A green approach to offset the perturbation action of 1-butyl-3-methylimidazolium iodide on α-chymotrypsin†
P. Madhusudhana
Reddy
,
R.
Umapathi
and
P.
Venkatesu
*
Department of Chemistry, University of Delhi, Delhi-110 007, India. E-mail: venkatesup@hotmail.com; pvenkatesu@chemistry.du.ac.in
First published on 24th October 2014
Abstract
Recently, studies have provided evidence for the negative effects of ionic liquids (ILs), “greener solvents,” on proteins [Phys. Chem. Chem. Phys., 2014, 16, 5514]. The search to offset the negative effects of ILs on proteins has come into limelight as the maintenance of a “green solvent medium” is a great challenge for chemists and biochemists. As the first step in this search, 1-butyl-3-methylimidazolium bromide ([Bmim][Br]) has been applied to offset the deleterious action of 1-butyl-3-methylimidazolium iodide ([Bmim][I]) on α-chymotrypsin (CT). Fluorescence and circular dichroism (CD) experiments results have indicated that [Bmim][Br] significantly offsets the deleterious action of [Bmim][I] at lower concentrations (0.025 M). Surprisingly, the stabilizing action of [Bmim][Br] turns into a deleterious action for CT at higher concentrations (>0.1 M). On the other hand, [Bmim][I] acted as a destabilizer for CT at all investigated concentrations (0.025–0.6 M). The results obtained from this study lead to valuable insights into the selection of suitable ILs to attenuate the deleterious action of another IL without disturbing the protein structure.
1. Introduction
Because of their ability to tailor and tune physical and chemical properties by the appropriate combination of cations and anions, ionic liquids (ILs) offer a variety of optimized technological features like solvation properties, viscosity, conductivity and thermal as well as electrochemical stability.1 In this context, room-temperature ILs have been a focal point for green chemistry and have stimulated interest in both academia and industry.2Their synthesis is highly accelerated in the laboratory and industry by biocatalytic reactions. Furthermore, biocatalysts have been optimized by nature in aqueous medium, although the use of alternative solvents may improve certain properties such as the selectivity of the reaction or the stability of the substrates. In this context, recently, the use of ILs in bioscience fields has focused on applications in biotechnology, such as biocatalysis and protein storage, because ILs provide not only a novel and highly efficient reaction medium but also serve as effective participants in various biological processes.3–5 Thus, the structural stability and activity of proteins in aqueous IL solutions have been investigated to determine the effect of ILs on biological reaction processes. According to recent studies, aqueous IL solutions increase the protein activity6,7 and protein stability,8–13 improve refolding,14–19 and inhibit aggregation.20
However, there are also reports in literature regarding the negative effects of ILs on proteins.21–26 For instance, serine protease cutinase can be destabilized in the presence of 1-butyl-3-methylimidazolium nitrate [Bmim][NO3], which suggests that there is a strong interaction between the [NO3]− anion and the enzyme donor groups.27 In another study, Klähn et al.28,29 reported the destabilization of Candida antarctica lipase B (CALB) in imidazolium or guanidinium-based ILs through MD simulations. Furthermore, very recently, our research group showed the destabilization of heme proteins in the presence of ammonium-based ILs.26 All these results will be an alarm for chemists and biochemists searching for novel methods to counteract the denaturation action of ILs on biomolecules. The counteracting effect is a situation in which an unfolded protein structure is counter-balanced by a tendency of the stabilizer to minimize the protein surface area in contact with water.30,31 However, finding a new agent that could counteract the deleterious action of IL is a great challenge for chemists and biochemists as the counteracting agent should not alter the “green solvent medium” concept.
