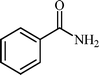
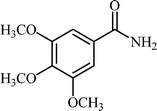
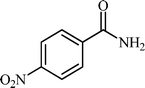
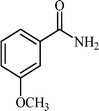
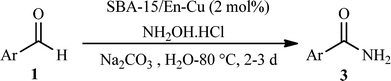Efficient and selective copper-grafted nanoporous silica in aqueous conversion of aldehydes to amides†
Sadegh
Rostamnia
*a,
Nasrin
Nouruzi
a,
Hongchuan
Xin
*b and
Rafael
Luque
c
aOrganic and Nano Group (ONG), Department of Chemistry, Faculty of Science, University of Maragheh, P.O. Box. 55181-83111, Maragheh, Iran. E-mail: rostamnia@maragheh.ac.ir; srostamnia@gmail.com; Fax: +98(421)2274893; Tel: +98(421)2278001(108)
bKey Laboratory of Biofuels, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao 266101, China. E-mail: xinhc@qibebt.ac.cn
cDepartamento de Quimica Organica, Universidad de Cordoba, Edificio Marie Curie, Ctra Nnal IV, Km 396, E-14014, Cordoba, Spain. E-mail: q62alsor@uco.es; Fax: +34 957212066; Tel: +34 957212065
First published on 4th September 2014
Abstract
The one-pot conversion of aldehydes to their corresponding primary amides has been studied using SBA-15-grafted ethylenediamine copper complexes (En–Cu). The heterogeneous catalyst could be readily isolated from the reaction mixture and reused at least 14 times without significant loss in activity. The influence of reaction parameters was studied and the conditions determining yields and selectivities, including quantity of catalyst, solvent and base, were optimized to afford amides in high yields. The structures of synthesized catalysts were investigated using different characterization techniques including Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), CHN, atomic absorption (AA), scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
Introduction
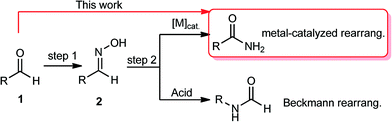
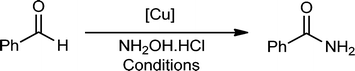
The formation of C–N bonds via C–H activation processes or related processes represents an indispensable tool for the synthesis of important target molecules that have significant pharmaceutical impact.1 Csp2–H bond conversion of aldehydes to the Csp2–N bond of amides can in principle be considered as a two-step oxidative amidation process in which the corresponding oxime forms from aldehydes in the first step. The second step leads to the formation of primary amides via nitrile intermediates based on a dehydration/hydration process. Alternatively, an acid-assisted Beckman rearrangement can take place to generate secondary amides (Scheme 1).2Amides are functional groups in organic synthesis that show a wide range of applications in pharmaceuticals, detergents and biologically active compounds.3 Au,4a Pd,4b Ru,4c Ir,4d and Rh4e catalysts have been recently reported to be effective for the rearrangement of aldoximes into primary amides. However, Au-, Pd-, Ir-, and Rh-containing catalytic materials are highly expensive. Cu(II)/Cu(0) mesoporous silica materials (unfunctionalized mesoporous impregnated copper nitrate and then reduced by H2) were disclosed as alternative catalytic systems for the conversion of aldoximes to primary amides in good yields and with good reusability (up to four cycles).5 Ramón, Ganguly and their coworkers6 later on utilised Cu(II) salts [Cu(OAc)2 and CuSO4] as direct catalysts for the one-pot transformation of aldehydes to primary amides via C–H modification of aldehydes. The metal-catalyzed single-pot conversion of aldehydes into primary amides, in comparison with aldoxime rearrangement, is a generally economical, simple and useful method.6 Nevertheless, the use of homogeneous metal salts requires laborious workup procedures to separate and isolate pure products. Furthermore, the use of environmentally unfriendly and biologically harmful solvents is one of the main issues in these chemical transformations together with the use of stoichiometric amounts of acids (as catalysts) and the use of hydroxylamine reagents in large excess.4,4a–e,5,5a,b,6
