Polyoxometalates as inorganic chiral ligands for the synthesis of chiral nanoparticles†
Lei
Shi
,
Bao
Li
and
Lixin
Wu
*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China. E-mail: wulx@jlu.edu.cn
First published on 31st October 2014
Abstract
Inherent chiral polyoxometalates, while acting as stabilizers, are developed into inorganic chiral ligands for the synthesis of chiral metal nanoparticles. The generated optical activity and stabilization of the nanoparticles are found to source from the strong interaction with polyoxometalate clusters.
Chiral nanoparticles (NPs), which hold substantial potential in heterogeneous enantioselective catalysis, sensing, nonlinear optics and composite materials, have become one of the important research topics in nanosciences.1 Nowadays, enantiopure NPs have been prepared by diverse chiral ligands such as chiral thiols, phosphine ligands, amines including amino acid derivatives, DNA templates and even polymers.2 However, these ligands are completely sourced from organic molecules and bio-molecules, and inorganic chiral ligands have not yet been well developed to date. Considering the advantages of inorganic compounds in high thermal stability, low acquisition cost, and multifunctions,3 exploiting inorganic chiral ligands for synergistic chiral nano-materials is of great significance.
Polyoxometalates (POMs) are a type of nano-sized inorganic clusters with abundant compositions and various frame structures.4 Due to their substantial potentials in chiral recognition, catalysis, and medicine, chiral POMs acquire special interests in POM chemistry.5 The covalent modification and/or electrostatic complexation of various POMs6 make them possess induced optical activity promising as asymmetric catalysts.7 Following this strategy, the photochromic chiral switch based on chiral surfactant-enwrapped POM complexes has been constructed accompanying the discovery of chiral heteropoly blue.8 However, it is still a challenge to obtain stable inherent chiral POMs (ICPOMs) and realize their functionalization.9
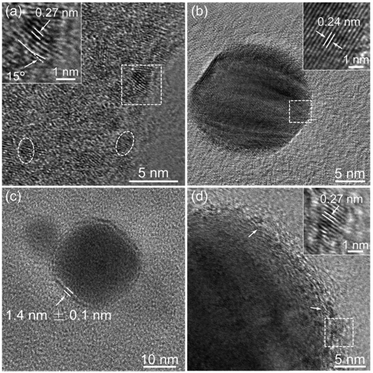
We herein demonstrate that ICPOMs induce the chirality of NPs by suitable selection of POMs, as illustrated in Scheme 1. To reach this end, a couple of inherent enantiopure POMs, [(CH3)2NH2]15{[α-P2W15O55(H2O)]Zr3(μ3-O)(H2O)(L-tartH)[α-P2W16O59]} (L-Zr-P2W15) and [(CH3)2NH2]15{[α-P2W15O55(H2O)]Zr3(μ3-O)(H2O)(D-tartH)[α-P2W16O59]} (D-Zr-P2W15), are prepared10 and confirmed using UV-Vis, FT-IR, and circular dichroism (CD) spectroscopy (Fig. S1–S3, ESI†). No obvious racemization of the chiral POMs in one day suggests the stability in aqueous solution (Fig. S4 and S5, ESI†). Single POM cluster exhibiting curved framework could be observed in HR-TEM image with size ca. 2.1 nm (Fig. 1a). The observed lattice fringes are discernible and the corresponding lattice distance 0.27 nm is consistent with the published result.7b
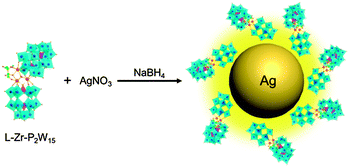 | ||
| Scheme 1 Schematic route for the synthesis of chiral silver NPs by using L-Zr-P2W15 as the stabilizer. | ||
In a typical procedure for the preparation of NPs stabilized by ICPOMs, L-Zr-P2W15 in 0.625 mM is added to Ag ion solution and controlled at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. After reduction of NaBH4, a characteristic absorption band appears at ca. 401 nm, suggesting the formation of silver NPs. The bands ascribed to 3d5/2 (367.5 eV) and 3d3/2 (373.6 eV) in the XPS spectrum indicate the metal state of Ag atoms.11 The zeta potential at −41.2 ± 2 mV implies the good stability of the prepared NPs. Spherical particles are found to be predominant in the HR-TEM images with an average size of ca. 12 nm and narrow distribution (Fig. S10, ESI†). The observed lattice plane spacing 0.24 nm corresponds to the Ag(111) plane (Fig. 1b). A monolayer shell is distinctly found at the periphery of silver NPs with a thickness of ca. 1.4 ± 0.1 nm (Fig. 1c). EDX measurements focused on a single NP exhibit characteristic peaks belonging to both POM and silver NPs (Fig. S11 and S12, ESI†), supporting the hypothesis that the monolayer on NPs is composed of POM clusters. The domains possessing a single oriented lattice fringe area are in perfect agreement with the size and lattice face distance of employed POM, further confirming the surrounding of the POM on silver NPs (Fig. 1d). The unchanged 31P NMR spectrum of L-Zr-P2W15 in the presence of NaBH4 and slight chemical shifting when anchored on NPs evidently confirm the structural completeness of chiral POMs in the reaction system (Fig. S13, ESI†). Gold NPs of a regular size are also found to be sufficiently synthesized in the presence of D-Zr-P2W15 POM clusters via the same procedure (Fig. S14 and S15, ESI†), revealing a general ability of ICPOMs for stabilizing NPs.
