photograph of the CIE chromaticity diagram for 2. | ||
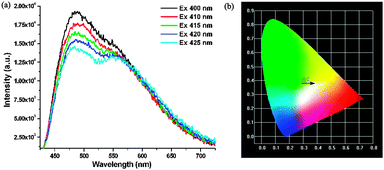
Compound 3 showed two obvious emission maxima at 410 and 580 nm upon varying the excitation wavelengths from 355 to 380 nm, which were located in the blue and yellow light region, respectively, Fig. 4a. As the excitation wavelength was increased, the intensities of the blue light emission reduced while the yellow upshot was enhanced. From the CIE chromaticity diagram (Fig. 4b), it was obvious that the PLs of 3 were all located at the white light region throughout changing the excitation wavelengths. The chromaticity coordinate for compound 3 was (0.30, 0.30) when irradiated by 370 nm light, which nearly reached the value for ideal white-light (0.33, 0.33). Along with the increase in the excited wavelength, the quantum yields (Φ) of 3 were 2.42% at 360 nm, 2.35% at 370 nm and 2.36% at 380 nm, respectively, which were comparable to those of the Pb, Zn–Ln and Cd–Ln–CPs.3c,d,4b The CRI of 89.41, 92.97, 93.12 and the CCT of 6622, 6106, 5606 K were monitored at 370 nm, 375 nm and 380 nm, respectively. Noticeably, for most of the compounds such as 1 and 2, the PLs changed gradually upon varying the excitation wavelengths,4 while for compound 3, the overall emissions remained well within the white domain of the chromaticity diagram though the irradiation wavelengths were varied from 355 to 380 nm (the inset of Fig. 4b). Such remarkable persistence of the white light feature is rare,4b which makes 3 advantageous in lighting applications. The thermal stabilities of PL response are important for white-light emissive materials. The PLs of compound 3 at varied temperatures monitored at an excitation wavelength of 360 nm were further measured (Fig. S7, ESI†), indicating sound thermally stable emissions up to 528 K.
The two emission bands in the PL spectra of the title compounds shall arise from different origins. The emission bands at around 500 nm could be assigned to ligand-centered emission features of the auxiliary ligands compared with the emissions of the free ligands (Fig. S12, ESI†).4a,10 While the low energy emission beyond 550 nm has a red-shift compared with the emissions of Mg/Cd-CPs based on the 1,4-NDC ligand11 and the emission of the 1,4-NDC ligand (Fig. S12, ESI†), possibly due to the LLCT process between 1,4-NDC and auxiliary N-donor ligands in compounds 1–3.10b,12 The PL spectra tunability of the title compounds could be presumably ascribed to the difference in the adsorption bands of the 1,4-NDC and N-donor ligands, which results in the tuning of the ratio of the two emission bands with varied excitation energies.7 For instance, for compound 1, the absorption spectrum of NDC shows a good overlap with bpy, which indicates an efficient energy transfer from bpy to 1,4-NDC, Fig. S13b (ESI†).
To deeply understand the luminescent properties of the title compounds from a theoretical aspect, the molecular orbital calculations were implemented by the density functional theory (DFT) at the B3LYP level.13 As shown in Fig. S14a (ESI†), in compound 1, the highest occupied molecular orbital (HOMO) is mainly associated with the π-bonding orbitals from the pyridine group of the bpy ligand, whereas the lowest unoccupied molecular orbital (LUMO) is on the π*-antibonding orbitals of corresponding naphthalene rings of the NDC ligand. Thus, the origin of the long-wave emission of 1 might be mainly attributed to the intraligand π*–π transitions, confirming the LLCT from the auxiliary ligands of bpy to the NDC ligand. The origin of the different emission of 2 might also be due to a similar mechanism, Fig. S13b and S14b (ESI†). The PLs of the title compounds responded differently to the variation of excitation wavelength, which might be due to the different conjugating abilities of the auxiliary ligands causing the different LLCT process.14 The DFT calculations of compound 3 could also confirm this conclusion (Fig. S14c, ESI†), the LUMO and HOMO of which were located on dppe and NDC ligands which are different from 1 and 2.15 Compared to the CPs that were constructed from the same ligands without tunable emission property, the specific 3D structures of the title compounds might be one of the main factors as well.16
In summary, the bpy, dpe and dppe were introduced as auxiliary ligands to synthesize three 3D Mg-CPs with the 1,4-NDCH2 ligand. The photoluminescence of the title compounds could be finely tunable by varying the excitation wavelength and direct white-light emission can be achieved. Future work will be focused on constructing more L-Mg-CPs based on mixed ligands. Our work would shed light on exploring L-CPs based on low-cost, non-toxic, and abundant Mg2+ ions, thus encouraging people to pay more attention towards constructing useful CPs based on environmentally and abundant metals.