The design of advanced catalysts with transition metal active centers on the walls of solid nanoporous material has attracted increasing attention in recent years.7,8 SBA-15 silicates have been extensively utilized as mesoporous materials for a large number of applications (e.g. support for catalysis, adsorption, drug delivery, etc.) due to their uniform pore structures and high surface areas. Nevertheless, purely siliceous SBA-15 is essentially not catalytically active and has also improvable mechanical and hydrothermal stabilities due to the high hydrophilicity from its rich silanol groups on the surfaces.9 Organic–inorganic hybrid SBA-15 materials featuring organic groups incorporated within the pores of the silica wall can comparably offer improved hydrothermal stability and hydrophobicity and consequently improved catalytic properties for organic reactions. Applications of hybrid SBA-15 materials in organic processes can provide a more efficient approach of the reactants to active sites as well as suitable mesochannels to facilitate mass transfer of products into the bulk solution for improved reusability.10
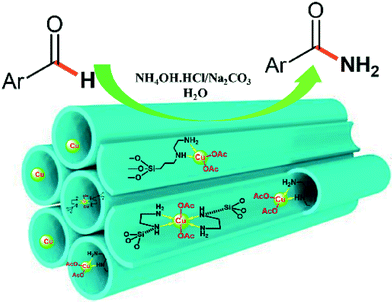
Herein, we report the design and preparation of an efficient heterogeneous Cu-containing catalyst for the mild aqueous conversion of aldehydes into primary amides. The use of widely available, less toxic and low-cost copper ethylenediamine complex-based heterogeneous catalysts has not been reported to date in the synthesis of amides. In this work, we confined copper complexes inside the channels of ethylenediamine functionalized silica SBA-15 (Fig. 1) to design highly active and reusable nanomaterials in the one-pot synthesis of amides from aldehydes.
Results and discussion
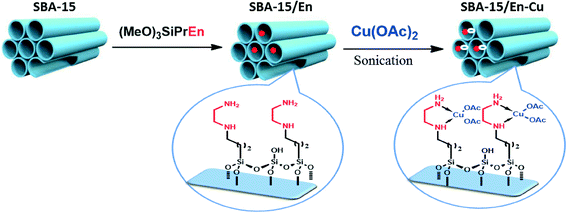
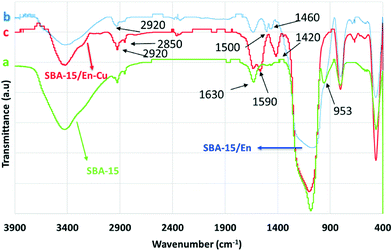
Ethylenediamine functionalized SBA-15 materials were previously reported to have excellent activities in a range of CO2 capture11a as well as aqueous removal of metal ions (e.g. Cu(II)).11b A similar complex system between grafted ethylenediamine and copper (SBA-15/En–Cu) was devised and synthesized for the selective aqueous transformation of aldehydes to amides (Fig. 2). The solid materials, characterized by a series of techniques including FT-IR, XRD, SEM, TEM and atomic absorption spectrometry (AAS), were found to be mostly composed of supported copper acetate complexes on SBA-15, which exhibited a well-ordered hexagonal structure typical of SBA-15 materials.FT-IR spectra of SBA-15 exhibit typical bands at 799 and ~1100 cm−1, correlated to (Si–O–Si) bond vibrations, with a small band at ca. 960 cm−1 attributed to functionalized (Si–OH) bonds. SiO–H groups can be visualized in the very broad IR absorption band in the 3000–3700 cm−1 region (Fig. 3). The presence of several bands with low intensity in the 1400–1600 cm−1 region corresponds to propyl ethylenediamine groups, with the band at 2900 cm−1 most possibly related to the presence of remaining traces of the surfactant and/or solvent in the material. Importantly, the decrease in intensity of the small bands at 850–950 and 1600 cm−1 in functionalized materials as compared to parent SBA-15 evidences a surface functionalization in the silicate. The characteristic peak of the C–N bond also experienced a strong shift from 1460 to 1420 cm−1 upon Cu2+ incorporation, which can be attributed to the strong interaction of –NH2 and –NH groups with Cu2+ ions.11b
XRD patterns of the unmodified SBA-15 sample exhibited three well-resolved peaks at 2θ = 0.90° and 1.5°–2.0°, consistent with (100), (110) and (200) diffraction lines, indicative of a highly ordered hexagonal SBA-15 mesoporous structure (see the ESI†).10 The structure was fully preserved upon functionalization. The morphology and microstructure of the catalyst before and after modification as well as metal loading were investigated by SEM and TEM (Fig. 4). TEM images confirm the presence of well-ordered hexagonal arrays of mesoporous channels, preserved upon functionalization, along the direction of the pore axis or in the direction perpendicular to the pore axis. TEM images could also confirm the absence of Cu0 nanoparticles in SBA-15/En–Cu.
