Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterisation of halide, separated ion pair, and hydride cyclopentadienyl iron bis(diphenylphosphino)ethane derivatives†
Dipti
Patel
a,
Ashley
Wooles
a,
Andrew D.
Cornish
a,
Lindsey
Steven
a,
E. Stephen
Davies
a,
David J.
Evans
*b,
Jonathan
McMaster
a,
William
Lewis
a,
Alexander J.
Blake
 a and
Stephen T.
Liddle
*a
a and
Stephen T.
Liddle
*a
aSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK
bDepartment of Chemistry, University of Hull, Hull, HU6 7RX, UK. E-mail: stephen.liddle@nottingham.ac.uk; david.evans@hull.ac.uk
First published on 17th July 2015
Abstract
Treatment of anhydrous FeX2 (X = Cl, Br, I) with one equivalent of bis(diphenylphosphino)ethane (dppe) in refluxing THF afforded analytically pure white (X = Cl), light green (X = Br), and yellow (X = I) [FeX2(dppe)]n (X = Cl, I; Br, II; I, III). Complexes I–III are excellent synthons from which to prepare a range of cyclopentadienyl derivatives. Specifically, treatment of I–III with alkali metal salts of C5H5 (Cp, series 1), C5Me5 (Cp*, series 2), C5H4SiMe3 (Cp′, series 3), C5H3(SiMe3)2 (Cp′′, series 4), and C5H3(But)2 (Cptt, series 5) afforded [Fe(Cp†)(Cl)(dppe)] 1Cl–5Cl, [Fe(Cp†)(Br)(dppe)] 1Br–5Br, and [Fe(Cp†)(I)(dppe)] 1I–5I (Cp† = Cp, Cp*, Cp′, Cp′′, or Cptt). Dissolution of 1I–5I in acetonitrile, or treatment of 1Cl–5Cl with Me3SiI in acetonitrile (no halide exchange reactions were observed in other solvents) afforded the separated ion pair complexes [Fe(Cp†)(NCMe)(dppe)][I] 1SIP–5SIP. Attempts to reduce 1Cl–5Cl, 1Br–5Br, and 1I–5I with a variety of reductants (Li-Cs, KC8, Na/Hg) were unsuccessful. Treatment of 1Cl–5Cl with LiAlH4 gave the hydride derivatives [Fe(Cp†)(H)(dppe)] 1H–5H. This report provides a systematic account of reliable methods of preparing these complexes which may find utility in molecular wire and metal–metal bond chemistries. The complexes reported herein have been characterised by X-ray diffraction, NMR, IR, UV/Vis, and Mössbauer spectroscopies, cyclic voltammetry, density functional theory calculations, and elemental analyses, which have enabled us to elucidate the electronic structure of the complexes and probe the variation of iron redox properties as a function of varying the cyclopentadienyl or halide ligand.
Introduction
One of the most popular and widely used organometallic transition metal anions in the literature is [FeCp(CO)2]− (Fp−).1 The Fp− anion has been used extensively in nucleophilic displacement reactions and in the preparation of metal–metal bonds.2 Regarding the latter point, although the nucleophilicity of the Fp anion should favour M–Fe bond formation, there is a significant possibility of iso-carbonyl bond formation with early, electropositive and oxophilic metals,3 an area which we have been investigating in recent years.4 One approach to avoid iso-carbonyl bridges is to employ cyclopentadienyl iron fragments that are supported by two monodentate or one bidentate phosphine ligand[s]. This approach has enabled the isolation of a range of novel linkages and has also been implemented in rare earth-ruthenium chemistry.5Recently, we reported four uranium–ruthenium bonds using the ruthenium analogue of Fp.6 As part of that study we found that the corresponding uranium–iron (Fp) linkages could not be isolated. We reasoned that substitution of the carbonyl groups with phosphines might increase the steric protection and thus stability of uranium–iron linkages which would necessitate the preparation of the corresponding iron precursors. Since substitution of the carbonyl groups in situ is not feasible, it would be necessary to start with a cyclopentadienyl–phosphine ligand set before constructing the uranium–iron bond.
Two methods of preparing uranium–iron bonds could be envisaged, either reductive cleavage of a Fe–Fe species, as has been accomplished with cobalt,10 or protonolysis of a uranium alkyl or amide with an iron hydride.4 One approach to iron cyclopentadienyl ligand derivatives would be to prepare the relevant iron cyclopentadienyl dicarbonyl halide or hydride then substitute the carbonyl with phosphines, but it is known that this approach can be complicated by the formation of mono-substituted complexes,7 or the formation of chelated derivatives with the retention of one CO ligand and expulsion of the halide ligand from the primary coordination sphere of iron.8 We therefore decided to prepare phosphine chelated iron(II) halides, introduce the cyclopentadienyl ligand, then either reduce or substitute the halide with hydride. This methodology has been shown to work in a few cases,7 but the data in the literature are fragmented and sometimes incomplete. In particular, solid state structures are often missing but would provide valuable benchmarking for previously reported spectroscopic and computational studies. Given the importance of cyclopentadienyl iron bis(diphenylphosphino)ethane (dppe) fragments in assembling molecular wires9 as well as representing synthons to iron–metal bonds it would be desirable to draw together a cohesive and comprehensive description of a reliable general methodology for the preparation of a range of well characterised and understood functionalised iron cyclopentadienyl dppe derivatives where the steric and electronic properties can be systematically varied.
Here, we report the synthesis of three iron(II) halide dppe complexes, and their utility in preparing a range of cyclopentadienyl derivatives. We describe attempts to reduce these cyclopentadienyl dppe halide complexes to the corresponding diiron derivatives, and also the synthesis of hydride congeners. In all, we describe the synthesis of twenty five iron cyclopentadienyl dppe complexes as either halide, separated ion pair, or hydride derivatives which has enabled us, through a structural and spectroscopic benchmarking study, to provide reliable synthetic methods and a detailed understanding of the electronic structure of these compounds. This report thus constitutes a cohesive account of compounds which could find extensive utility in molecular wire and metal–metal bond chemistries.
Results and discussion
Synthesis of iron(II) halide dppe adducts
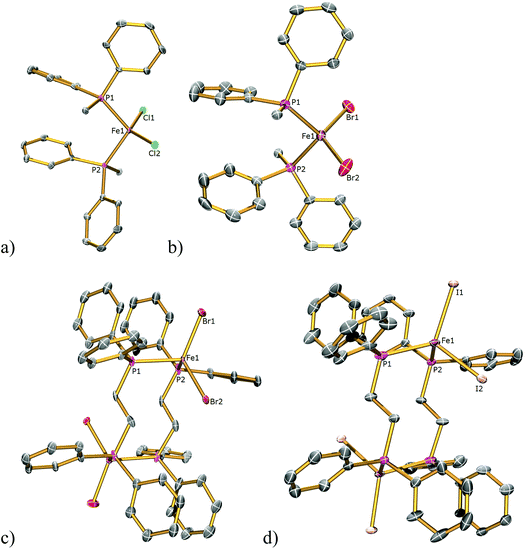
We began by preparing dppe adducts of iron(II) halides from the reaction between anhydrous FeX2 (X = Cl, Br, I) and one equivalent of dppe in refluxing THF, Scheme 1. Following filtration, removal of solvent, washing with toluene, and drying, analytically pure white (X = Cl), light green (X = Br), and yellow (X = I) solids were obtained in ∼95% yield in each case. It is notable that although these simple coordination complexes [FeX2(dppe)]n (X = Cl, I; Br, II; I, III) are often mentioned in the literature,7 detailed descriptions of their synthesis and characterisation are scant and the structure of II is unknown. In all three cases we were able to determine the solid state structures by X-ray crystallography (Fig. 1). The solid state structures of I and III were determined from crystals grown from THF, for I, an infinite polymer is observed in the solid state with tetrahedral iron centres and bridging dppe ligands whereas in III a dimer is formed containing two tetrahedral iron centres which are each bridged by two dppe ligands. In contrast, the bromide compound II could be isolated as the polymeric (II) or dimeric (IIa) forms from THF/hexane or toluene solutions, respectively. Langer and co-workers have recently independently found that I co-crystallises with [FeCl2(dppe)2] from a chloroform/acetone mixture in a polymeric chain similar to our findings, and also solely if the reaction is completed in the correct stoichiometry, with similar bond lengths and angles to those found in I.7 Pohl et al. elucidated the solid state structure of [Fe2I2(dppe)4] from toluene solution and also found it to be dimeric in nature, with similar bond lengths and angles to those found in III which crystallised from THF solvent,7 suggesting that the isolation of polymeric or dimeric forms of I–III is due to the halide and not solvent effects.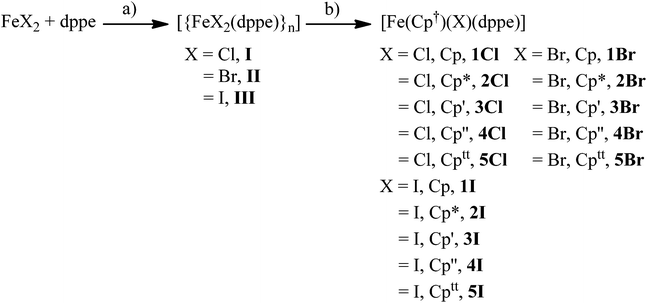 | ||
| Scheme 1 Synthesis of I, II, III, 1Cl–5Cl, 1Br–5Br, 1I–5I. Reagents and conditions: (a) THF, Δ; (b) LiCp/NaCp/KCp†, toluene, −78 °C, –LiCl/KCl/NaI/KCl/KBr/KI. | ||
Synthesis and characterisation of halide and separated ion pair complexes
With the synthesis of I–III in-hand we first targeted cyclopentadienyl derivatives using C5H5 (Cp, series 1), C5Me5 (Cp*, series 2), C5H4SiMe3 (Cp′, series 3), C5H3(SiMe3)2 (Cp′′, series 4), and C5H3(But)2 (Cptt, series 5) since this selection provides a wide variation of steric and electronic (σ-donor, π-acceptor) properties. Thus, treatment of I–III with one equivalent of the relevant lithium/sodium/potassium (Cp) or potassium (Cp*/′/′′/tt) cyclopentadienyl reagent in toluene at −78 °C afforded, after filtration and removal of toluene, generally black solids, Scheme 1. Recrystallisation from toluene or dichloromethane layered with hexane (1Cl) afforded the target complexes [Fe(Cp)(Cl)(dppe)] (1Cl), [Fe(Cp*)(Cl)(dppe)] (2Cl), [Fe(Cp′)(Cl)(dppe)] (3Cl), [Fe(Cp′′)(Cl)(dppe)] (4Cl), [Fe(Cptt)(Cl)(dppe)] (5Cl), [Fe(Cp)(Br)(dppe)] (1Br), [Fe(Cp*)(Br)(dppe)] (2Br), [Fe(Cp′)(Br)(dppe)] (3Br), [Fe(Cp′′)(Br)(dppe)] (4Br), [Fe(Cptt)(Br)(dppe)] (5Br), [Fe(Cp)(I)(dppe)] (1I), [Fe(Cp*)(I)(dppe)] (2I), [Fe(Cp′)(I)(dppe)] (3I), [Fe(Cp′′)(I)(dppe)] (4I), and [Fe(Cptt)(I)(dppe)] (5I), in crystalline yields of ca. 50–70%. We note that apart from some minor decomposition, the bulk products are fairly pure (ca. 90%) and that the crystalline yields principally reflect the solubility of the cyclopentadienyl derivatives. During this work we noted that the temperature of the initial salt elimination reaction is important since the formation of ferrocenes and free dppe from ligand scrambling is a competing side reaction which becomes a major by-product at room temperature. However, ferrocenes formation is suppressed and indeed essentially eliminated if reactions are initially conducted at −78 °C. Compound 1I can also be prepared from [Fe(CO)2(Cp)]2 with treatment with iodine followed by addition of dppe in refluxing toluene, however in lower yields of 48% compared to the above method in which yields of 1I reach 67%.11Halide exchange in iron cyclopentadienyl phosphine derivatives has previously been effected by mixing complexes such as 2Cl with, for example, KI,12 whereas the approach outlined above gives direct access to chloride, bromide, and iodide derivatives. In an attempt to ascertain whether any other methods have applicability here, we screened the reactivity of the chlorides with trimethylsilyl iodide. Interestingly, although 1Cl–5Cl are inert with respect to halide exchange in THF, toluene, and dichloromethane, in acetonitrile spontaneous displacement of the chloride ligand by acetonitrile and facile halide exchange occurs to afford the separated ion pair (SIP) complexes [Fe(Cp)(NCMe)(dppe)][I] (1SIP), [Fe(Cp*)(NCMe)(dppe)][I] (2SIP), [Fe(Cp′)(NCMe)(dppe)][I] (3SIP), [Fe(Cp′′)(NCMe)(dppe)][I] (4SIP), and [Fe(Cptt)(NCMe)(dppe)][I] (5SIP) as red powders following work-up (Scheme 2). Recrystallisation of these powders from acetonitrile afforded 1SIP–5SIP in crystalline yields of typically 65–75%, although 3SIP is a notable outlier (36% crystalline yield). Since SIPs form readily we did not investigate this avenue further with the bromides, but it is germane to note that complexes 1SIP–5SIP represent valuable precursors to a range of separated ion pair species such as known PF6-derivatives.