As a first step in the search for a counteracting agent for the deleterious action of IL, herein, we successfully counteracted the deleterious action of IL, 1-butyl-3-methyl imidazolium iodide [Bmim][I] on α-chymotrypsin (CT) by another IL, 1-butyl-3-methyl imidazolium bromide [Bmim][Br]. Furthermore, [Bmim][Br] can also act as a counteracting agent against the thermal unfolding of CT. Proteolytic enzyme, CT, is one of the key valuable biological substances for understanding the mechanism of protein folding or unfolding with the addition of cosolvents.32 CT is composed of two juxtaposed β-barrel domains with catalytic residue bridging and disulfide bridges that join the three polypeptide chains. The polypeptide chain of CT comprises 245 amino acids and a catalytic triad is formed by His 57, Asp 102 and Ser 195, which is the reactive group and part of the second domain.33,34 A combination of fluorescence and circular dichroism (CD) spectroscopy was applied to elucidate the action of each ILs individually and mixture of both the ILs on thermal stability of CT. Our results demonstrated that the addition of [Bmim][Br] in a relatively small amount (0.025 M) enhances thermal stability, whereas it diminishes the thermal stability of CT at a relatively higher concentration (>0.025 M). On the other hand, the addition of [Bmim][I] decreases the thermal stability of CT at all studied concentrations (0.025–6 M). Interestingly, the deleterious action of [Bmim][I] on CT was successfully offset by the addition of [Bmim][Br].
2. Experimental section
2.1. Materials
Essentially, salt-free α-chymotrypsin (CT) (MW of 25 kDa) from bovine pancreas type II, 1-butyl-3-methylimidazolium bromide (≤200 ppm water) and 1-butyl-3-methylimidazolium iodide (≤0.5% water) were provided by the Sigma-Aldrich Chemical Company (USA). All materials, with high purity, were used without further purification. Distilled deionized water, with a resistivity of 18.3 MΩ cm, was used for the preparation of 50 mM tris-HCl buffer of pH 8.2. A Mettler Toledo balance with a precision of ±0.0001 g was used to prepare all the sample solutions. The protein concentration was maintained at 0.5 mg mL−1 for all the measurements, and the required amount of IL was added for the desired concentration of IL.2.2. Fluorescence spectroscopy
A Cary Eclipse spectrofluorometer (Varian optical spectroscopy instruments, Mulgrave, Victoria, Australia) was used to monitor the spectral changes of CT in tris-HCl buffer induced by the addition of the ILs and the mixture of ILs. The instrument was equipped with a thermostated cell holder and the desired temperature was obtained by a Peltier device attached to the sample holder of the fluorimeter. The experiments were performed at excitation and emission slit widths of 2.5 nm. The temperature dependent fluorescence intensities were measured by heating the sample at a rate of 2 °C min−1. The Trp of CT was excited at 295 nm (λexc) for the emission spectra, and the thermodynamic parameters were calculated using the fluorescence intensities. The detailed procedure to calculate the thermodynamic parameters can be found in our recent study.262.3. Circular dichroism spectroscopy
A PiStar-180 spectrophotometer (Applied Photophysics, UK) was used to record the structural changes of CT in the tris-HCl buffer induced by the addition of the ILs and the mixture of ILs. Note that the temperature was controlled by the Peltier device. (1S)-(+)-10-Camphorsulphonic acid (Aldrich, Milwaukee, WI), which exhibits a 34.5 M cm−1 molar extinction coefficient at 285 nm and 2.36 M cm−1 molar ellipticity (Θ) at 295 nm, was used for calibration. The scan speed was fixed for all the samples with a response time of 1 s and 1 nm bandwidth. Each sample spectrum was obtained by subtracting the appropriate blank medium (without CT) from the experimental spectrum and was collected by averaging six spectra.3. Results and discussion