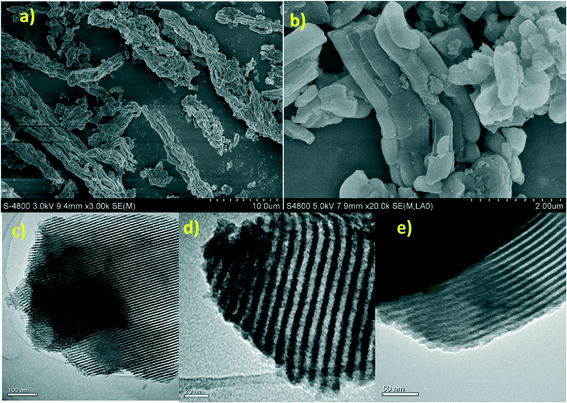 | ||
| Fig. 4 SEM images of (a) SBA-15/En and (b) SBA-15/En–Cu. TEM image of (c) SBA-15/En; (d) SBA-15/En–Cu and (e) SBA-15/En–Cu after 14 reuses in the aqueous conversion of benzaldehyde to benzamide. | ||
These results are in good agreement with results from XRD patterns and confirm that the characteristic pore dimensions and channel structures of the support materials remained intact upon organic ethylenediamine and metal complex functionalization onto SBA-15. Amine contents determined by CHN elemental analysis (1.58 mmol N g−1) also indicate that amine moieties can efficiently chelate copper ions (0.74 mmol of Cu(II) loading per gram of support) in SBA-15/Cu–En. The thermal stability of SBA-15/Cu–En was subsequently examined by thermal gravimetric (TG) studies between RT and 800 °C in a static nitrogen atmosphere (see the ESI†). TG/DTG analysis shows a remarkable thermal stability for SBA-15/Cu–En up to 400 °C in which the decomposition/degradation of the organic part of the material starts to take place.
A preliminary screening of SBA-15/En–Cu in the selected aqueous transformation of aldehydes to amides, particularly for the model reaction of benzaldehyde to benzamide under different conditions, has been included in Table 1. The first studies of the influence of the [Cu] content in the model reaction were approached by screening a wide range of supported copper-containing materials (Table 1, entry 1–4). For comparative purposes, a propylamine-grafted SBA-15 material (NH2/SBA-15/Cu) was prepared and its reactivity and stability were compared to those of SBA-15/En–Cu.
| Entry | [Cu]b | Solvent | Base | Cu (mol%) | T (°C) | Time (d) | Yield of amideb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), NH2OH·HCl (1 mmol), base (1.1 mmol) in 3 mL of solvent. b Isolated yield. c Catalyst synthesized using Cu(NO3)2via impregnation, ref. 5. | |||||||
| 1 | NH2–SBA-15/Cu | MeOH | Na2CO3 | 5 | 80 | 2 | 66 |
| 2 | SiO2/Cuc | MeOH | Na2CO3 | 5 | 80 | 2 | 33 |
| 3 | SBA-15/Cuc | MeOH | Na2CO3 | 5 | 80 | 2 | 38 |
| 4 | SBA-15/En–Cu | MeOH | Na2CO3 | 5 | 80 | 2 | 74 |
| 5 | SBA-15/En–Cu | Dioxane | Na2CO3 | 2 | 80 | 2 | 83 |
| 6 | SBA-15/En–Cu | Toluene | Na2CO3 | 2 | 80 | 3 | 62 |
| 7 | SBA-15/En–Cu | Ethanol | Na2CO3 | 2 | 60 | 2 | 51 |
| 8 | SBA-15/En–Cu | DMSO | Na2CO3 | 2 | 110 | 3 | 49 |
| 9 | SBA-15/En–Cu | DMSO/H2O (2/1) | Na2CO3 | 2 | 80 | 3 | 20 |
| 10 | SBA-15/En–Cu | H2O | Na2CO3 | 2 | 80 | 2 | 95 |
| 11 | SBA-15/En–Cu | H2O | NaHCO3 | 2 | 80 | 2 | 72 |
| 12 | SBA-15/En–Cu | H2O | K2CO3 | 2 | 80 | 2 | 93 |
| 13 | SBA-15/En–Cu | H2O | NaOAc | 2 | 80 | 2 | 33 |
| 14 | SBA-15/En–Cu | H2O | Cs2CO3 | 2 | 80 | 2 | >99 |
| 15 | SBA-15/En–Cu | H2O | Na2CO3 | 1 | 80 | 2 | 66 |
| 16 | SBA-15/En–Cu | H2O | Na2CO3 | 0.5 | 80 | 2 | 47 |
| 17 | SBA-15/En–Cu | H2O | Na2CO3 | 0.5 | 110 | 2 | 50 |
| 18 | SBA-15/En–Cu | H2O | — | 2 | 80 | 3 | Trace |
| 19 | SBA-15/En–Cu | H2O | Na2CO3 | 2 | r.t. | 3 | Trace |
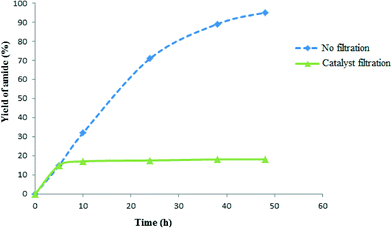
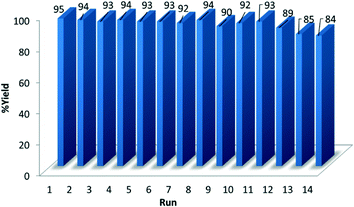