We investigated the reduction of 1Cl–5Cl, 1Br–5Br, and 1I–5I with a variety of reductants (Li-Cs, KC8, Na/Hg), but in all cases either no reaction occurred, or on extended stirring decomposition was observed. This might be attributed to the iron centres being electron rich, and more so than in Fp because of less effective back-bonding to dppe compared to two CO ligands. This would also be consistent with the relative ease of oxidising these complexes (see below). We therefore focussed on preparing the hydrides.
Synthesis of hydride complexes
With the aforementioned halide complexes in hand we examined their conversion to the corresponding hydrides. Accordingly, treatment of the chloride series 1Cl–5Cl with 5 equivalents of lithium aluminium hydride in THF afforded, after removal of solvent, extraction into hexane, filtration and removal of solvent, the hydride complexes [Fe(Cp)(H)(dppe)] (1H), [Fe(Cp*)(H)(dppe)] (2H), [Fe(Cp′)(H)(dppe)] (3H), [Fe(Cp′′)(H)(dppe)] (4H), and [Fe(Cptt)(H)(dppe)] (5H) as orange solids that are approximately 90% pure, Scheme 2. Recrystallisation of 1H–5H from hexane afforded orange crystalline materials in yields typically ranging from 41 to 73%, but, as was the case for the SIP complex 3SIP, the crystalline yield of the Cp′ and Cp′′ derivatives 3H and 4H are notably low (35 and 41%, respectively). It should be noted that 3H and 4H are far less stable than the other three hydride derivatives and decompose in solution over a few hours, which is, in part, reflected in their crystalline yields. Complexes 1H and 2H have been previously reported, however, in the preparation of 1H an excess of NaBH4 was reacted with the chloroform adduct of 1Cl giving only a 50% yield13 and 2H was reported to have been synthesised by the same method outlined above or alternatively by reacting I with Cp*H in a colloidal dispersion of potassium metal in THF; a reaction which reportedly proceeds via the formation of a “Fe(dppe)” intermediate followed by oxidative addition of Cp*H.14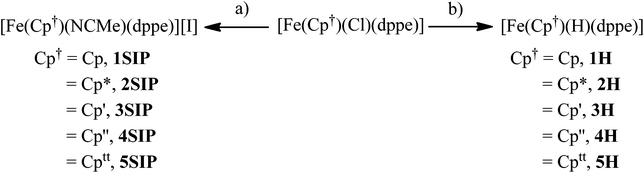 | ||
| Scheme 2 Synthesis of 1SIP–5SIP, 1H–5H. Reagents and conditions: (a) TMSI, MeCN, –TMSCl; (b) excess LiAlH4, THF, –LiCl. | ||
Solid state structures
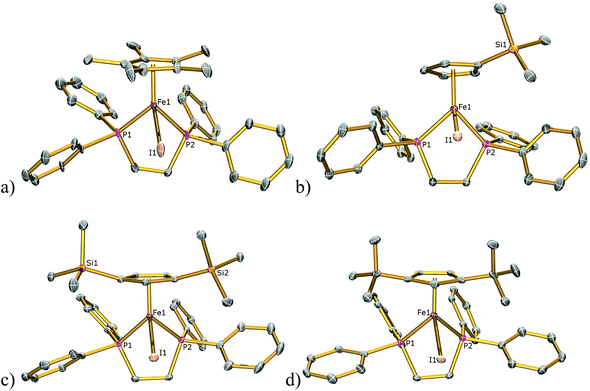
The solid-state structures of 1Cl, 3Cl–5Cl, 1Br, 3Br–5Br, 2I–5I were determined by single-crystal X-ray diffraction studies and are illustrated in Fig. 2–4, respectively, and key metrical data are presented in Table 1. The structures of 2Cl14 and 1I15 were determined previously and the experimental data obtained of crystals of 2Br were of insufficient quality to determine meaningful metrical parameters. Each complex displays a piano-stool geometry, and in comparison to the ideal bond angle of a classical tetrahedral geometry of 109.5°, the X1–Fe1–P1, X1–Fe1–P2, P1–Fe1–P2 angles span the ranges 86.03(4)–93.12(2), 84.18(4)–92.12(10) and 80.78(5)–86.22(3)° in 1Cl–5Cl, 1Br–5Br and 1I–5I respectively, due in part to the restrictive bite angle of the dppe ligand but mainly due to the large size of the various substituted Cp ligands forcing the other ligands closer together. This results in large Ct–Fe1–X1, Ct–Fe1–P1, Ct–Fe1–P2 angles ranging 118.97(9)–123.9(9), 126.37(7)–132.6(11) and 124.8(2)–132.98(13)° respectively. The Fe1–Ct bond lengths span the narrow range of 1.699(3)–1.743(12) Å in all of the complexes, which compares well to the Fe–Cpcentroid average distance of 1.740 Å in complexes containing both dppe and Cp ligands.16 The Fe–P bond distances span the narrow range of 2.184(2)–2.2580(9) Å, which compares to Fe–P bond distances in complexes containing both Cp and dppe ligands in the range 2.1168(19)–2.3105(14) Å.16 The Fe–Cl, Fe–Br, Fe–I distances of 2.294(2)–2.346(1), 2.2464(6)–2.481(3) and 2.643(1)–2.661(2) Å respectively increase as expected according to increasing ion size in the order I > Br > Cl. Although a range of different substituted Cp ligands have been studied and characterised the difference in their steric properties appears to have no major effect on the bond angles and lengths of the other ligands.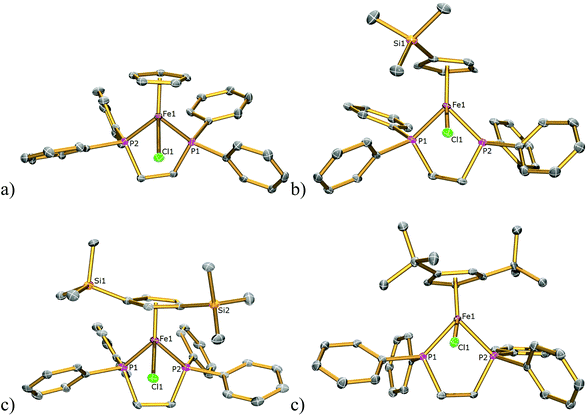 | ||
| Fig. 2 Solid state structures of (a) 1Cl, (b) 3Cl, (c) 4Cl, (d) 5Cl with ellipsoids set at 40% probability. Hydrogen atoms are omitted for clarity. | ||
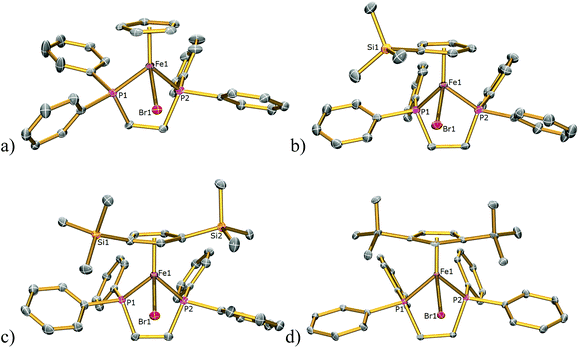 | ||
| Fig. 3 Solid state structures of (a) 1Br, (b) 3Br, (c) 4Br, (d) 5Br with ellipsoids set at 40% probability. Hydrogen atoms are omitted for clarity. | ||
 | ||
| Fig. 4 Solid state structures of (a) 2I, (b) 3I, (c) 4I, (d) 5I with ellipsoids set at 40% probability. Hydrogen atoms are omitted for clarity. | ||
| Compound | Fe–Ct | Fe–X | Fe1–P1 | Fe1–P2 |
|---|---|---|---|---|
| a Ct = centroid of the cyclopentadienyl ring; X = Cl, Br, I, acetonitrile N or H. | ||||
| 1Cl | 1.699(3) | 2.3317(9) | 2.1963(10) | 2.1846(10) |
| 3Cl | 1.716(5) | 2.3298(16) | 2.1980(16) | 2.1881(15) |
| 4Cl | 1.704(7) | 2.294(2) | 2.184(2) | 2.194(2) |
| 5Cl | 1.739(4) | 2.3423(10) | 2.2107(11) | 2.2358(10) |
| 1Br | 1.699(5) | 2.4647(6) | 2.1909(10) | 2.1958(10) |
| 3Br | 1.709(18) | 2.481(3) | 2.1909(5) | 2.2059(5) |
| 4Br | 1.720(5) | 2.464(8) | 2.201(13) | 2.210(13) |
| 5Br | 1.734(4) | 2.476(8) | 2.2273(13) | 2.2578(13) |
| 2I | 1.743(12) | 2.661(2) | 2.221(3) | 2.208(3) |
| 3I | 1.712(2) | 2.6478(4) | 2.1874(7) | 2.1956(7) |
| 4I | 1.728(2) | 2.6464(4) | 2.2018(7) | 2.2116(7) |
| 5I | 1.734(3) | 2.6603(5) | 2.2266(10) | 2.2580(9) |
| 1SIP | 1.713(3) | 1.900(2) | 2.2188(7) | 2.2194(7) |
| 2SIP | 1.732(4) | 1.896(3) | 2.2034(13) | 2.2214(11) |
| 3SIP | 1.709(4) | 1.908(4) | 2.1986(12) | 2.1940(12) |
| 4SIP | 1.730(2) | 1.9063(19) | 2.2392(7) | 2.2429(7) |
| 5SIP | 1.727(7) | 1.892(7) | 2.240(2) | 2.247(2) |
| 1H | 1.702(4) | 1.60(4) | 2.1455(1) | 2.1292(10) |
| 2H | 1.712(7) | 1.56(7) | 2.1255(18) | 2.1448(19) |
| 3H | 1.698(3) | 1.50(4) | 2.1404(9) | 2.1196(9) |
| 4H | 1.701(5) | 1.39(2) | 2.1333(13) | 2.1302(13) |
| 5H | 1.707(8) | 1.52(14) | 2.142(2) | 2.153(2) |
The solid-state structures of 1SIP–5SIP were determined by single-crystal X-ray diffraction studies and are illustrated in Fig. 5 with key bond distances listed in Table 1. The geometries of these complexes are similar to those featured in the halide complexes except that an acetonitrile, not a halide, ligand is coordinated to the iron centres. The Fe1–Ct bond lengths range 1.709(4)–1.732(4) Å and are comparable to those found in 1I–5I (1.707(3)–1.743(12) Å) and the Fe–P bond lengths range from 2.1986(12)–2.247(2) Å and are comparable to those found in 1I–5I (2.188(1)–2.2580(9) Å. The Fe–N bond lengths range from 1.892(7)–1.908(4) Å and are comparable to those found in the literature with complexes containing dppe and Cp ligands (1.881(5)–1.909(8) Å).16 The Ct–Fe–N and Ct–Fe–P angles range 120.8(15)–123.4(10) and 124.27(13)–132.27(12)° respectively, and are comparable with the Ct–Fe–I and Ct–Fe–P bond angles of 118.97(7)–123.78(7), 126.37(7)–133.93(4)° respectively found in 1I–5I. The N–Fe–P and P1–Fe–P2 bond angles of 88.79(10)–93.24(12) and 85.48(4)–86.47(4)° respectively lie within the range shown for the I–Fe–P and P1–Fe–P2 angles in 1I–5I (88.82(2)–93.12(2), 80.85(3)–86.22(3)° respectively).