1 molar ratio. After reduction of NaBH4, a characteristic absorption band appears at ca. 401 nm, suggesting the formation of silver NPs. The bands ascribed to 3d5/2 (367.5 eV) and 3d3/2 (373.6 eV) in the XPS spectrum indicate the metal state of Ag atoms.11 The zeta potential at −41.2 ± 2 mV implies the good stability of the prepared NPs. Spherical particles are found to be predominant in the HR-TEM images with an average size of ca. 12 nm and narrow distribution (Fig. S10, ESI†). The observed lattice plane spacing 0.24 nm corresponds to the Ag(111) plane (Fig. 1b). A monolayer shell is distinctly found at the periphery of silver NPs with a thickness of ca. 1.4 ± 0.1 nm (Fig. 1c). EDX measurements focused on a single NP exhibit characteristic peaks belonging to both POM and silver NPs (Fig. S11 and S12, ESI†), supporting the hypothesis that the monolayer on NPs is composed of POM clusters. The domains possessing a single oriented lattice fringe area are in perfect agreement with the size and lattice face distance of employed POM, further confirming the surrounding of the POM on silver NPs (Fig. 1d). The unchanged 31P NMR spectrum of L-Zr-P2W15 in the presence of NaBH4 and slight chemical shifting when anchored on NPs evidently confirm the structural completeness of chiral POMs in the reaction system (Fig. S13, ESI†). Gold NPs of a regular size are also found to be sufficiently synthesized in the presence of D-Zr-P2W15 POM clusters via the same procedure (Fig. S14 and S15, ESI†), revealing a general ability of ICPOMs for stabilizing NPs.
To detect the interaction between POM and silver NP, XPS spectra of POM before and after complexation with NPs are also recorded. Compared with pure POM, all peaks belonging to W, O, Zr and P elements shift to lower energies in the binding state (Table S1, ESI†). The binding energies of W shift to a lower energy by ca. 0.5 eV while the binding states of C1s show no obvious shift (Fig. S19, ESI†), confirming that the POM framework rather than organic moiety directly binds to silver NPs. The W4f (7/2 and 5/2) doublets with binding energies of 35.6 and 37.7 eV prove that the L-Zr-P2W15 remains intact and no heteropoly blue forms during the preparation process of silver NPs.12 The cyclic voltammetry of L-Zr-P2W15 exhibits two couples of redox peaks in the potential range between −0.8 and −1.1 V (Fig. S22, ESI†), consistent with the stiff POMs. Thus, the apparent binding energy shift can be ascribed to the electron pulling effect of POM clusters, which enhances the intermolecular interaction between POM and silver NP. This interaction not only stabilizes the silver NPs efficiently, but also provides the driving force for the chiral induction of POM-covered silver NPs.