Amine– and ethylenediamine–copper complexes supported on SBA-15 exhibited superior reactivity. Interestingly, the catalytic performance of recycled NH2/SBA-15/Cu and SBA-15/En–Cu catalysts was also remarkably different. The catalytic activity of NH2–SBA-15/Cu slowly decreased after the third run. Hot filtration test studies revealed the leaching of active species to be the main reason for the observed gradual deactivation. Comparably, SBA-15/En–Cu could be successfully reused 14 times in the reaction without any significant decrease in activity or selectivity under the investigated reaction conditions (Fig. 5 and 6). The observed stability was also confirmed by hot filtration tests (no Cu leaching detected in solution after repeated reactions) and TEM (Fig. 4e, the long-range order and structure were preserved after 14 uses).
SBA-15/En–Cu was subsequently utilized as a catalyst in a number of experiments under various conditions to optimize the reaction conditions. Various solvents were screened in the reaction including H2O, dioxane, toluene, DMSO and others. Comparing results from Table 1, water was found to be the optimum solvent for the reaction (Table 1, entry 4–10). In order to explore the effect of the base, the reaction was conducted using different bases. No amide product was obtained under base-free conditions. Cs2CO3 was found to be most effective to provide quantitative yields to benzamide (Table 1, entry 14). These optimum results for cesium carbonate are in good agreement with previously reported results using homogeneous Pd catalysts.4 However, no significant differences were observed for the use of other bases in the reaction which provided excellent yields of products (93% to 95%), as shown in Table 1 (entries 10 and 12). Na2CO3 was consequently selected as a suitable base for optimization studies based on price and availability.
The amount of the catalyst had a critical effect on the yields of isolated amides. Catalyst quantities could be reduced to 2 mol% without a significant influence on product yields (Table 1, entry 10), while a further reduction (<1 mol%) led to remarkably reduced amide yields. Interestingly, reactions conducted with 0.5 mol% catalyst could provide good yields of oximes (intermediate products) which did not undergo the final reaction step to amides (Table 1, entry 17).
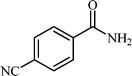
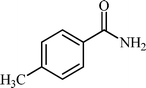
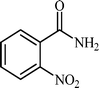
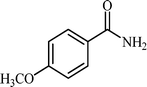
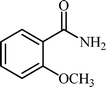
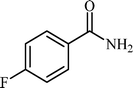
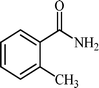
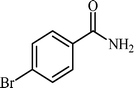
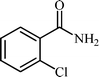
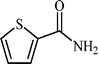
With these results in hand, the optimized reaction conditions comprised the utilization of 1 mmol of aldehyde, 1 mmol of hydroxylamine hydrochloride, 2 mol% SBA-15/En–Cu, 1.1 mmol of Na2CO3 and 3 mL of water as solvent. Optimum conditions were subsequently extended for a range of aryl aldehydes to assess the scope of the reaction. Good to excellent yields of products were obtained using aromatic aldehydes bearing electron-withdrawing (Table 2, entry 2–4) or electron-donating (Table 2, entry 5–10) functional groups. Ortho-substituted aryl aldehydes (Table 2, entry 4–7) gave comparably lower isolated yields than those of para-substituted compounds, which can be attributed to steric effects. Similar results were obtained with the reactions of heteroaromatic aldehydes including thiophene-2-carbaldehyde (Table 2, 14).