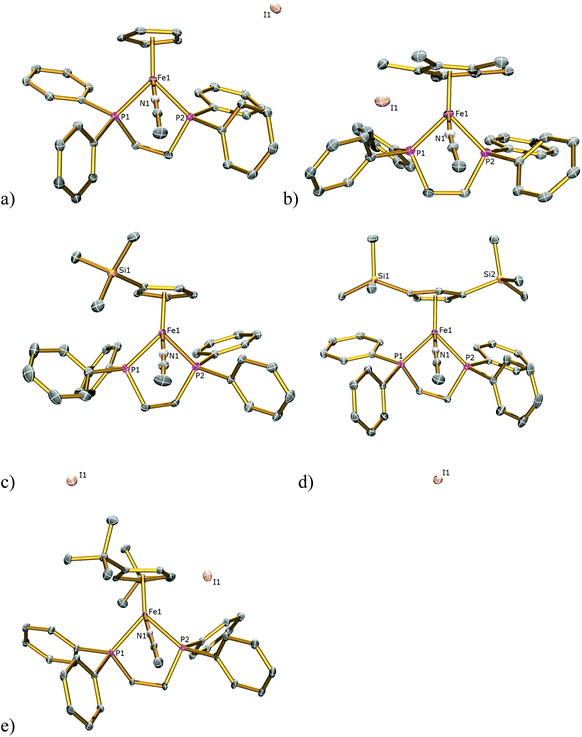 | ||
| Fig. 5 Solid state structures of (a) 1SIP, (b) 2SIP, (c) 3SIP, (d) 4SIP, (e) 5SIP with ellipsoids set at 40% probability. Hydrogen atoms are omitted for clarity. | ||
Although complexes 1H and 2H were synthesised previously their solid-state structures were not reported and hence the solid-state structures of 1H–5H were also determined by single-crystal X-ray diffraction studies and are illustrated in Fig. 6 with pertinent bond lengths compiled in Table 1. In each instance the hydride atom was located in the difference map and was allowed to refine freely. In each complex the FeII centre adopts a classical piano stool geometry. The Fe–H bond distances in 1H–5H span the range 1.39(2)–1.60(4) Å. There are only two other examples of terminal iron hydride bonds with bidentate phosphine and cyclopentadienyl ligands with which to compare these values; [Fe(Cp*)(H)2(dppe)][BF4] has Fe–Hhydride distances of 1.48 and 1.50 Å![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 and [Fe(Cp*)(H)2(dippe)][BPh4], where dippe = 1,2-bis(diisopropylphosphino)ethane, has Fe–Hhydride distances of 1.41(5) and 1.35(6) Å.18 The Fe–H bond distance in 4H of 1.39(2) Å, is significantly shorter than those in the rest of the series; however, it is not outside the limited range reported for Fe–Hhydride bonds in complexes containing the dppe ligand (1.28(8)–1.65(9) Å).17,19–35
17 and [Fe(Cp*)(H)2(dippe)][BPh4], where dippe = 1,2-bis(diisopropylphosphino)ethane, has Fe–Hhydride distances of 1.41(5) and 1.35(6) Å.18 The Fe–H bond distance in 4H of 1.39(2) Å, is significantly shorter than those in the rest of the series; however, it is not outside the limited range reported for Fe–Hhydride bonds in complexes containing the dppe ligand (1.28(8)–1.65(9) Å).17,19–35
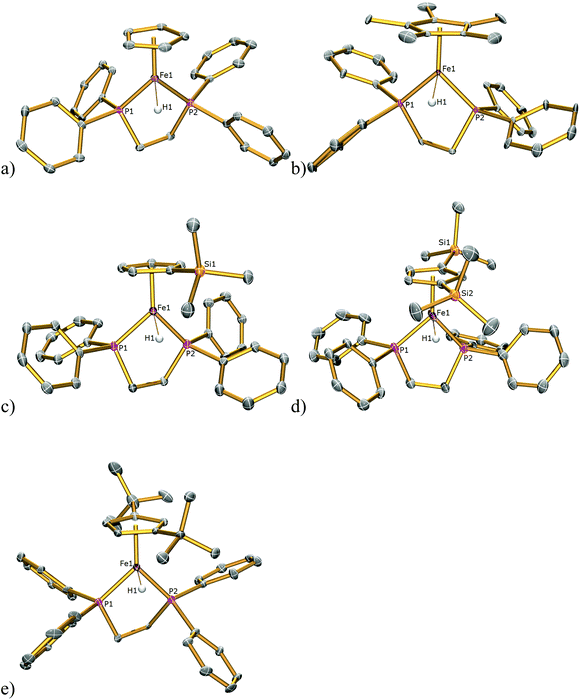 | ||
| Fig. 6 Solid state structures of (a) 1H, (b) 2H, (c) 3H, (d) 4H, (e) 5H with ellipsoids set at 40% probability. All hydrogen atoms except those for the hydride atoms are omitted for clarity. | ||
Electrochemical investigations
Cyclic voltammetric studies were performed on 1Cl–5Cl, 1Br–5Br, 1I–5I and 1H–5H as THF solutions containing 0.5 M of electrolyte, [NBun4][BF4], in the potential range from −2.55 to 1.2 V vs. Fc+/Fc. The results of these studies are summarised in Table 2 and plotted in Fig. 7. THF was chosen as the solvent as it provides good solubility for all of the complexes and remains relatively inert with respect to nucleophilic attack on electrogenerated cations.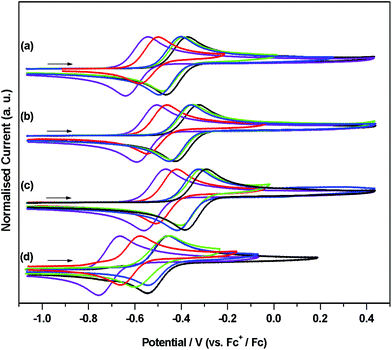 | ||
| Fig. 7 Cyclic voltammograms showing OX for (a) [Fe(Cp†)(Cl)(dppe)], (b) [Fe(Cp†)(Br)(dppe)], (c) [Fe(Cp†)(I)(dppe)] and (d) [Fe(Cp†)(H)(dppe)] (Cp† = Cp (black), Cp* (violet), Cp′ (blue), Cp′′ (green), Cptt (red)). In THF containing [nBu4N][BF4] (0.5 M) as supporting electrolyte, at 0.1 V s−1 (except 4H, at 1 V s−1). Currents are normalised to Iap for clarity. Typical currents obtained from CV experiments for [Fe(Cp†)(X)(dppe)] compounds are shown in Fig. S2† for 1Cl, as are designations of OX, OX′, OX′′ and RED for 1Cl–5Cl, 1Br–5Br, 1I–5I and 1H–5H used in Table S1.† | ||
| Compound | E 1/2 (V) | HOMO (eV) | Compound | E 1/2 (V) | HOMO (eV) |
|---|---|---|---|---|---|
| a Solutions were ca. 0.1 mM of the complex in THF containing 0.5 M [NBun4][BF4] as the electrolyte for 1Cl–5Cl, 1Br–5Br, 1I–5I and 1H–5H and ca. 0.1 mM of 1I–5I in MeCN containing 0.1 M [NBun4][BF4] as the electrolyte for 1SIP–5SIP. The working electrode was glassy carbon and potentials are reported against the Fc+/Fc redox couple at ambient temperature. The HOMO energies are derived from the DFT analyses (see below) and are in the gas-phase with no solvent-shell correction. | |||||
| 1Cl | −0.42 | −3.732 | 4I | −0.36 | −3.868 |
| 2Cl | −0.59 | −3.537 | 5I | −0.46 | −3.741 |
| 3Cl | −0.45 | −3.694 | 1H | −0.50 | −3.838 |
| 4Cl | −0.44 | −3.747 | 2H | −0.71 | −3.616 |
| 5Cl | −0.54 | −3.545 | 3H | −0.50 | −3.789 |
| 1Br | −0.38 | −3.788 | 4H | −0.53 | −3.706 |
| 2Br | −0.55 | −3.561 | 5H | −0.62 | −3.638 |
| 3Br | −0.41 | −3.741 | 1SIP | 0.26 | −7.218 |
| 4Br | −0.40 | −3.787 | 2SIP | 0.06 | −6.958 |
| 5Br | −0.50 | −3.665 | 3SIP | 0.25 | −7.125 |
| 1I | −0.34 | −3.836 | 4SIP | 0.26 | −7.073 |
| 2I | −0.51 | −3.625 | 5SIP | 0.20 | −6.997 |
| 3I | −0.37 | −3.781 | |||
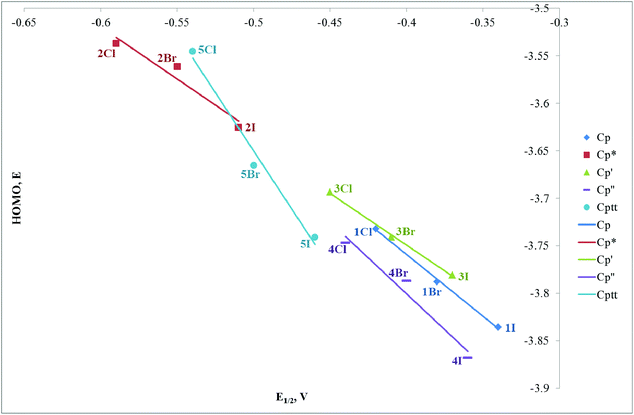
The potentials obtained from cyclic voltammetric studies are consistent with results of square wave voltammetry (±0.01 V, except 4H, see below) for the process designated OX and in all cases the potentials are quoted against an appropriate internal standard (see Table S1†). For OX, analysis of the current response in a range of scan rates between 0.02 and 0.3 V s−1 suggest that this process is, in general, electrochemically reversible under these conditions, 4H being an notable exception. At slow scan rates (<0.1 V s−1), the cyclic voltammogram of 4H has no reduction wave associated with this oxidation suggesting that the electrogenerated cation is unstable. This was confirmed at faster scan rates (1 V s−1), corresponding to shorter timescales, when OX appeared as a redox couple. Data for [Fe(Cp*)(X)(dppe)] (X = Cl, Br, I, and H) have been reported previously wherein this process was assigned as a one electron oxidation to give the corresponding cationic species, [Fe(Cp*)(X)(dppe)].36 Our results are consistent with these, albeit with a small difference in reported potentials, therefore we assign OX for the series of compounds 1Cl–5Cl, 1Br–5Br, 1I–5I and 1H–5H as a one-electron oxidation of Fe(II) to Fe(III) corresponding to a [Fe(Cp†)(X)(dppe)]+/0 couple. The negative values of these potentials, particularly noticeable when referenced against the Fe(III)/Fe(II) couple of ferrocene, are evidence of an electron rich Fe centre, where the loss of an electron is facile. The trend in OX for [Fe(Cp†)(X)(dppe)] (X = H, Cl, Br and I) always follows H < Cl < Br < I, and it is worthy of note that the difference between OX for [Fe(Cp†)(Cl)(dppe)]+/0 and [Fe(Cp†)(Br)(dppe)]+/0 and between [Fe(Cp†)(Br)(dppe)]+/0 and [Fe(Cp†)(I)(dppe)]+/0 is +0.04 V and that this difference is independent of the substitutents on the cyclopentadienyl ring. This trend does not extend to the hydrides, where differences between potentials for [Fe(Cp†)(H)(dppe)]+/0 and [Fe(Cp†)(Cl)(dppe)]+/0 vary between +0.05 (Cp′) and +0.12 (Cp*).