This work was granted by the NNSF of China (no. 21221001 and 21403233), and the 973 program (no. 2012CB821702).
Notes and references
- (a) W. H. Green, K. P. Le, J. Grey, T. T. Au and M. J. Sailor, Science, 1997, 276, 1826–1828 CrossRef CAS; (b) S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lussem and K. Leo, Nature, 2009, 459, 234–238 CrossRef CAS PubMed; (c) L. D. Carlos, R. A. S. Ferreira, V. D. Bermudez, B. Julian-Lopez and P. Escribano, Chem. Soc. Rev., 2011, 40, 536–549 RSC; (d) J. M. Phillips, M. E. Coltrin, M. H. Crawford, A. J. Fischer, M. R. Krames, R. Mueller-Mach, G. O. Mueller, Y. Ohno, L. E. S. Rohwer, J. A. Simmons and J. Y. Tsao, Laser Photonics Rev., 2007, 1, 307–333 CrossRef CAS; (e) Q. Wang and D. G. Ma, Chem. Soc. Rev., 2010, 39, 2387–2398 RSC.
- (a) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC; (b) J. Heine and K. Moüller-Buschbaum, Chem. Soc. Rev., 2013, 42, 9232–9242 RSC; (c) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed.
- (a) Y. Liu, M. Pan, Q.-Y. Yang, L. Fu, K. Li, S.-C. Wei and C.-Y. Su, Chem. Mater., 2012, 24, 1954–1960 CrossRef CAS; (b) H.-B. Xu, X.-M. Chen, Q.-S. Zhang, L.-Y. Zhang and Z.-N. Chen, Chem. Commun., 2009, 7318–7320 RSC; (c) A. Ablet, S.-M. Li, W. Cao, X.-J. Zheng, W.-T. Wong and L.-P. Jin, Chem. – Asian J., 2013, 8, 95–100 CrossRef CAS PubMed; (d) H.-B. Xu, Y.-T. Zhong, W.-X. Zhang, Z.-N. Chen and X.-M. Chen, Dalton Trans., 2010, 39, 5676–5682 RSC; (e) S.-M. Li, X.-J. Zheng, D.-Q. Yuan, A. Ablet and L.-P. Jin, Inorg. Chem., 2012, 51, 1201–1203 CrossRef CAS PubMed; (f) X. T. Rao, Q. Huang, X. L. Yang, Y. J. Cui, Y. Yang, C. D. Wu, B. L. Chen and G. D. Qian, J. Mater. Chem., 2012, 22, 3210–3214 RSC; (g) D. F. Sava, L. E. S. Rohwer, M. A. Rodriguez and T. M. Nenoff, J. Am. Chem. Soc., 2012, 134, 3983–3986 CrossRef CAS PubMed.
- (a) M.-S. Wang, S.-P. Guo, Y. Li, L.-Z. Cai, J.-P. Zou, G. Xu, W.-W. Zhou, F.-K. Zheng and G.-C. Guo, J. Am. Chem. Soc., 2009, 131, 13572–13573 CrossRef CAS PubMed; (b) J. He, M. Zeller, A. D. Hunter and Z. T. Xu, J. Am. Chem. Soc., 2012, 134, 1553–1559 CrossRef CAS PubMed.
- (a) N. Yanai, K. Kitayama, Y. Hijikata, H. Sato, R. Matsuda, Y. Kubota, M. Takata, M. Mizuno, T. Uemura and S. Kitagawa, Nat. Mater., 2011, 787–793 CrossRef CAS PubMed; (b) M.-J. Dong, M. Zhao, S. Ou, C. Zou and C.-D. Wu, Angew. Chem., Int. Ed., 2014, 53, 1575–1579 CrossRef CAS PubMed; (c) C.-Y. Sun, X.-L. Wang, X. Zhang, C. Qin, P. Li, Z.-M. Su, D.-X. Zhu, G.-G. Shan, K.-Z. Shao, H. Wu and J. Li, Nat. Commun., 2013, 4, 2717–2724 Search PubMed; (d) Y. Takashima, V. M. Martínez, S. Furukawa, M. Kondo, S. Shimomura, H. Uehara, M. Nakahama, K. Sugimoto and S. Kitagawa, Nat. Commun., 2011, 2, 168–176 CrossRef PubMed.
- (a) K. V. Rao, K. K. R. Datta, M. Eswaramoorthy and S. J. George, Adv. Mater., 2013, 25, 1713–1718 CrossRef CAS PubMed; (b) Y. S. Zhao, H. B. Fu, F. Q. Hu, A. D. Peng, W. S. Yang and J. N. Yao, Adv. Mater., 2008, 20, 79–83 CrossRef CAS.