A hot filtration test12 was subsequently performed to determine the stability in terms of the amount of Cu leached in SBA-15/En–Cu under the investigated reaction conditions. The solid catalyst was removed after 5 h reaction (~17% conversion) and amide formation followed, maintaining the same reaction conditions for a further 90 h. No further increase in product yield was observed upon catalyst removal. Atomic absorption (AAS) analysis only showed 4.8 ppm copper in solution, demonstrating that essentially no Cu leached out into the reaction mixture (Fig. 5, Cu content >1 mg in 2 mol% catalyst per reaction). These studies showed that copper(II) ions are strongly coordinated with ethylenediamine and that the trace amounts of copper leached into solution are unable to further catalyze the reaction. Comparatively, the use of the recovered catalyst in another reaction run with fresh substrates provided identical results to those obtained for fresh SBA-15/En–Cu (Table 2). These findings support the truly heterogeneous nature of the catalytic system under the investigated reaction conditions.
The recyclability of SBA-15/En–Cu was further investigated in the model reaction. Results showed that apart from being highly stable (in terms of Cu leaching) under the investigated reaction conditions, the catalyst could be reused at least 14 times without a significant loss of catalytic activity and selectivity. After the 14th run, the catalytic activity considerably decreased, although amide yields could be slightly increased by prolonging the time of reaction (Fig. 6). A plausible mechanistic explanation for the one-pot preparation of primary amides from aldehydes using SBA-15/En–Cu has been proposed in Scheme 2.
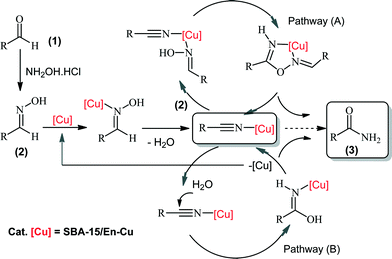 | ||
| Scheme 2 Proposed mechanisms for the catalytic conversion of aldehydes to amides.4 | ||
From a mechanistic point of view, two different metal-promoted reaction pathways have been previously reported.4 The first one involves a metal-containing five-membered cyclic intermediate, which decomposes into the final amide product (pathway A), while a dehydration/hydration pathway (pathway B) followed by subsequent rearrangement to the amide product was also proposed.4 We believe that pathway B (dehydration/hydration) seems to be the most plausible on the basis of obtained results and previously reported Cu activities in chemical processes.13
The catalytic activity of SBA-15/En–Cu was finally compared with previously reported catalytic protocols. Interestingly, the reported activities in this reaction using the designed SBA-15/En–Cu system were comparable to those of optimum homogeneous copper catalysts reported in the literature, with remarkable differences in terms of stability and reusability (Table 3).