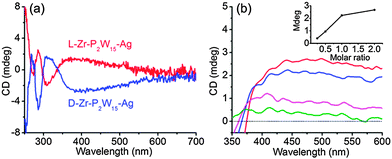
The optical activity of L-Zr-P2W15 stabilized silver NPs is examined using CD spectroscopy. In addition to the signals of isolated chiral POMs below 360 nm, a positive cotton effect appears at the wavelength corresponding to the absorption of silver NPs (Fig. 2a). A mirror symmetric CD signal is obtained from D-Zr-P2W15 stabilized silver NPs, demonstrating that enantiopure silver NPs are successfully prepared. When a racemic mixture of L- and D-Zr-P2W15 was employed, no CD signal could be observed, suggesting that enantiopure POMs contribute to the NP chirality (Fig. S23, ESI†). The induced chirality of gold NPs is also detected though the chiral intensity is weaker than that of silver NPs (Fig. S24, ESI†). This difference of induced chiral intensity can be well explained by the weaker interaction of POMs with gold NPs.13 To figure out the effect of POM concentration on induced optical activity, we modulate the molar ratio of L-Zr-P2W15 to Ag ion at 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The chiral signal intensity of silver NPs at 440 nm increases in almost linear proportion to the molar ratio of POMs up to 1
1. The chiral signal intensity of silver NPs at 440 nm increases in almost linear proportion to the molar ratio of POMs up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (inset of Fig. 2b). The coverage density of POMs is apparently responsible for the chirality saturation of NPs and the direct correlation of induced chirality with chiral POMs just matches their characteristic as a chiral ligand.
1 (inset of Fig. 2b). The coverage density of POMs is apparently responsible for the chirality saturation of NPs and the direct correlation of induced chirality with chiral POMs just matches their characteristic as a chiral ligand.
The L-tartaric acid that is used to induce chirality of POMs is employed isolatedly for the synthetic process to examine its possible influence on the chiral induction of silver NPs. The silver particle architecture is evaluated by UV-Vis spectrum and EDX measurements (Fig. S25 and S26, ESI†). Large aggregates with irregular shape in the HR-TEM images suggest an inadequate capability of L-tartaric acid for stabilizing silver NPs. Almost identical CD spectra between L-tartaric acid and silver NPs prepared in the presence of this molecule demonstrate its helplessness for yielding the chirality of NPs (Fig. S28, ESI†). Another unit of ICPOMs, α-P2W15, and a well-known achiral POM, H3PW12O40, were both found to have the capability to stabilize NPs efficiently, but they could not create any induced CD signals (Fig. S29–S32, ESI†). Apparently, it is the whole chiral POM that plays the role of a chiral ligand for optical activity of NPs.
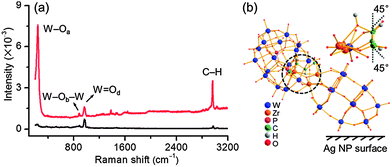
The binding mode of the chiral POM ligand on silver NPs is subsequently identified. According to the crystal structure, the long and short axes of this POM are ca. 2.1 and 1.0 nm.10 Considering that the observed POM covering layer from the above HR-TEM results is in between the two size scales, a tilt orientation of POMs on silver NPs could be simply drawn. The strong interaction of POM with NPs makes the surface enhanced Raman scattering (SERS) measurements suitable.14 The Raman bands of solid L-Zr-P2W15 at 237, 887 and 967 cm−1 can be well ascribed to vs(W–Oa), vas(W–Ob–W) and vs(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Od) vibrations, while the band at 2970 cm−1 can be attributed to C–H characteristic vibration (Fig. 3a).15 After complexation with silver NP, some Raman bands of L-Zr-P2W15 shift to lower wavenumbers and undergo enhancement (Fig. S34, ESI†), which should result from the distortion of the molecular framework of the chiral POM in the binding state.16 The change of vibration bands confirms again that the chiral POMs have strong interactions with silver NPs. Remarkably, among all vibrations in SERS spectrum, the bands of W–Oa and C–H undergo much larger enhancement.
Od) vibrations, while the band at 2970 cm−1 can be attributed to C–H characteristic vibration (Fig. 3a).15 After complexation with silver NP, some Raman bands of L-Zr-P2W15 shift to lower wavenumbers and undergo enhancement (Fig. S34, ESI†), which should result from the distortion of the molecular framework of the chiral POM in the binding state.16 The change of vibration bands confirms again that the chiral POMs have strong interactions with silver NPs. Remarkably, among all vibrations in SERS spectrum, the bands of W–Oa and C–H undergo much larger enhancement.