In the present study, the thermal and structure stability of CT polypeptide was investigated in tris-HCl in the presence of [Bmim][Br] and [Bmim][I]. Furthermore, the counteracting ability of [Bmim][Br] on the deleterious action of [Bmim][I] on CT was explored.3.1. Effect of [Bmim][Br], [Bmim][I] and [Bmim][I] in the presence of [Bmim][Br] on the thermal stability of CT
In this study, we used fluorescence spectroscopy to systematically characterize the effect on the thermal behavior of CT of both individual ILs and [Bmim][I] in the presence of [Bmim][Br]. Fig. 1 and Table S1 (ESI†) demonstrate how the Tm of CT varies with the addition of [Bmim][Br], [Bmim][I], and [Bmim][I] in the presence of [Bmim][Br]. These Tm values were extracted from the graphs of the fraction unfolded vs. temperature, as shown in Fig. S1 and S2 (ESI†). Tm was defined as the temperature where the fraction unfolded reached 0.5 in the heating process. The unfolded fraction was calculated from the temperature dependent florescence intensities. It can be observed from the inset of Fig. 1 that the buffered solutions of up to ∼0.1 M of [Bmim][Br] caused CT to be in a folded conformation. However, the folded conformation was disturbed by the higher concentration of [Bmim][Br] as smaller Tm values were observed in Fig. 1 and Table S1 (ESI†). This indicates that, depending upon the concentration, [Bmim][Br] played a dual role as a stabilizer and destabilizer. On the other hand, [Bmim][I] acted as a destabilizer in all the concentrations. The expected underlying mechanism for the deleterious action of [Bmim][I] is to have favorable electrostatic interactions towards proteins, which inevitably affects the strength of the intraprotein electrostatic interactions. For the intraprotein electrostatic interactions, the backbone hydrogen bonding between the carbonyl and amide groups is the main structural element and its stability is crucial for the secondary structure. In addition to denaturant molecules, water molecules also can act as a strong hydrogen bond donor and/or acceptor, and the hydrogen bonding with the protein backbone could interrupt the backbone hydrogen bonds and thus denature the protein structure.35–37 Moreover, protein denaturation in a chaotropic salt solution is considered as a consequence of the direct binding of the cation to the protein backbone.38,39Interestingly, [Bmim][I] in the presence of [Bmim][Br] substantially increases the Tm values and offsets the [Bmim][I]-induced denaturation of CT. Moreover, it can be seen from Fig. 1 and Table S1 (ESI†) that the deleterious effect of [Bmim][I] on CT was greatly compensated by 0.025 M [Bmim][Br]. This suggests that 0.025 M [Bmim][Br] was strongly counteracting the detrimental effects of [Bmim][I] on CT. For instance, a significant increase was observed in Tm value by 1.8 °C when compared the Tm (45.6 °C) of CT in the presence of 0.4 M [Bmim][I] with that (47.4 °C) of CT in the presence of 0.025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 M [Bmim][Br]
0.4 M [Bmim][Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Bmim][I] mixture. Based on these results, a preliminary conclusion can be obtained that the deleterious action of [Bmim][I] was successfully offset by [Bmim][Br]. Although the molecular mechanism for the offset action of [Bmim][Br] is yet to be established, at present, we speculate that a synergetic effect might be the reason.
[Bmim][I] mixture. Based on these results, a preliminary conclusion can be obtained that the deleterious action of [Bmim][I] was successfully offset by [Bmim][Br]. Although the molecular mechanism for the offset action of [Bmim][Br] is yet to be established, at present, we speculate that a synergetic effect might be the reason.