| Catalyst | Cat. (mol%) | Yield (%) | Reaction conditions | Ref. |
|---|---|---|---|---|
| Pd(OAc)2 | 5 | 98 | 100 °C/DMSO–H2O | 4b |
| Co(OAc)2 | 2 | 31 | 110 °C/H2O | 6a |
| Cu(OAc)2 | 2 | 99 | 110 °C/H2O | 6a |
| Ru(DMSO)4Cl2 | 2 | 24 | 110 °C/toluene | 14 |
| [RuCl(CO)(PPh3)(TAC)] | 1 | 95 | 110 °C/toluene | 15 |
| TerpyRu(PPh3)Cl2 | 1 | 88 | 110 °C/toluene | 4e |
| CuSO4·5H2O | 5 | 95 | 110 °C/solventless | 16 |
| SBA-15/En–Cu (heterogeneous) | 2 | 95 | 80 °C/H2O | This work |
Conclusions
A highly active heterogeneous SBA-15/En–Cu catalyst was prepared by grafting copper acetate complexes onto diamine-modified SBA-15. Fully characterized SBA-15/En–Cu exhibited excellent activity as a heterogeneous catalyst in the conversion of aldehydes to amides. The catalyst could be reused at least 14 times without a significant loss in catalytic activity. The present method features several advantages including simplicity and one-pot protocol requiring no activation or modification of intermediate products. We envisaged that this catalytic system can be potentially extended further to C–H activation and C–C and C–heteroatom coupling chemistries that will be reported in due course.Experimental
Preparation of SBA-15, SBA-15/En and SBA-15/En–Cu
Mesoporous silica SBA-15 was prepared using the hydrothermal method according to Zhao et al.17 Diamine group-grafted SBA-15 materials were obtained by refluxing 1 g of SBA-15 silica in 250 mL of toluene with N-(2-aminoethyl)-3-aminopropyltrimethoxysilane [(MeO)3SiPrEn] for 20 h, based on our previous report.10d Complexation of the diamine-modified SBA-15 (SBA-15/En) with copper is depicted in Fig. S1 (see the ESI†).In a typical synthesis, 1 g of SBA-15/En was activated at 60 °C for 5 h and then dispersed in MeOH/H2O (25 mL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 mL). 1 mmol of copper acetate was separately dissolved in 10 mL of H2O and subsequently added dropwise to the dispersion of SBA-15/En under continuous stirring at RT for 24 h. The solvent was then removed by filtration and the resulting solid was thoroughly washed with H2O (×2 with 5 min bath sonication), ethanol and dichloromethane several times, followed by oven vacuum drying at 60 °C.
25 mL). 1 mmol of copper acetate was separately dissolved in 10 mL of H2O and subsequently added dropwise to the dispersion of SBA-15/En under continuous stirring at RT for 24 h. The solvent was then removed by filtration and the resulting solid was thoroughly washed with H2O (×2 with 5 min bath sonication), ethanol and dichloromethane several times, followed by oven vacuum drying at 60 °C.
General procedure for amide synthesis catalyzed by SBA-15/En–Cu
In a typical run, benzaldehyde (1.0 mmol), hydroxylamine hydrochloride (1 mmol), sodium carbonate (1.1 mmol) and SBA-15/En–Cu (0.027 g, 2 mol%) were added to 3 mL of H2O. The reaction mixture was stirred at 80 °C for 2 days. Upon reaction completion, the catalyst was separated by centrifugation and the solution was quenched with dichloromethane. The organic phase was evaporated under reduced pressure. Evaporation of the organic phase was followed by purification on column chromatography using silica gel to provide the desired products (see the ESI†).Once the reaction was complete, the catalyst was recovered (by filtration or centrifugation), washed with methanol and dried at 60 °C. The catalyst was transferred to a flask and then fresh reagents including the base (1.1 mmol), benzaldehyde (1 mmol) and hydroxylamine hydrochloride (1 mmol) were added and the mixture was again heated under stirring for a certain time. Products were separated following the procedure described in the first cycle and the catalyst was again collected and reused in another reaction run.
2-Thiophenecarboxamide
White crystalline powder. MP: 182–184 °C. 1H NMR (400 MHz, DMSO-d6): δ = 8.00 (1H, s, 1H of NH), 7.75–7.74 (2H, m, 2H of CH aromatic), 7.40 (1H, s, 1H of NH), 7.14 (1H, t, 1H of CH aromatic, 3JHH = 4.2 Hz); 13C NMR (100 MHz; DMSO-d6): d = 163.3 (CONH2), 140.68 (ArCCONH2), 131.47 (ArCH), 129.19 (ArCH), 128.39 (ArCH).References
- C. Suzuki, K. Morimoto, K. Hirano, T. Satoh and M. Miura, Adv. Synth. Catal., 2014, 356, 1521 Search PubMed.
- (a) E. C. Horning and V. L. Stromberg, J. Am. Chem. Soc., 1952, 74, 5151 Search PubMed; (b) D. S. Hoffenberg and C. R. Hauser, J. Org. Chem., 1955, 20, 1496 Search PubMed; (c) L. Field, P. B. Hughmark, S. H. Shumaker and W. S. Marshall, J. Am. Chem. Soc., 1961, 83, 1983 CrossRef CAS.
- A. Vinu, K. Z. Hossain and V. Ariga, J. Nanosci. Nanotechnol., 2005, 5, 347 Search PubMed.