- S.-Q. Zhang, F.-L. Jiang, Y. Bu, M.-Y. Wu, J. Ma, X.-C. Shan, K.-C. Xiong and M.-C. Hong, CrystEngComm, 2012, 14, 6394–6396 RSC.
- (a) N. B. Shustova, A. F. Cozzolino, S. Reineke, M. Baldo and M. Dincă, J. Am. Chem. Soc., 2013, 135, 13326–13329 CrossRef CAS PubMed; (b) Z.-F. Wu, B. Tan, M.-L. Feng, A.-J. Lan and X.-Y. Huang, J. Mater. Chem. A, 2014, 2, 6426–6431 RSC; (c) K. Jayaramulu, P. Kanoo, S. J. George and T. K. Maji, Chem. Commun., 2010, 46, 7906–7908 RSC.
- N. L. Rosi, J. Kim, M. Eddaoudi, B.-L. Chen, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 1504–1518 CrossRef CAS PubMed.
- (a) J.-J. Wang, T.-L. Hu and X.-H. Bu, CrystEngComm, 2011, 13, 5152–5161 RSC; (b) J.-J. Wang, C.-S. Liu, T.-L. Hu, Z. Chang, C.-Y. Li, L.-F. Yan, P.-Q. Chen, X.-H. Bu, Q. Wu, L.-J. Zhao, Z. Wang and X.-Z. Zhang, CrystEngComm, 2008, 10, 681–692 RSC.
- (a) J. Yang, G.-D. Li, J.-J. Cao, Q. Yue, G.-H. Li and J.-S. Chen, Chem. – Eur. J., 2007, 13, 3248–3261 CrossRef CAS PubMed; (b) K.-H. He, W.-C. Song, Y.-W. Li, Y.-Q. Chen and X.-H. Bu, Cryst. Growth Des., 2012, 12, 1064–1068 CrossRef CAS; (c) X.-G. Guo, W.-B. Yang, X.-Y. Wu, K. Zhang, L. Lin, R.-M. Yu and C.-Z. Lu, CrystEngComm, 2013, 15, 3654–3663 RSC; (d) B. Tan, Z.-L. Xie, X.-Y. Huang and X.-R. Xiao, Inorg. Chem. Commun., 2011, 14, 1001–1003 CrossRef CAS PubMed.
- (a) S. J. Farley, D. L. Rochester, A. L. Thompson, J. A. K. Howard and J. A. G. Williams, Inorg. Chem., 2005, 44, 9690–9703 CrossRef CAS PubMed; (b) D. L. Rochester, S. Develay, S. Zalis and J. A. G. Williams, Dalton Trans., 2009, 1728–1741 RSC.
- (a) C. T. Lee, W.-T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS; (b) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS PubMed.
- (a) J. Gordo, J. Avó, A. J. Parola, J. C. Lima, A. Pereira and P. S. Branco, Org. Lett., 2011, 13, 5112–5115 CrossRef CAS PubMed; (b) K.-R. Wee, H.-C. Ahn, H.-J. Son, W.-S. Han, J.-E. Kim, D.-W. Cho and S.-O. Kang, J. Org. Chem., 2009, 74, 8472–8475 CrossRef CAS PubMed.
- (a) B.-S. Du, J.-L. Liao, M.-H. Huang, C.-H. Lin, H.-W. Lin, Y. Chi, H.-A. Pan, G.-L. Fan, K.-T. Wong, G.-H. Lee and P.-T. Chou, Adv. Funct. Mater., 2012, 22, 3491–3499 CrossRef CAS; (b) J.-L. Liao, Y. Chi, S.-H. Liu, G.-H. Lee, P.-T. Chou, H.-X. Huang, Y.-D. Su, C.-H. Chang, J.-S. Lin and M.-R. Tseng, Inorg. Chem., 2014, 53, 9366–9374 CrossRef CAS PubMed.
- (a) P. Kanoo and T. K. Maji, Eur. J. Inorg. Chem., 2010, 3762–3769 CrossRef CAS; (b) N.-Y. Li, Y. Ge, T. Wang, S.-J. Wang, X.-Y. Ji and D. Liu, CrystEngComm, 2014, 16, 2168–2175 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data in CIF format, detailed synthetic procedures, more structural details, PXRD, TGA curves, elemental analyses results, more PL spectra, absorption spectra and more DFT calculation details. CCDC 1025192–1025194. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc07634f |
| This journal is © The Royal Society of Chemistry 2015 |