3.2. Steady state fluorescence spectroscopy of CT in [Bmim][Br], [Bmim][I], and [Bmim][I] in the presence of [Bmim][Br]
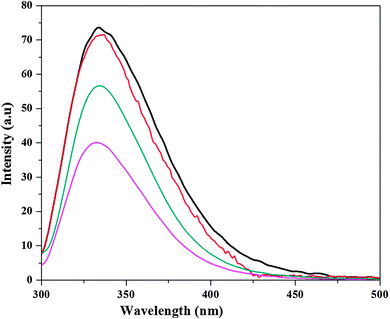
Steady state fluorescence spectroscopy was employed to study the protein unfolding process in each of the solvent media and to understand the conformational transitions that affect the tertiary structure of the protein. For proteins that contain fluorophore residues (e.g., Trp, Tyr, or Phe), such as CT, the denaturation process can be followed by changes in both the maximal intensity of the fluorescence (Imax) and the maximal emission wavelength (λmax).40 According to the selective excitation of Trp residues at 295 nm, both fluorescence parameters can be related to changes in the polarity of the microenvironment of these residues in the protein globule. First, the fluorescence intensity curve was obtained in tris-HCl solution in the absence of ILs, to evaluate the effect of ILs on protein stability. The steady state fluorescence spectra of CT in ILs are displayed in Fig. 2 and Fig. S3 (ESI†), respectively. From Fig. 2a, it can be seen that the pattern of the intensity curves of CT in the presence of [Bmim][Br] is identical, within the experimental noise, to that seen in the aqueous tris-HCl buffer control, which reflects minimal changes in the chemical environment of Trp group active-site structure in the presence of 0.025–0.1 M [Bmim][Br]. As the [Bmim][Br] concentration increases to higher than 0.1 M, the fluorescence spectrum was characterized by a marked decrease in the intensity, indicating the denaturation of CT. On the other hand, in [Bmim][I], CT consistently showed the lowest fluorescence, as shown in Fig. 2b, indicating that CT was in a denatured state. [Bmim][I] and higher concentrations of [Bmim][Br] denature the protein, and thus the Trp environment is more perturbed and leads to the diminishment in intensity. Similar observations were seen in our previous study, in which heme proteins were denatured with the addition of ammonium-based ILs and contributed to the low fluorescence intensity.26In order to evaluate the offset action of [Bmim][Br], the fluorescence spectra of CT in buffer, [Bmim][Br], [Bmim][I] and [Bmim][I] in the presence of [Bmim][Br] are illustrated in Fig. 3. As shown in Fig. 3, the fluorescence maximum (Imax) for CT in buffer was 73.6 a.u.; in 0.025 M [Bmim][Br], it was 71.5 a.u.; and in 0.2 M [Bmim][I], it was 39.7 a.u. It is necessary to take into account that under denaturing conditions, the absolute value of Imax was reduced to half with respect to that of the native CT because of the enhanced exposure of Trp residues to the bulk solvent. Interestingly, after the addition of 0.025 M [Bmim][Br] to 0.2 M [Bmim][I], it is noticeable how the fluorescence spectra of the CT was clearly modified compared with the remaining spectra, especially with regard to the enhancement of the Imax parameter of the enzyme in the presence of [Bmim][Br], which is in contrast with the decrease of this parameter in the presence of 0.2 M [Bmim][I]. Although the intensity was lower in the presence of [Bmim][Br] + [Bmim][I] than that of the native protein, it was certainly larger than that of [Bmim][I]. The main reason behind the enhancement of intensity with the addition of [Bmim][Br] into the protein solution with [Bmim][I] is the movement of Trp towards the more hydrophobic environment, and therefore a high fluorescence intensity is observed due to the higher quantum yield.