- (a) R. S. Ramón, J. Bosson, S. Díez-González, N. Marion and S. P. Nolan, J. Org. Chem., 2010, 75, 1197 Search PubMed; (b) M. A. Ali and T. Punniyamurthy, Adv. Synth. Catal., 2010, 352, 288 Search PubMed; (c) H. Fujiwara, Y. Ogasawara, K. Yamaguchi and N. Mizuno, Angew. Chem., 2007, 119, 5294 Search PubMed; (d) N. A. Owston, A. J. Parker and J. M. J. Williams, Org. Lett., 2007, 9, 73 Search PubMed; (e) D. Gnanamgari and R. H. Crabtree, Organometallics, 2009, 28, 922 Search PubMed.
- (a) G. Saidulu, N. Anand, K. Seetha, R. Rao, A. Burri, S.-E. Park and D. R. Burri, Catal. Lett., 2011, 141, 1865 Search PubMed; (b) H. Peng, D. Wang, L. Xu and P. Wu, Chin. J. Catal., 2013, 34, 2057 Search PubMed.
- (a) A. Martínez-Asencio, M. Yus and D. J. Ramón, Tetrahedron, 2012, 68, 3948 Search PubMed; (b) N. C. Ganguly, S. Roy and P. Mondal, Tetrahedron Lett., 2012, 53, 1413 CrossRef CAS PubMed.
- N. Linares, E. Serrano, M. Rico, A. M. Balu, E. Losada, R. Luque and J. García-Martínez, Chem. Commun., 2011, 47, 9024 Search PubMed.
- R. Luque and J. Garcia-Martinez, ChemCatChem, 2013, 5, 827–829 CrossRef CAS.
- (a) F. Rajabi, S. Naserian, A. Primo and R. Luque, Adv. Synth. Catal., 2011, 353, 2060 CrossRef CAS; (b) F. Rajabi, N. Karimi, M. R. Saidi, A. Primo, R. S. Varma and R. Luque, Adv. Synth. Catal., 2012, 354, 1707 Search PubMed.
- (a) B. Karimi and M. Khorasani, ACS Catal., 2013, 3, 1657 Search PubMed; (b) B. Karimi and M. Vafaeezadeh, RSC Adv., 2013, 3, 23207 Search PubMed; (c) B. Karimi and H. Mirzaei, RSC Adv., 2013, 3, 20655 Search PubMed; (d) S. Rostamnia and H. Xin, Appl. Organomet. Chem., 2013, 27, 348 CrossRef CAS; (e) S. Rostamnia, H. Xin, X. Liu and K. Lamei, J. Mol. Catal. A: Chem., 2013, 374–375, 85 Search PubMed; (f) S. Rostamnia and F. Pourhassan, Chin. Chem. Lett., 2013, 24, 401 Search PubMed.
- (a) R. Sanz, G. Calleja, A. Arencibia and E. S. Sanz-Pérez, Microporous Mesoporous Mater., 2012, 158, 309 Search PubMed; (b) Z. Wang, M. Wang, G. Wu, D. Wu and A. Wu, Dalton Trans., 2014, 43, 8461 Search PubMed.
- (a) B. Karimi and D. Enders, Org. Lett., 2006, 6, 1237 Search PubMed; (b) J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 198, 317 CrossRef CAS; (c) J. D. Webb, S. M-Quarrie, K. M-Eleney and C. M. Crudden, J. Catal., 2007, 252, 97 Search PubMed.
- (a) J. M. Bermudez, J. A. Menéndez, A. A. Romero, E. Serrano, J. Garcia-Martinez and R. Luque, Green Chem., 2013, 15, 2786 RSC; (b) A. Yepez, A. Garcia, M. S. Climent, A. A. Romero and R. Luque, Catal. Sci. Technol., 2014, 4, 428 Search PubMed.
- J. F. Hull, S. T. Hilton and R. H. Crabtree, Inorg. Chim. Acta, 2010, 363, 1243 CrossRef CAS PubMed.
- N. Raja, M. U. Raja and R. Ramesh, Inorg. Chem. Commun., 2012, 19, 51 Search PubMed.
- N. C. Ganguly, S. Roy and P. Mondal, Tetrahedron Lett., 2012, 53, 1413 Search PubMed.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4cy00963k |
| This journal is © The Royal Society of Chemistry 2015 |