The alignment of [(P2W15O56)Zn4(H2O)2(P2W15O56)]16− cluster on gold NPs has been reported,13 in which tetrad-terminal oxygens co-contribute to a planar equidistant binding to maximize the interaction of the POM with the gold surface. Considering that the employed ICPOM possessed structural similarity to the Finke–Droege ion in both overall cluster size and the total number of negative charges, a similar binding mode on the silver NP surface could be assumed. Three binding motifs as marked in Fig. 3b and Fig. S35 (ESI†) could match the model. Compared with other enhanced vibrations, vs(C–H) stretching has a smaller bond number and greater enhancement (Fig. S36, ESI†) and thus we use it to estimate the most likely model. By defining one vs(C–H) stretching mode as R, the total value of stretching mode perpendicular to NP surface should be 2R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45° = 1.4R. Clearly, this model could yield more SERS enhancement because the value for other two possible models is just 2R
45° = 1.4R. Clearly, this model could yield more SERS enhancement because the value for other two possible models is just 2R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60° = 1.0R. We evaluate all the vibrations and classify them into three orientation types (0°–30°, 30°–60° and 60°–90°) according to the tilt angle with respect to the NP surface (Table S2, ESI†). The percentage of vs(W–Oa), vas(W–Ob–W) and vs(W
60° = 1.0R. We evaluate all the vibrations and classify them into three orientation types (0°–30°, 30°–60° and 60°–90°) according to the tilt angle with respect to the NP surface (Table S2, ESI†). The percentage of vs(W–Oa), vas(W–Ob–W) and vs(W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Od) vibrations within tilting angle of 30°–60° is very similar, whereas many more vibrations of vs(W–Oa) are nearly perpendicular to the NP surface. Thus, the remarkable enhancement of the vs(W–Oa) mode becomes rational.
Od) vibrations within tilting angle of 30°–60° is very similar, whereas many more vibrations of vs(W–Oa) are nearly perpendicular to the NP surface. Thus, the remarkable enhancement of the vs(W–Oa) mode becomes rational.
The binding mode of POM clusters on the NP surface is further confirmed by HR-TEM measurements (Fig. 4 and Fig. S37, ESI†). Isolated clusters such as domains marked as 1, 2 and 3 could be identified. Though the lattice fringe in domain 1 overlaps with silver NP, those in domains 2 and 3 are distinguishable. Based on the shape and width spacing of lattice fringes, we can precisely determine the orientation of the POM against the NP surface. The size of domains 2 and 3 is about 2.1 nm, consistent with that of the employed POM. Both domains adopt a tilt orientation towards the surface of NPs. The tilting angle for domain 2 is approximately 30°, which confidently confirms the proposed binding mode. A similar orientation for domains 2 and 3 and the statistics of several POMs indicate that chiral POMs interact with silver NPs through their four identical terminal oxygen atoms, and the self-adaptive POM frameworks exhibit tilt orientation.
 | ||
| Fig. 4 (a) HR-TEM image of a selected L-Zr-P2W15 stabilized silver NP and (b) the proposed binding mode of L-Zr-P2W15 anchoring on silver NP. | ||
Based on the above binding mode analysis, strong multiple Ag–O binding motifs could distort the surface silver atoms, forming a locally chiral pattern. For grown-up NPs, two possible mechanisms, formation of the chiral surface on NPs and the induction of chiral ligand to asymmetric NPs, could be used to explain the generation of chirality of NPs.17 To further trace the formation of chirality of NPs, the chiral POM ligands around NPs are exchanged by thioglycolic acid. And it is found that despite the absence of chiral POM ligands, the silver NPs stabilized by achiral thioglycolic acid are still CD active (Fig. S40 and S41, ESI†). Therefore, the chiral “footprint” created by the chiral POM ligands should be retained as responsible for the NP chirality.
In short, we develop a new kind of inorganic chiral ligand for chiral NPs. For the first time, the attached chiral POM clusters with distinguishable lattice fringes are observed, which helps to uncover the binding mode of POMs on the NP surface. Importantly, enantiopure silver NPs can be prepared and modulated by controlling molar ratio of chiral POMs to silver ions. Apparently, other chiral POMs can be used for the purpose only if the binding interaction is strong enough. Apparently, ICPOMs can be utilized for chirality transfer to other acceptors through intermolecular interactions. As a logical extension, different inorganic chiral compounds which yield strong interactions with the NPs can be also used to fabricate surface stabilized chiral NP systems.