3.3. CD studies of CT in [Bmim][Br], [Bmim][I], and [Bmim][I] in the presence of [Bmim][Br]
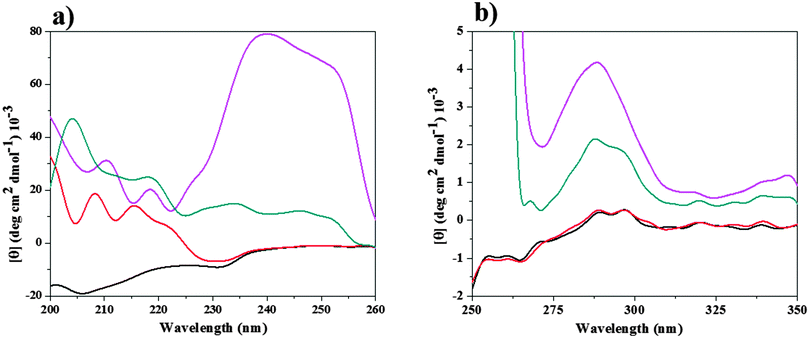
CD spectroscopy has been widely used as a biological technique in molecular biology, cell biology and biochemistry on account of its significant role in determining the structure of proteins. The use of the CD technique makes it easy for biologists to analyze the conformational changes and especially to quantify the secondary structures of proteins from the changes observed in the CD spectrum.40 The far-UV region (190–240 nm), which corresponds to the peptide bond absorption, can be used to extract knowledge regarding regular secondary structural features such as the α-helix and β-sheet. Moreover, the CD spectrum in the near region (260–320 nm) reflects the environments of the aromatic amino acid side chains, and thus gives information about the tertiary structure of the protein.41Fig. 4 and 5 show the CD spectral changes of CT in the presence of [Bmim][Br] and [Bmim][I], respectively. In low concentration of [Bmim][Br], both the CD spectra (near and far) of CT were similar to those in tris-HCl buffer, whereas the spectra were completely different in the presence of high concentrations of [Bmim][Br] from that in buffer, as shown in Fig. 4. On the other hand, as can be seen in Fig. 5, both spectra of CT in the presence of [Bmim][I] were completely different from that in buffer, thus contributing to the unfolded conformation.To understand more clearly the offset action of [Bmim][Br] on the denaturing action of [Bmim][I], the CD spectra of CT in buffer in the presence of 0.025 M [Bmim][Br], 0.2 M [Bmim][I] and 0.025 M [Bmim][Br] + 0.2 M [Bmim][I] were compared, as shown in Fig. 6. The CD spectra for CT in other combinations of molar ratios of [Bmim][Br] + [Bmim][I] are given in Fig. S4 (ESI†). As shown in Fig. 6, the CT exhibits two negative peaks at 202 and 230 nm in tris-HCl buffer. Similar kinds of negative peaks have been observed in the presence of [Bmim][Br], whereas these peaks completely vanish in the presence of [Bmim][I]. These results indicate that the secondary structure of CT is retained in the presence of [Bmim][Br], whereas it is altered in the presence of [Bmim][I]. Interestingly, by the addition of 0.025 M [Bmim][Br] to the protein solution with 0.2 M [Bmim][I], the alteration of these bands are minimum when compared to [Bmim][I], which is an indication that [Bmim][Br] acts as a counteracting agent to the deleterious action of [Bmim][I] by enhancing the β-sheet. The ellipticity values for CT in the far-UV region decreased in the presence of the mixture of [Bmim][Br] + [Bmim][I]. Moreover, an absorption band reappeared in the ∼230 nm region, which indicates the presence of β sheets in the protein structure. The present results corroborate those reported in previous studies.42
As shown in Fig. 6, CT shows one negative peak and two positive peaks at 260 and 287, 296 nm, respectively, in both the buffer and in 0.025 M [Bmim][Br]. This reflects that the orientations of aromatic amino acids (tertiary structure) has not been changed with the addition of [Bmim][Br]. On the other hand, in the presence of 0.2 M [Bmim][I], all the peaks were completely lost in the near-UV region of CD. This indicates that [Bmim][I] alters the structure of CT, and thus exposes the amino acids to the solvent media and finally leads to an increase in the magnitude of the positive ellipticity value. A similar type of enhancement was observed when CT was denatured in the presence of 5 M urea.13 The peaks were restored with the addition 0.025 M [Bmim][Br] to the protein solution with 0.2 M [Bmim][I], as shown in Fig. 6. This reestablishment of peaks with the addition of [Bmim][Br] in protein solution having [Bmim][I] indicates that [Bmim][Br] helps in keeping the protein in its folded form by counteracting the denaturation action of [Bmim][I].