The trend in OX for each halide series appears to be inversely correlated with the electronegativity of the halide thus making the chlorides easiest to oxidise and indicating an increase in electron density on the iron centre relative to the corresponding bromides and iodides. This has been rationalised by the involvement of π-based orbitals in the bonding of the halide to the iron centre (see DFT calculations below) thus allowing donation of electron density from the halide to the metal centre. The hydrides should have no contribution from π-bonding so the relatively negative values for OX in [Fe(Cp†)(H)(dppe)] compounds reflect the purely σ-bonded nature of the hydrides. The trend in OX for [Fe(Cp†)(Cl)(dppe)], [Fe(Cp†)(Br)(dppe)] and [Fe(Cp†)(I)(dppe)] (Cp† = Cp, Cp*, Cp′, Cp′′, Cptt) follow the series Cp* < Cptt < Cp′ < Cp′′ < Cp and compounds containing Cp* are −0.05 V easier to oxidise than compounds containing Cptt, Cptt compounds are −0.09 V easier to oxidise than compounds containing Cp′, Cp′ compounds are −0.01 V easier to oxidise than compounds containing Cp′′, and Cp′′ compounds are −0.02 V easier to oxidise than compounds containing Cp. Hence compounds containing Cp*, with five electron donating methyl groups are easiest to oxidise whilst compounds containing Cp are most difficult. The latter result is unexpected since TMS is electron withdrawing37 relative to hydrogen (in Cp) and would be expected to reduce the electron density at iron, thus making oxidation more difficult (hence occurring at a higher potential). However, the difference in OX between [Fe(Cp′)(X)(dppe)] and [Fe(Cp′′)(X)(dppe)] (X = Cl, Br and I) is only 0.01 V for the addition of a second TMS group therefore the inductive effect of these substitutents may not be transferred effectively to the redox centre, which is reflected by only minor changes in the energies of the HOMO orbitals of the Cp′ and Cp′′ derivatives of 1Cl, 1Br, and 1I.
The electrochemistry of the separated ion pairs, [Fe(Cp†)(NCMe)(dppe)][I] (Cp† = Cp, Cp*, Cp′, Cp′′, Cptt) was studied by cyclic and square wave voltammetries and the results of these investigations are presented in Table 2 and Fig. S1.† The separated ion pairs were generated in situ by dissolving the corresponding [Fe(Cp†)(I)(dppe)] (Cp† = Cp, Cp*, Cp′, Cp′′, Cptt) compound in MeCN containing 0.1 M [NBun4][BF4] as the supporting electrolyte. Analysis of these solutions showed an absence of redox chemistry associated with [Fe(Cp†)(I)(dppe)] indicating that the equilibrium exclusively favours the formation of the separated ion pair under these conditions. We confirmed the electrochemistry obtained by this method was that associated with a separated ion pair in MeCN solution by dissolving crystals of 2SIP in MeCN containing 0.1 M [NBun4][BF4] and repeating the electrochemical experiment; the results of this experiment are essentially identical to those obtained starting from [Fe(Cp*)(I)(dppe)] (Fig. S1†). The displacement of iodide from coordination at the metal centre into the outer sphere significantly complicates the electrochemistry of these compounds since both iodide and [Fe(Cp†)(NCMe)(dppe)]+ exhibit oxidation chemistry in the range of potentials from ca. 0 to +0.5 V. Fig. S1† shows the cyclic voltammetry of the separated ion pairs and [NBun4][I]. The electrochemistry of iodide gives two oxidation processes (−0.02 and +0.31 V) and a broad reduction, centred around ca. −0.6 V and these may represent electrochemical processes resulting from components of an [I−]/[I2]/[I3−] equilibrium.38 The electrochemistry of [Fe(Cp†)(NCMe)(dppe)]+ (Cp† = Cp, Cp*, Cp′, Cp′′, Cptt) appears as a redox couple in the range of potentials between +0.06 V (Cp*) and +0.26 V (Cp and Cp′′). These potentials are consistent with results of square wave voltammetry (±0.01 V) for the process designated OX (Table S1†). The nature of the electron transfer process in these compounds is difficult to determine given the presence of OX′, a process we assign to iodide oxidation, however it is noted that the separation between Eap and Ecp for OX in [Fe(Cp†)(NCMe)(dppe)]+ (Cp† = Cp, Cp*, Cp′, Cp′′, Cptt) compounds (0.09–0.10 V at 0.1 V s−1) is greater than that for the decamethylferrocene couple (0.07 V) used as the internal standard and this suggest that the process is not simply diffusion controlled. For 2SIP, the electron donating effect of the five methyl groups shift OX to a more negative potential than others in the series and result in OX overlapping with OX′. For the other members of the series OX overlaps with the second oxidation process of the iodide anion. Redox potentials for the separated ion pair complexes are considerably more positive (ca. 0.5 V) than those of their [Fe(Cp†)(I)(dppe)] precursors since oxidation involves the loss of an electron from an already cationic species i.e. the [Fe(Cp†)(MeCN)(dppe)]2+/+ redox couple however these potentials are significantly lower than those obtained previously for the 16-electron cationic complex [Fe(Cp*)(dppe)][PF6]39 (0.64 V vs. Fc+/Fc in THF) cf. 0.06 V for [Fe(Cp*)(NCMe)(dppe)] in MeCN.