The authors acknowledge financial support from the National Basic Research Program (2013CB834503), NSFC (91227110, 21221063) and ME (20120061110047) of China. The authors thank Dr W. Song at SKL-SSM for Raman measurements.
Notes and references
- C. Noguez and I. L. Garzón, Chem. Soc. Rev., 2009, 38, 757 RSC.
- (a) C. Gautier and T. Bürgi, ChemPhysChem, 2009, 10, 483 CrossRef CAS PubMed; (b) Y. Y. Li, Y. L. Zhou, H. Y. Wang, S. Perrett, Y. L. Zhao, Z. Y. Tang and G. J. Nie, Angew. Chem., Int. Ed., 2011, 50, 5860 CrossRef CAS PubMed.
- Y. X. Hu, X. L. Dong, J. P. Nan, W. Q. Jin, X. M. Ren, N. P. Xua and Y. M. Lee, Chem. Commun., 2011, 47, 737 RSC.
- (a) M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34 CrossRef; (b) P. C. Yin, D. Li and T. B. Liu, Chem. Soc. Rev., 2012, 41, 7368 RSC.
- B. Hasenknopf, K. Micoine, E. Lacôte, S. Thorimbert, M. Malacria and R. Thouvenot, Eur. J. Inorg. Chem., 2008, 5001 CrossRef CAS.
- (a) F. P. Xiao, J. Hao, J. Zhang, C. L. Lv, P. C. Yin, L. S. Wang and Y. G. Wei, J. Am. Chem. Soc., 2010, 132, 5956 CrossRef CAS PubMed; (b) C. Jahier, M. Cantuel, N. D. McClenaghan, T. Buffeteau, D. Cavagnat, F. Agbossou, M. Carraro, M. Bonchio and S. Nlate, Chem. – Eur. J., 2009, 15, 8703 CrossRef CAS PubMed.
- (a) Y. Z. Wang, H. L. Li, W. Qi, Y. Yang, Y. Yan, B. Li and L. X. Wu, J. Mater. Chem., 2012, 22, 9181 RSC; (b) L. Shi, Y. Z. Wang, B. Li and L. X. Wu, Dalton Trans., 2014, 43, 9177 RSC.
- Y. Z. Wang, H. L. Li, C. Wu, Y. Yang, L. Shi and L. X. Wu, Angew. Chem., Int. Ed., 2013, 125, 4675 CrossRef.
- H. Q. Tan, Y. G. Li, W. L. Chen, D. Liu, Z. M. Su, Y. Lu and E. B. Wang, Chem. – Eur. J., 2009, 15, 10940 CrossRef CAS PubMed.
- X. K. Fang, T. M. Anderson and C. L. Hill, Angew. Chem., Int. Ed., 2005, 44, 3540 CrossRef CAS PubMed.
- G. L. Liu, D. Q. Feng, W. J. Zheng, T. F. Chen and D. Li, Chem. Commun., 2013, 49, 7941 RSC.
- S. W. Li, X. L. Yu, G. J. Zhang, Y. Ma, J. N. Yao, B. Keita, L. Nadjo and H. Zhao, J. Mater. Chem., 2011, 21, 2282 RSC.
- S. Sharet, E. Sandars, Y. F. Wang, O. Zeiri, A. Neyman, L. Meshi and I. A. Weinstock, Dalton Trans., 2012, 41, 9849 RSC.
- C. L. Haynes, A. D. McFarland and R. P. V. Duyne, Anal. Chem., 2005, 77, 338A CrossRef CAS.
- C. M. Teague, X. Li, M. E. Biggin, L. Lee, J. W. Kim and A. A. Gewirth, J. Phys. Chem. B, 2004, 108, 1974 CrossRef CAS.
- G. C. Lica, K. P. Browne and Y. Y. Tong, J. Cluster Sci., 2006, 17, 349 CrossRef CAS PubMed.
- A. Guerrero-Martínez, J. L. Alonso-Gómez, B. Auguié, M. M. Cid and L. M. Liz-Marzán, Nano Today, 2011, 6, 381 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cc07750d |
| This journal is © The Royal Society of Chemistry 2015 |