The counteraction of IL on the deleterious action of the other ILs on protein likely results from a diminishment in the alteration in the protein hydration levels and structural compaction, although the underlying reasons are still largely speculative and may include additional factors such as free volume contributions, ionic interactions (salt bridges) and confinement effects. Nevertheless, this remarkable stabilization against chemical inactivation suggests a general and notable alternative to engineer solvents for protein stabilization.
4. Conclusions
For the first time, we have shown the counteracting effect of [Bmim][Br] on the deleterious action of [Bmim][I] on CT. Our fluorescence and CD spectral results demonstrated that [Bmim][Br] acts as a stabilizer at low concentrations, while it acts as a destabilizer at high concentrations. On the other hand, [Bmim][I] acts as a destabilizer at all concentrations. However, the denaturing ability of [Bmim][I] was compensated by [Bmim][Br]. Furthermore, the offset action of [Bmim][Br] on the deleterious action of [Bmim][I] is more pronounced at lower concentrations (0.025 M) than at higher concentrations. These results improve our knowledge of the excellent properties of ILs mixtures as stabilizers for the native conformation of protein, since ILs mixtures are able to stabilize enzymes and are suitable as reaction media for enzymatic biotransformations of industrial interest.Acknowledgements
We gratefully acknowledge the Council of Scientific Industrial Research (CSIR), New Delhi, through the grant No. 01(2713)/13/EMR-II, for financial support.References
- V. Govinda, P. Attri, P. Venkatesu and P. Venkateswarlu, J. Phys. Chem. B, 2013, 117, 12535 CrossRef CAS PubMed.
- Ionic Liquids: Industrial Applications for Green Chemistry, ed. R. D. Rogers and K. R. Seddon, American Chemical Society, Washington DC, 2002 Search PubMed.
- S. Park and R. J. Kazlauskas, Curr. Opin. Biotechnol., 2003, 14, 432 CrossRef CAS.
- F. van Rantwijk, R. M. Lau and R. A. Sheldon, Trends Biotechnol., 2003, 21, 131 CrossRef CAS.
- F. van Rantwijk and R. A. Sheldon, Chem. Rev., 2007, 107, 2757 CrossRef CAS PubMed.
- P. D. de Maria, Angew. Chem., Int. Ed., 2008, 47, 6960 CrossRef PubMed.
- K. Fujita and H. Ohno, Biopolymers, 2010, 93, 1093 CrossRef CAS PubMed.
- K. Fujita, D. R. MacFarlane and M. Forsyth, Chem. Commun., 2005, 4804 RSC.
- K. Fujita, D. R. MacFarlane, M. Forsyth, M. Yoshizawa-Fujita, K. Murata, N. Nakamura and H. Ohno, Biomacromolecules, 2007, 8, 2080 CrossRef CAS PubMed.
- H. S. Kim, S. H. Ha, L. Sethaphong, Y.-M. Koo and Y. G. Yingling, Phys. Chem. Chem. Phys., 2014, 16, 2944 RSC.
- A. Kumar and P. Venkatesu, RSC Adv., 2014, 4, 4487 RSC.
- S. R. Tomlinson, C. W. Kehr, M. S. Lopez, J. R. Schlup and J. L. Anthony, Ind. Eng. Chem. Res., 2014, 53, 2293 CrossRef CAS.
- P. Attri, P. Venkatesu, A. Kumar and N. Byrne, Phys. Chem. Chem. Phys., 2011, 13, 17023 RSC.
- C. Lnge, G. Patil and R. Rudolph, Protein Sci., 2005, 14, 2693 CrossRef PubMed.
- R. Buchfink, A. Tischer, G. Patil, R. Rudolph and C. Lange, J. Biotechnol., 2010, 150, 64 CrossRef CAS PubMed.
- C. A. Summers and R. A. Flowers, Protein Sci., 2000, 9, 2001 CrossRef CAS PubMed.