Mössbauer studies
The Mössbauer parameters at 80 K for all of the compounds are collected in Table 3. The isomer shift (I.S.) and quadruple splitting (Q.S.) values for all compounds are fully consistent with derivatives of iron(II)–cyclopentadienyl in a piano-stool geometry.13,14,40 As expected the I.S. decreases and the Q.S. is invariant on raising the temperature from 80 to 298 K.41,42 On changing halide from chloride to bromide to iodide and on varying substituents on the cyclopentadienyl ligand there are only slight changes in I.S. and Q.S. When halide is replaced by acetonitrile to form a separated ion pair there is a slight decrease in I.S. but a much larger decrease is observed when halide is replaced by hydride; this can be attributed to the higher σ-donor ability of the hydride ligand increasing the relative electron density at the iron centre.| Compound | I.S. (mms−1) | Q.S. (mms−1) | H.W.H.M. (mms−1) |
|---|---|---|---|
| a I.S. = isomer shift; Q.S. = quadrupole splitting; H.W.H.M = half-width at half-maxima with errors ≤±0.01 mms−1. | |||
| 1Cl | 0.44 | 1.92 | 0.13 |
| 2Cl | 0.48 | 2.04 | 0.15 |
| 3Cl | 0.46 | 1.88 | 0.13 |
| 4Cl | 0.48 | 1.73 | 0.13 |
| 5Cl | 0.50 | 2.08 | 0.15 |
| 1Br | 0.44 | 1.94 | 0.13 |
| 2Br | 0.50 | 2.10 | 0.14 |
| 3Br | 0.47 | 1.84 | 0.12 |
| 4Br | 0.48 | 1.70 | 0.13 |
| 5Br | 0.53 | 2.00 | 0.14 |
| 1I | 0.43 | 1.89 | 0.12 |
| 2I | 0.52 | 2.09 | 0.13 |
| 3I | 0.45 | 1.85 | 0.13 |
| 4I | 0.49 | 1.69 | 0.12 |
| 5I | 0.53 | 1.95 | 0.13 |
| 1SIP | 0.39 | 2.01 | 0.14 |
| 2SIP | 0.42 | 2.00 | 0.13 |
| 3SIP | 0.39 | 1.91 | 0.14 |
| 4SIP | 0.44 | 1.86 | 0.14 |
| 5SIP | 0.45 | 1.97 | 0.13 |
| 1H | 0.26 | 1.91 | 0.13 |
| 2H | 0.25 | 1.92 | 0.13 |
| 3H | 0.26 | 1.70 | 0.14 |
| 4H | 0.28 | 1.71 | 0.16 |
| 5H | 0.27 | 1.96 | 0.15 |
UV-Vis spectroscopic studies
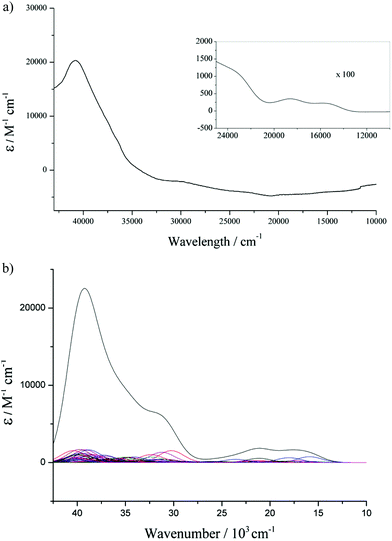
The UV-vis spectra of all the complexes were recorded in THF solutions at two different concentrations (1.25 mM and 0.0125 mM) to enable observation of all the relevant transitions without interference from solvent overtones. Theoretical UV-vis spectra have also been plotted from time dependant DFT (TD-DFT) calculations. The representative experimental and calculated UV-vis spectra of 2Cl are illustrated in Fig. 8. At dilute concentration an intense band is observed at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 cm−1 (ε = 20
816 cm−1 (ε = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1) in the UV region, and at higher concentrations relatively weak bands can be observed in the visible region at 16
300 M−1 cm−1) in the UV region, and at higher concentrations relatively weak bands can be observed in the visible region at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502 (ε = 221 M−1 cm−1) and 18
502 (ε = 221 M−1 cm−1) and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587 cm−1 (ε = 352 M−1 cm−1). By comparison of these experimental data with the combined total of the calculated data it can be seen that they are in good agreement, and that the calculations are a good approximation of the experimental spectra. With this in hand it is then possible to assign these absorptions from the calculated spectra, and calculations suggest that the band at 40
587 cm−1 (ε = 352 M−1 cm−1). By comparison of these experimental data with the combined total of the calculated data it can be seen that they are in good agreement, and that the calculations are a good approximation of the experimental spectra. With this in hand it is then possible to assign these absorptions from the calculated spectra, and calculations suggest that the band at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 cm−1, is a result of π–π* transitions in the aromatic carbon systems; and the bands in the visible region can be predominantly ascribed to metal–ligand charge transfer (MLCT) from the Fe 3d orbitals to the phenyl rings and the Cp* ligand.
816 cm−1, is a result of π–π* transitions in the aromatic carbon systems; and the bands in the visible region can be predominantly ascribed to metal–ligand charge transfer (MLCT) from the Fe 3d orbitals to the phenyl rings and the Cp* ligand.
The UV-vis spectra for the bromide and iodide series of complexes present similar observations as found in the spectra of the chloride series where variation of the Cp ligands has an effect on the absorptions observed in the visible region (see ESI† for further spectra). However, there is no obvious trend in the electronic absorptions observed upon change in halide across the series, therefore we can conclude that the Fe to Cp MLCT bands are the dominant features in the visible region and the halide has very little effect on the absorptions, Table 4. Although one notable feature is the value of the extinction coefficient for the π–π* transitions in the bromide complexes (1Br–5Br) are on average larger than their chloride and iodide counterparts. For example the π–π* transition in 4Br at 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 cm−1 has a molar absorptivity of 46
152 cm−1 has a molar absorptivity of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 M−1 cm−1 which is at least twice as large as the analogous transition in 4Cl of ε = 13
300 M−1 cm−1 which is at least twice as large as the analogous transition in 4Cl of ε = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 M−1 cm−1 at 39
200 M−1 cm−1 at 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 682 cm−1, and 4I of ε = 19
682 cm−1, and 4I of ε = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 M−1 cm−1 at 40
100 M−1 cm−1 at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 cm−1.
486 cm−1.
| Compound | 1Cl | 2Cl | 3Cl | 4Cl | 5Cl |
|---|---|---|---|---|---|
| a Epsilon is quoted to 3 significant figures. | |||||
| Experimental/cm−1 (ε/M−1 cm−1) | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 007 (247) 007 (247) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502 (221) 502 (221) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978 (376) 978 (376) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (191) 500 (191) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (113) 000 (113) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 (307) 880 (307) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587 (352) 587 (352) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 (463) 417 (463) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 903 (339) 903 (339) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148 (183) 148 (183) |
|
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (21 323 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 (20 816 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 (15 152 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 682 (13 682 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 682 (21 682 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
|
| Theoretical/cm−1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (478) 200 (478) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (1340) 900 (1340) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1170) 400 (1170) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (1460) 600 (1460) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (412) 400 (412) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1150) 400 (1150) |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (1650) 600 (1650) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (1310) 100 (1310) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1340) 400 (1340) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (1580) 500 (1580) |
|
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (9590) 100 (9590) |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (22 200 (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (11 300 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (11 600 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (11 500 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
|
| Compound | 1Br | 2Br | 3Br | 4Br | 5Br |
| Experimental/cm−1 (ε/M−1 cm−1) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155 (200) 155 (200) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 456 (156) 456 (156) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898 (389) 898 (389) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898 (334) 898 (334) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 456 (271) 456 (271) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 (391) 960 (391) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587 (283) 587 (283) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 (617) 417 (617) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 (621) 939 (621) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051 (425) 051 (425) |
|
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (21 000 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 (46 152 (46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 (30 152 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
|||
| Theoretical/cm−1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (577) 300 (577) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (921) 200 (921) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (1310) 600 (1310) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1260) 400 (1260) |
|
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (1030) 600 (1030) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1320) 400 (1320) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (1390) 200 (1390) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (1450) 900 (1450) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (1810) 200 (1810) |
|
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (8390) 900 (8390) |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 (11 800 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 (11 800 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (9930) 500 (9930) |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (10 700 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
|
| Compound | 1I | 2I | 3I | 4I | 5I |
| Experimental/cm−1 (ε/M−1 cm−1) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (134) 000 (134) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 (264) 106 (264) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 848 (199) 848 (199) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (172) 000 (172) |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (202) 500 (202) |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 (238) 120 (238) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 519 (500) 519 (500) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569 (421) 569 (421) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 (445) 048 (445) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051 (448) 051 (448) |
|
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (19 323 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 (25 486 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 (25 486 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 (19 486 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (21 323 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
|
| Theoretical/cm−1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 (219) 800 (219) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (441) 400 (441) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (556) 500 (556) |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (1140) 300 (1140) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (949) 000 (949) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (982) 200 (982) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (1090) 200 (1090) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 (1180) 800 (1180) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (1360) 100 (1360) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (1880) 500 (1880) |
|
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (7060) 500 (7060) |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (10 400 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (10 000 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (10 300 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (10 000 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
|
| Compound | 1SIP | 2SIP | 3SIP | 4SIP | 5SIP |
| Experimental/cm−1 (ε/M−1 cm−1) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (95) 000 (95) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244 (160) 244 (160) |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 (123) 015 (123) |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 (221) 514 (221) |
|
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 202 (392) 202 (392) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553 (368) 553 (368) |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 921 (658) 921 (658) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 (391) 120 (391) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 (491) 083 (491) |
|
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (552) 500 (552) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 (809) 750 (809) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (25 323 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (458) 000 (458) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (715) 500 (715) |
|
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (22 323 (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (32 323 (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (26 000 (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (28 000 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
||
| Theoretical/cm−1 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (1180) 200 (1180) |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (705) 900 (705) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (1440) 300 (1440) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (1530) 000 (1530) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1050) 400 (1050) |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (1170) 100 (1170) |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (2030) 400 (2030) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (1530) 000 (1530) |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (1800) 600 (1800) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (1830) 900 (1830) |
|
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (10 700 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (11 500 (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (10 400 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (10 900 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 (12 800 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
|
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (12 600 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) 400) |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (14 600 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (15 700 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (12 100 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (12 500 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
|
| Compound | 1H | 2H | 3H | 4H | 5H |
| Experimental/cm−1 (ε/M−1 cm−1) | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 011 (2760) 011 (2760) |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (805) 000 (805) |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096 (1660) 096 (1660) |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 (2610) 474 (2610) |
|
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (27 683 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 841 (27 841 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 161 (8730) 161 (8730) |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (14 683 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) 200) |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (20 683 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) 500) |
|
| Theoretical/cm−1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1190) 400 (1190) |
||||
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (3640) 600 (3640) |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (2590) 600 (2590) |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (3550) 200 (3550) |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (3430) 300 (3430) |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (2190) 900 (2190) |
|
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (1050) 300 (1050) |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (2520) 600 (2520) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (1790) 700 (1790) |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (2130) 600 (2130) |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 (2110) 900 (2110) |
|
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (13 700 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (1210) 400 (1210) |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 (12 700 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300) 300) |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 (12 400 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600) 600) |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (1620) 200 (1620) |
|
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (5950) 300 (5950) |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (15 000 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
||||
IR spectroscopic studies
The IR spectra of all the complexes studied in this work were recorded as Nujol mulls. The IR spectra of 1H–2H contain bands at 1845, 1844, 1849, 1854 and 1872 cm−1 respectively and these absorptions can be assigned to the νFe–H stretching absorptions for the terminally bonded metal hydride. All these bands are very weak, and are comparable to the νFe–H reported for other structurally authenticated iron terminal hydride complexes containing the dppe ligand which span the range 1833–1919 cm−1.20,30,34 The only other example of an iron hydride complex with both dppe and cyclopentadienyl ligands with which to compare is [Fe(Cp*)(H)(dppe)][PF6] that has a comparable band at 1869 cm−1.43DFT calculations
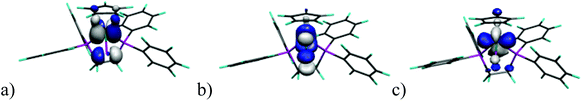
We have undertaken DFT calculations for 1Cl–5Cl, 1Br–5Br, 1I–5I, 1SIP–5SIP and 1H–5H to provide a qualitative description of the structure and bonding within these complexes and to provide a framework to interpret the electrochemical properties of these compounds. The DFT calculations reproduce the magnitude of the crystallographically determined bond lengths and the interbond angles of these complexes to within ∼0.05 Å and ∼1.5°, respectively. We therefore conclude that the calculations afford reliable qualitative descriptions of the electronic manifolds of 1Cl–5Cl, 1Br–5Br, 1I–5I, 1SIP–5SIP and 1H–5H.The frontier orbital manifolds of 1Cl–5Cl, 1Br–5Br and 1I–5I are similar with the HOMO, HOMO−1 and HOMO−2 orbitals in each compound possessing dominant Fe character derived from the 3d orbitals that form the t2g set in Oh symmetry (for representative frontier orbitals see Fig. 9).44 These orbitals are non-bonding with respect to σ-interactions but can exhibit π-interactions with X = Cl, Br, I. Thus, in each 1Cl–5Cl, 1Br–5Br and 1I–5I the HOMO possess Fe and X character (61.9–74.0% and 10.7–24.9%, Table S3†) and for a homogeneous series with a fixed cyclopentadienyl-derived ligand the contribution to the HOMO typically varies as I > Br > Cl as would be expected from the π-donor ability for each halogen donor. These calculations are in broad agreement with those accomplished previously for CpFe(dpe)X (dpe = 1,2-diphospinoethane; X = Cl, Br, I).28 The energies of the HOMO orbital for a fixed Cp ligand and E½ for the oxidation process (see above) show clear trends as X is varied (Fig. 10). Thus, the energy of the HOMO varies as Cl > Br > I with a reduction potential order of Cl < Br < I. Thus, for a fixed cyclopentadienyl-derived ligand complexes with X = Cl are more readily oxidised to their cationic counterparts and this observation is borne out by the relative energies of the HOMO orbitals in this series of compounds. The variation in energy of the HOMO with X-ligand appears counterintuitive given the percentage X-ligand character in the HOMO and the relative π-donor abilities of the halide donors. This inverse halide order has been noted previously,39 and has been ascribed to the ionic nature of the Fe–X bond, in which the X-ligand may be viewed as acting as a point negative charge that destabilizes the t2g set of orbitals which results in an inverse of the energy order expected from π-donor effects alone.45–50
Conclusions
To conclude, we have reported the synthesis of three iron(II) halide dppe complexes, and their utility in preparing a range of cyclopentadienyl derivatives. In all, we describe the synthesis of twenty five iron cyclopentadienyl dppe complexes as either halide (Cl, Br, I), separated ion pair, or hydride derivatives, thus providing a cohesive and reliable approach to the preparation of such molecules. This report has enabled a comprehensive structural and spectroscopic benchmarking study, providing a detailed understanding of the electronic structure of these compounds in one common framework. The Mössbauer studies suggest that the bonding is these complexes is predominantly ionic. Nevertheless, it is evident that the cyclopentadienyl and X-ligands modulate the electronic structure and redox properties of iron. Surprisingly, it is clear that although the cyclopentadienyl substituents do influence the redox properties of the iron centre, their influence is modest, and does not follow the trend that might be anticipated. In the pantheon of cyclopentadienyl ligands, taking C5H5 as the reference point, the pentamethyl and di-tert-butyl variants are usually described as better donor and poorer acceptor ligands, whereas the silyl-substituted variants are often viewed as poorer donors and better acceptor ligands. This should result in pentamethyl and di-tert-butyl cyclopentadienyl ligands leading to more electron rich iron centres whereas the silyl-substituted variants should give more electron deficient iron centres. However, on the basis of the electrochemical data presented here although the alkyl-cyclopentadienyls do fit this trend, this view is clearly overly simplistic where the silyl-substituted variants are concerned since their behaviour more closely resembles that of C5H5. This observation helps to codify the unpredictability of cyclopentadienyl-substituent effects on the redox potentials of transition metal complexes for a widely employed ligand class. The identity of the X-ligand, however, has a substantial and direct effect on the redox properties of the iron centre, which reflects the potential π-donor capcacity of the halides and their direct negative point charge to iron; indeed for the halides there is an essentially linear electrochemical relationship on moving from Cl to Br to I and these studies support the notion of hydride as a strong donor ligand. The complexes reported in this study could find extensive utility in molecular wire and metal–metal bond chemistry, where control of redox properties is important; the data presented here provide a database that enables the selection of a particular iron complex with an appropriate reduction potential for a given application. We are currently investigating the utility of the iron-hydride complexes presented here for the synthesis of uranium–iron bonds by alkane or amine elimination routes.Experimental
General experimental details
All manipulations were carried out using standard Schlenk techniques or an MBraun UniLab glovebox, under an atmosphere of dry nitrogen. THF, toluene, hexane and diethyl ether were dried by passage through activated alumina towers and degassed before use and then were stored over potassium mirrors (except THF which was stored over activated 4 Å molecular sieves). Acetonitrile and dichloromethane were distilled from CaH2 and stored over 3 Å (DCM) or 4 Å (MeCN) activated molecular sieves. Benzene-d6 was dried over potassium, distilled, degassed and stored under nitrogen. The compounds CpH*,51,52 KCp*,53 C5H5(SiMe3),54 KC5H4(SiMe3),54 C5H4(SiMe3)2,54 KC5H3(SiMe3)2,54 C5H4(tBu)2,55 KC5H3(tBu)2,55 and [FeCl2(dppe)]55 were synthesised according to published procedures. The compounds LiCp, NaCp and KCp were purchased from Sigma Aldrich and were used without any further purification. Compounds II, III, 2Cl, 1I, and 1H have previously been reported and are well characterised, the syntheses described for these compounds below are for completeness and to report any additional characterisation.NMR spectra were recorded on either a Bruker DPX300 spectrometer [operating at 300.1 MHz (1H), 75.5 MHz (13C{1H}) and 121.5 MHz (31P{1H})] or a Bruker DPX400, AV400 spectrometer [operating at 400.2 MHz (1H), 100.6 MHz {13C{1H}), 162.0 MHz (31P{1H}) and 79.5 MHz (29Si{1H})]. Chemical shifts are quoted in ppm and are relative to TMS (1H, 13C{1H} and 29Si{1H}) and external 85% H3PO4 (31P{1H}). IR spectra were recorded on a Bruker Tensor 27 FTIR spectrometer, where samples were prepared in the glovebox using a Nujol mull between two KBr discs. UV-Vis/NIR spectra were recorded on a Perkin Elmer LAMBDA 750 spectrometer. Data were collected in THF in 1 cm path length quartz cuvettes which were prepared in the glovebox. Elemental microanalyses were carried out by Mr Stephen Boyer at the Microanalysis Service, London Metropolitan University, UK or Dr Tong Liu, University of Nottingham. Mössbauer spectra were recorded in a zero magnetic field at 80 K or 298 K on an ES-Technology MS-105 Mössbauer spectrometer with a 25 MBq 57Co source in a rhodium matrix at ambient temperature. Spectra were referenced against a 25 μm iron foil at 298 K and spectrum parameters were obtained by fitting with Lorentzian lines. Samples were prepared by grinding with boron nitride before mounting.
Preparation of [FeBr2(dppe)], II
THF (40 mL) was added to a mixture of FeBr2 (3.45 g, 16.0 mmol) and dppe (6.43 g, 16.0 mmol) at room temperature and then refluxed overnight. The mixture was filtered, the solids washed with toluene and dried in vacuo to yield a light green solid in excellent yield (9.28 g, 94%).Preparation of [FeI2(dppe)], III
THF (40 ml) was added to a mixture of FeI2 (5.00 g, 16.0 mmol) and dppe (6.43 g, 16.0 mmol) at room temperature and stirred for 18 hours. The mixture was filtered and the solids were dried in vacuo to yield a yellow solid in excellent yield (10.8 g, 95%).General preparative method for 1Cl–5Cl, 1Br–5Br, 1I–5I
Toluene (20 ml) was added dropwise to a mixture of FeX2(dppe) (where X = Cl, Br or I) and MCp† (where M = Li, K or Na, Cp = C5H5, M = K, Cp = C5Me5, C5H4(SiMe3), C5H3(SiMe3)2 or C5H3(tBu)2) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at −78 °C with stirring, the resultant mixture was allowed to warm to room temperature slowly over 18 hours. The mixture was filtered and the solids washed with toluene (2 × 5 ml), the volatiles were removed in vacuo and the resulting solids were recrystallized from the minimum amount of toluene with storage at −30 °C, with the exception of 1Cl which is best recrystallized from a DCM/hexane layer (1
1 molar ratio at −78 °C with stirring, the resultant mixture was allowed to warm to room temperature slowly over 18 hours. The mixture was filtered and the solids washed with toluene (2 × 5 ml), the volatiles were removed in vacuo and the resulting solids were recrystallized from the minimum amount of toluene with storage at −30 °C, with the exception of 1Cl which is best recrystallized from a DCM/hexane layer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) with storage at −30 °C. The black crystals were isolated by filtration, washed with hexanes (2 × 1–2 mL) and dried in vacuo.
3 ratio) with storage at −30 °C. The black crystals were isolated by filtration, washed with hexanes (2 × 1–2 mL) and dried in vacuo.
1Cl: I (2.63 g, 5.0 mmol) and LiCp (0.36 g, 5.0 mmol), yield: 1.75 g, 66%. Anal. calc'd for C31H29FeClP2: C, 67.11; H, 5.27%. Found: C, 66.85; H, 5.16%. 1H NMR (C6D6, 298 K): δH 2.29 (m, 4H, CH2), 4.28 (s, 5H, C5H5), 7.01–8.23 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 27.65 (t, CH2, 1JCP = 81 Hz), 76.94 (C5H5), 127.88 (Ar–C), 128.22 (Ar–C), 128.90 (Ar–C), 129.60 (Ar–C), 132.31 (t, Ar–C, 2JCP = 18 Hz), 134.62 (t, Ar–C, 2JCP = 18 Hz), 142.31 (Ar–C), 142.67 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 111.31. FTIR ν/cm−1 (Nujol): 1403 (s), 861 (s), 694 (s), 667 (s) 651 (s), 588 (s), 529 (s) 518 (s), 491 (s), 462 (s) and 438 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 007 (247), 19
007 (247), 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881 (307), 40
881 (307), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (21
323 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Mössbauer (80 K, mm s−1) I.S. = 0.44, Q.S. = 1.92. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.42 V.
000). Mössbauer (80 K, mm s−1) I.S. = 0.44, Q.S. = 1.92. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.42 V.
2Cl: I (2.63 g, 5.0 mmol) and KCp* (0.87, 5.0 mmol), yield: 2.28 g, 73%. 1H NMR (C6D6, 298 K): δH 1.40 (s, 15H, CH3), 2.75 (m, 4H, CH2), 7.00–8.05 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 30.2 (CH2), 83.60 (C5H5), 131.3–140.2 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 91.6. FTIR ν/cm−1 (Nujol): 1483(w), 1432 (s), 1091 (s), 1068 (w), 1027 (m), 787 (w), 471 (s), 694 (vs), 658 (s), 616 (w), 528 (vs), 519 (w), 484 (vs), 450 (w) and 428 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502 (221), 18
502 (221), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587 (352), 40
587 (352), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 (20
816 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). Mössbauer (80 K, mm s−1) I.S. = 0.48, Q.S. = 2.04. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.59 V.
300). Mössbauer (80 K, mm s−1) I.S. = 0.48, Q.S. = 2.04. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.59 V.