- S. Yamaguchi, E. Yamamoto, S. Tsukiji and T. Nagamune, Biotechnol. Prog., 2008, 24, 402 CrossRef CAS PubMed.
- N. Byrne and C. A. Angell, J. Mol. Biol., 2008, 378, 707 CrossRef CAS PubMed.
- P. Attri, P. Venkatesu and A. Kumar, Org. Biomol. Chem., 2012, 10, 7475 CAS.
- N. Byrne, L.-M. Wang, J.-P. Belieres and C. A. Angell, Chem. Commun., 2007, 2714 RSC.
- R. A. Sheldon, R. Madeira Lau, M. J. Sorgedrager, F. V. Rantwijk and K. R. Seddon, Green Chem., 2002, 4, 147 RSC.
- R. M. Lau, M. J. Sorgedrager, G. Carrea, F. van Rantwijk, F. Secundo and R. A. Sheldon, Green Chem., 2004, 6, 483 RSC.
- M. B. Turner, S. K. Spear, J. G. Huddleston, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5, 443 RSC.
- D. Constantinescu, H. Weingartner and C. Herrmann, Angew. Chem., Int. Ed., 2007, 46, 8887 CrossRef CAS PubMed.
- D. Sate, M. H. A. Janssen, G. Stephens, R. A. Sheldon, K. R. Seddon and J. R. Lu, Green Chem., 2007, 9, 859 RSC.
- I. Jha, P. Attri and P. Venkatesu, Phys. Chem. Chem. Phys., 2014, 16, 5514 RSC.
- N. M. Micaelo and C. M. Soares, J. Phys. Chem. B, 2008, 112, 2566 CrossRef CAS PubMed.
- M. Klähn, G. S. Lim, A. Seduraman and P. Wu, Phys. Chem. Chem. Phys., 2011, 13, 1649 RSC.
- M. Klähn, G. S. Lim, A. Seduraman and P. Wu, Phys. Chem. Chem. Phys., 2011, 13, 18647 RSC.
- P. Venkatesu, M. J. Lee and H. M. Lin, Arch. Biochem. Biophys., 2007, 466, 106 CrossRef CAS PubMed.
- P. Zancan, F. V. Almeida, J. Faber-Barata, J. M. Dellias and M. Sola-Penna, Arch. Biochem. Biophys., 2007, 467, 275 CrossRef CAS PubMed.
- V. Y. Levitsky, A. A. Panova and V. V. Mozhaev, Eur. J. Biochem., 1994, 219, 231 CrossRef CAS PubMed.
- C. Branden and J. Tooze, Introduction to Protein Structure, Garland Publishing, Taylor & Francis Group, New York, 1999 Search PubMed.
- T. E. Creighton, Proteins, Structures and Molecular Properties, W. H. Freeman, New York, 1993 Search PubMed.
- H. Wei, Y. Fan and Y. Q. Gao, J. Phys. Chem. B, 2010, 114, 557 CrossRef CAS PubMed.
- H. Wei, Q. Shao and Y. Q. Gao, Phys. Chem. Chem. Phys., 2010, 12, 9292 RSC.
- H. Wei, L. Yang and Y. Q. Gao, J. Phys. Chem. B, 2010, 114, 11820 CrossRef CAS PubMed.
- W. J. Xie and Y. Q. Gao, Faraday Discuss., 2013, 160, 191 RSC.
- Y. Q. Gao, J. Phys. Chem. B, 2012, 116, 9934 CrossRef CAS PubMed.
- P. Lozano, T. de Diego and J. L. Iborra, Eur. J. Biochem., 1997, 248, 80 CAS.
- B. A. Wallace, J. G. Lees, A. J. W. Orry, A. Lobley and R. W. Janes, Protein Sci., 2003, 12, 875 CrossRef CAS PubMed.
- G. M. Rather, J. Mukherjee, P. J. Halling and M. N. Gupta, PLoS One, 2012, 7, 49241 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cp04180a |
| This journal is © the Owner Societies 2015 |