3Cl: I (1.05 g, 2.0 mmol) and KC5H4(SiMe3) (0.35 g, 2.0 mmol), yield: 0.66 g, 48%. Anal. calc'd for C33H37FeClP2Si: C, 65.13; H, 5.95%. Found: C, 64.85; H, 6.12%. 1H NMR (CDCl3, 298 K): δH 0.08 (s, 9H, CH3), 2.39 (m, 4H, CH2), 4.09 (t, 2H, CH), 4.31 (t, 2H, CH), 7.27–8.14 (m, 20H, Ar–H). 13C{1H} NMR (CDCl3, 298 K): δC 1.03 (SiCH3), 27.20 (CH2), 68.95 (CH), 77.25 (CSiMe3), 88.78 (CH), 127.86 (Ar–C), 127.97 (Ar–C), 129.12 (Ar–C), 129.41 (Ar–C), 132.29 (Ar–C), 134.24 (Ar–C). 31P{1H} NMR (CDCl3, 298 K): δP 93.9. 29Si{1H} (CDCl3, 298 K): δSi −21.94. FTIR ν/cm−1 (Nujol): 1658 (s), 1433 (s), 1304 (m), 1245 (s), 1180 (s), 1158 (s), 1070 (s), 1041 (s), 900 (s), 871 (s), 817 (s), 737 (s), 697 (s) 669 (s), 645 (s), 635 (s), 609 (s), 530 (s), 520 (s), 489 (s), 452 (s), 436 (s) and 424 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978 (376), 19
978 (376), 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 (463), 41
417 (463), 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 (15
152 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Mössbauer (80 K, mm s−1) I.S. = 0.46, Q.S. = 1.88. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.45 V.
000). Mössbauer (80 K, mm s−1) I.S. = 0.46, Q.S. = 1.88. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.45 V.
4Cl: I (0.53 g, 1.0 mmol) and KC5H3(SiMe3)2 (0.25 g, 1.0 mmol), yield: 0.36 g, 51%. Anal. calc'd for C37H45FeClP2Si2: C, 63.56; H, 6.49%. Found: C, 60.26; H, 6.61%. 1H NMR (CDCl3, 298 K): δH 0.25 (s, 18H, CH3), 2.75 (m, 4H, CH2), 4.31 (d, 2H, CH), 4.85 (s, 1H, CH), 7.25–7.94 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 0.04 (CH3), 28.95 (t, CH2, 1JCP = 36 Hz), 77.04 (CH), 81.43 (CSiMe3), 102.97 (CH), 127.74 (Ar–C), 129.29 (Ar–C), 133.38 (Ar–C), 134.33 (Ar–C), 140.13 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 93.6. 29Si{1H} (CDCl3, 298 K): δSi −2.79. FTIR ν/cm−1 (Nujol): 1434 (s), 1401 (s), 1251 (s), 1188 (s), 1156 (s), 916 (s), 883 (s), 834 (s), 762 (s), 751 (s), 738 (s), 696 (s), 661 (s), 632 (s), 616 (s), 598 (s), 530 (s), 518 (s), 485 (s), 459 (s), 443 (s) and 426 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (191), 18
500 (191), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904 (339), 39
904 (339), 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (13
683 (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). Mössbauer (80 K, mm s−1) I.S. = 0.48, Q.S. = 1.73. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.44 V.
200). Mössbauer (80 K, mm s−1) I.S. = 0.48, Q.S. = 1.73. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.44 V.
5Cl: I (1.05 g, 2.0 mmol) and KC5H3(tBu)2 (0.43 g, 2.0 mmol), yield: 0.76 g, 57%. Anal. calc'd for C39H45FeClP2: C, 70.23; H, 6.80%. Found: C, 70.39; H, 6.72%. 1H NMR (CDCl3, 298 K): δH 0.94 (s, 18H, CH3), 2.26 (s, 4H, CH2), 3.14 (s, 2H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.99 (d, 1H, C
CH), 3.99 (d, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.30–7.68 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 1.18 (CH3), 31.25 (CH2), 61.21 (CH), 63.14 (CH), 76.27 (CtBu), 109.55 (CMe3), 126.12 (Ar–C), 129.00 (Ar–C), 130.15 (Ar–C), 134.34 (Ar–C), 139.80 (Ar–C). 31P{1H} NMR (CDCl3, 298 K): δP 85.6. FTIR ν/cm−1 (Nujol): 1433 (s), 1183 (s), 1160 (s), 937 (s), 919 (s), 875 (s), 833 (s), 748 (s), 694 (s), 671 (s), 648 (s), 636 (s), 616 (s), 528 (s), 517 (s), 508 (s), 489 (s), 475 (s) and 438 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15
CH), 7.30–7.68 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 1.18 (CH3), 31.25 (CH2), 61.21 (CH), 63.14 (CH), 76.27 (CtBu), 109.55 (CMe3), 126.12 (Ar–C), 129.00 (Ar–C), 130.15 (Ar–C), 134.34 (Ar–C), 139.80 (Ar–C). 31P{1H} NMR (CDCl3, 298 K): δP 85.6. FTIR ν/cm−1 (Nujol): 1433 (s), 1183 (s), 1160 (s), 937 (s), 919 (s), 875 (s), 833 (s), 748 (s), 694 (s), 671 (s), 648 (s), 636 (s), 616 (s), 528 (s), 517 (s), 508 (s), 489 (s), 475 (s) and 438 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (114), 18
000 (114), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 (183), 39
149 (183), 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (21
683 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). Mössbauer (80 K, mm s−1) I.S. = 0.50, Q.S. = 2.08. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.54 V.
900). Mössbauer (80 K, mm s−1) I.S. = 0.50, Q.S. = 2.08. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.54 V.
1Br: II (1.23 g, 2.0 mmol) and KCp (0.21 g, 2.0 mmol), yield: 0.52 g, 68%. Anal. Calc'd for C31H29P2FeBr: C, 62.13; H, 4.88%. Found: C, 62.25; H, 5.03%. 1H NMR (C6D6, 298 K): δH 2.40 (m, 4H, CH2,), 4.28 (s, 5H, CH), 7.01–8.23 (m, 20H, CH). 13C{1H} NMR (C6D6, 298 K): δC 76.64 (CH), 128.57 (Ar–C), 129.37 (Ar–C), 131.97 (Ar–C), 134.43 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 98.2. FTIR ν/cm−1 (Nujol): 1433 (s), 1179 (s), 1156 (s), 861 (s), 846 (s), 831 (s), 812 (s), 787 (s), 743 (s), 727 (s), 694 (s), 669 (s), 652 (s), 588 (s), 528 (s), 518 (s), 489 (s) and 461 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 155 (200), 19
155 (200), 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 (392). Mössbauer (80 K, mm s−1) I.S. = 0.44, Q.S. = 1.94. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.38 V.
960 (392). Mössbauer (80 K, mm s−1) I.S. = 0.44, Q.S. = 1.94. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.38 V.
2Br: II (1.23 g, 2.00 mmol) and KCp* (0.35 g, 2.00 mmol), yield: 0.40 g, 30%. Anal. Calc'd for C36H39P2FeBr: C, 64.60; H, 5.87%. Found: C, 64.52; H, 6.02%. 1H NMR (C6D6, 298 K): δH 1.58 (s, 15H, CH3), 2.35 (m, 4H, CH2), 7.11–7.40 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 11.24 (CH3), 31.90 (CH2), 83.30 (CH), 127.09 (Ar–C), 127.18 (Ar–C), 128.68 (Ar–C), 129.08 (Ar–C), 134.45 (t, Ar–C, 2JCP = 20 Hz), 135.00 (t, Ar–C, 2JCP = 16 Hz), 140.20 (Ar–C), 140.50 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 95.0. FTIR ν/cm−1 (Nujol): 1432 (s), 1179 (s), 1153 (s), 1067 (s), 865 (s), 740 (s), 699 (s), 662 (s), 617 (s), 528 (s), 490 (s), 468 (s) and 433 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455 (157), 18
455 (157), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587 (284), 40
587 (284), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (21
000 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). Mössbauer (80 K, mm s−1) I.S. = 0.52, Q.S. = 2.09. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.55 V.
900). Mössbauer (80 K, mm s−1) I.S. = 0.52, Q.S. = 2.09. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.55 V.
3Br: II (1.23 g, mmol) and KC5H4(SiMe3) (0.35 g, 2.0 mmol), yield: 0.52 g, 39%. Anal. Calc'd for C34H36P2SiFeBr: C, 60.82; H, 5.55%. Found: C, 61.95; H, 5.40%. 1H NMR (C6D6, 298 K): δH 0.39 (9H, s, CH3), 2.41 (m, 4H, CH2), 3.29 (d, 2H, CH), 5.15 (d, 2H, CH), 7.02–8.18 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 0.15 (CH3), 27.93 (t, CH2, 1JCP = 76 Hz), 70.42 (CH), 86.86 (CH), 128.68 (Ar–C), 129.34 (Ar–C), 132.39 (Ar–C), 134.51 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 95.9. 29Si{1H} NMR (C6D6, 298 K): δSi −2.3. FTIR ν/cm−1 (Nujol): 1245 (s), 1180 (s), 1154 (s), 914 (s), 885 (s), 831 (s), 740 (s), 659 (s), 655 (s), 635 (s), 532 (s), 517 (s), 482 (s) and 455 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898 (390), 19
898 (390), 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 (618). Mössbauer (80 K, mm s−1) I.S. = 0.47, Q.S. = 1.84. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.41 V.
417 (618). Mössbauer (80 K, mm s−1) I.S. = 0.47, Q.S. = 1.84. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.41 V.
4Br: II (1.23 g, 2.0 mmol) and KC5H3(SiMe3)2 (0.50 g, 2.0 mmol), yield: 0.69 g, 46%. Anal. Calc'd for C37H45P2Si2FeBr: C, 59.76; H, 6.10%. Found: C, 59.85; H, 5.91%. 1H NMR (C6D6, 298 K): δH 0.22 (s, 18H, CH3), 2.35 (m, 4H, CH2), 4.02 (s, 2H, CH), 5.43 (s, 1H, CH), 7.08–7.40 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 0.41 (CH3), 29.45 (CH2), 78.61 (CH), 81.12 (CH), 101.60 (Ar–C), 127.56–134.67 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 90.2. 29Si{1H} NMR (C6D6, 298 K): δSi −2.6. FTIR ν/cm−1 (Nujol): 1432 (s), 1366 (s), 1331 (s), 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 (s), 1238 (s), 1187 (s), 1161 (s), 1068 (s), 1051 (s), 968 (s), 956 (s), 923 (s), 887 (s), 833 (s), 751 (s), 733 (s), 692 (s), 656 (s), 629 (s), 611 (s), 585 (s), 532 (s), 519 (s), 483 (s), 448 (s), 438 (s) and 425 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15
580 (s), 1238 (s), 1187 (s), 1161 (s), 1068 (s), 1051 (s), 968 (s), 956 (s), 923 (s), 887 (s), 833 (s), 751 (s), 733 (s), 692 (s), 656 (s), 629 (s), 611 (s), 585 (s), 532 (s), 519 (s), 483 (s), 448 (s), 438 (s) and 425 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 898 (334), 18
898 (334), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 (622), 41
939 (622), 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 (46
152 (46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). Mössbauer (80 K, mm s−1) I.S. = 0.49, Q.S. = 1.69. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.40 V.
300). Mössbauer (80 K, mm s−1) I.S. = 0.49, Q.S. = 1.69. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.40 V.
5Br: II (1.23 g, 2.0 mmol) and KC5H3(tBu)2 (0.42 g, 2.0 mmol), yield: 0.79 g, 56%. Anal. Calc'd for C39H45P2FeBr: C, 65.82; H, 6.38%. Found: C, 65.67; H, 6.36%. 1H NMR (C6D6, 298 K): δH 1.26 (s, 18H, CH3), 2.23 (m, 4H, CH2), 3.39 (s, 2H, CH), 5.29 (s, 1H, CH), 7.12–7.27 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 1.17 (CH3), 31.54 (t, CH2, 1JCP = 278 Hz), 60.93 (CH), 80.63 (CH), 107.06 (Ar–C), 127.88 (Ar–C), 129.01 (Ar–C), 134.26 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 82.7. FTIR ν/cm−1 (Nujol): 1659 (s), 1433 (s), 1286 (s), 1251 (s), 1187 (s), 1160 (s), 1047 (s), 998 (s), 934 (s), 919 (s), 872 (s), 843 (s), 832 (s), 791 (s), 741 (s), 691 (s), 664 (s), 655 (s), 642 (s), 615 (s), 528 (s), 482 (s), 450 (s) and 428 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 456 (271), 18
456 (271), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051 (425), 41
051 (425), 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 152 (30
152 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). Mössbauer (80 K, mm s−1) I.S. = 0.53, Q.S. = 1.95. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.50 V.
200). Mössbauer (80 K, mm s−1) I.S. = 0.53, Q.S. = 1.95. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.50 V.
1I: III (1.42 g, 2.0 mmol) and NaCp (0.16 g, 2.0 mmol), yield: 0.84 g, 67%. 1H NMR (C6D6, 298 K): δH 2.60 (m, 4H, CH2), 4.30 (s, 5H, C5H5), 6.99–8.05 (m, 20H, Ar–H); 31P{1H} NMR (C6D6, 298 K): δP 99.1. UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (69.2), 20
000 (69.2), 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 (219), 40
120 (219), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (19
323 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). Mössbauer (80 K, mm s−1) I.S. = 0.43, Q.S. = 1.89. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.34 V.
300). Mössbauer (80 K, mm s−1) I.S. = 0.43, Q.S. = 1.89. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.34 V.
2I: III (0.71 g, 1.0 mmol) and KCp* (0.17 g, 1.0 mmol), yield: 0.46 g, 64%. Anal. calc'd for C36H39FeIP2: C, 60.36; H, 5.49%. Found: C, 60.26; H, 5.37%. 1H NMR (C6D6, 298 K): δH 1.17 (s, 15H, CH3), 2.50 (m, 4H, CH2), 6.82–8.14 (m, 20H, Ar–H); 13C{1H} NMR (C6D6, 298 K): δC −11.24 (CH3), 31.90 (t, CH2) 83.30 (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 128.81 (p-Ar–C), 129.48 (m-Ar–C), 133.14 (o-Ar–C), 134.73 (i-Ar–C); 31P{1H} NMR (C6D6, 298 K): δP 95.0. FTIR ν/cm−1 (Nujol): 1179 (s), 1153 (s), 1069 (s), 893 (s), 863 (s), 739 (s), 699 (s), 660 (s), 618 (s), 527 (s), 518 (s), 490 (s), 468 (s), 451 (s) and 431 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15
C) 128.81 (p-Ar–C), 129.48 (m-Ar–C), 133.14 (o-Ar–C), 134.73 (i-Ar–C); 31P{1H} NMR (C6D6, 298 K): δP 95.0. FTIR ν/cm−1 (Nujol): 1179 (s), 1153 (s), 1069 (s), 893 (s), 863 (s), 739 (s), 699 (s), 660 (s), 618 (s), 527 (s), 518 (s), 490 (s), 468 (s), 451 (s) and 431 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 (264), 18
106 (264), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 (500), 40
518 (500), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 (35
486 (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Mössbauer (80 K, mm s−1) I.S. = 0.52, Q.S. = 2.09. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.51 V.
000). Mössbauer (80 K, mm s−1) I.S. = 0.52, Q.S. = 2.09. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.51 V.
3I: III (0.71 g, 1.0 mmol) and KC5H4(SiMe3) (0.18 g, 1.0 mmol), yield: 0.26 g, 36%. Anal. calc'd for C34H37FeIP2Si: C, 56.84; H, 5.19%. Found: C, 57.50; H, 5.18%. 1H NMR (C6D6, 298 K): δH 0.38 (s, 9H, CH3), 2.57 (m, 4H, CH2), 3.45 (s, 2H, CH), 5.14 (s, 2H, CH), 6.99–8.14 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 0.59 (SiCH3), 28.78 (t, CH2, 1JCP = 84 Hz), 71.71 (CH), 81.94 (t, CSiMe3, 2JCP = 8 Hz), 86.50 (CH), 127.63 (Ar–C), 127.73 (Ar–C), 128.58 (Ar–C), 129.32 (Ar–C), 132.35 (t, Ar–C, 2JCP = 16 Hz), 132.90 (t, Ar–C, 2JCP = 32 Hz) 134.48 (t, Ar–C, 2JCP = 16 Hz), 139.33 (t, Ar–C, 2JCP = 24 Hz), 139.74 (t, Ar–C, 2JCP = 20 Hz) 142.00 (t, Ar–C, 1JCP = 52 Hz), 142.33 (t, Ar–C, 1JCP = 52 Hz). 31P{1H} NMR (C6D6, 298 K): δP 96.8. 29Si{1H} (C6D6, 298 K): δSi −1.79. FTIR ν/cm−1 (Nujol): 1482 (w), 1431 (s), 1308 (w), 1243 (m), 1156 (m), 1096 (m), 1069 (sh), 1037 (sh), 1026 (w), 999 (w), 900 (w), 890 (w), 833 (s), 741 (s), 693 (vs), 661 (s), 632 (m), 617 (sh), 566 (w), 518 (vs), 485 (s) and 436 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 848 (199), 19
848 (199), 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 569 (421), 40
569 (421), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 (25
486 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). Mössbauer (80 K, mm s−1) I.S. = 0.45, Q.S. = 1.85. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.37 V.
900). Mössbauer (80 K, mm s−1) I.S. = 0.45, Q.S. = 1.85. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.37 V.
4I: III (0.71 g, 1.0 mmol) and KC5H3(SiMe3)2 (0.25 g, 1.0 mmol), yield: 0.41 g, 52%. Anal. calc'd for C37H45FeIP2Si2: C, 56.21; H, 5.74%. Found: C, 56.30; H, 5.65%. 1H NMR (C6D6, 298 K): δH 0.24 (s, 18H, CH3), 2.50 (m, 4H, CH2), 4.04 (s, 2H, CH), 5.56 (s, H, CH), 6.95–8.28 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 0.70 (SiCH3), 30.46 (CH2), 79.00 (CH), 79.61 (CH), 101.70 (CSiMe3) 127.70 (Ar–C), 127.94 (Ar–C), 128.55 (Ar–C), 129.51 (Ar–C), 133.14 (t, Ar–C, 2JCP = 16 Hz), 134.73 (t, Ar–C, 2JCP = 16 Hz). 31P{1H} NMR (C6D6, 298 K): δP 92.3. 29Si{1H} (C6D6, 298 K): δSi −2.34. FTIR ν/cm−1 (Nujol): 915 (s), 886 (s), 696 (s), 661 (s), 635 (s), 532 (s), 517 (s), 484 (s) and 454 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (172), 19
500 (172), 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 048 (445), 40
048 (445), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 (19
486 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). Mössbauer (80 K, mm s−1) I.S. = 0.49, Q.S. = 1.69. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.36 V.
100). Mössbauer (80 K, mm s−1) I.S. = 0.49, Q.S. = 1.69. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.36 V.
5I: III (0.71 g, 1.0 mmol) and KC5H3(tBu)2 (0.22 g, 1.0 mmol), yield: 0.37 g, 49%. Anal. calc'd for C39H45FeIP2: C, 61.76; H, 5.98%. Found: C, 61.65; H, 6.06%. 1H NMR (C6D6, 298 K): δH 1.29 (s, 18H, CH3), 2.50 (m, 4H, CH2), 3.45 (s, 2H, CH), 5.38 (s, H, CH), 6.95–8.36 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 21.20 (CMe3), 31.91 (t, CH21JCP = 132 Hz), 62.69 (CH), 83.77 (CH), 104.20 (CtBu), 127.41 (Ar–C), 128.32 (Ar–C), 129.09 (Ar–C), 133.57 (Ar–C), 134.64 (Ar–C), 140.26 (Ar–C), 140.57 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 80.5. FTIR ν/cm−1 (Nujol): 1157 (s), 880 (s), 842 (s), 738 (s), 696 (s), 657 (s), 528 (s), 517 (s), 489 (s) and 467 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (202), 18
500 (202), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 051 (448), 40
051 (448), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (210
323 (210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Mössbauer (80 K, mm s−1) I.S. = 0.53, Q.S. = 1.95. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.46 V.
000). Mössbauer (80 K, mm s−1) I.S. = 0.53, Q.S. = 1.95. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.46 V.
General preparative method for 1SIP–5SIP
To a solution of 1Cl–5Cl in MeCN (10 ml), TMSI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) was added dropwise at −30 °C with stirring, the mixture was allowed to warm to room temperature slowly over 18 hours. Volatiles were removed in vacuo to yield red solids which were recrystallized from the minimum amount of MeCN with storage at −30 °C to afford red crystals. The crystals were isolated by filtration and dried in vacuo.
1 molar ratio) was added dropwise at −30 °C with stirring, the mixture was allowed to warm to room temperature slowly over 18 hours. Volatiles were removed in vacuo to yield red solids which were recrystallized from the minimum amount of MeCN with storage at −30 °C to afford red crystals. The crystals were isolated by filtration and dried in vacuo.
1SIP: 1Cl (0.46 g, 0.8 mmol) and TMSI (0.12 mL, 0.8 mmol), yield: 0.35 g, 65%. Anal. calc'd for C33H32FeINP2: C, 57.67; H, 4.69; N, 2.04%. Found: C, 57.50; H, 4.78; N, 2.18%. 1H NMR (CDCl3, 298 K): δH 0.08 (s, 3H, CH3), 2.02 (s, 3H, CH3), 2.18 (m, 4H, CH2), 4.41 (s, 5H, C5H5), 7.31–7.86 (m, 20H, Ar–H). 13C{1H} NMR (CDCl3, 298 K): δC 6.25 (CH3), 28.22 (t, CH2, 1JCP = 88 Hz), 78.87 (C5H5), 129.10 (t, Ar–C, 2JCP = 20 Hz), 129.26 (t, Ar–C, 2JCP = 18 Hz), 130.48 (Ar–C), 130.72 (Ar–C), 131.22 (t, Ar–C, 2JCP = 20 Hz), 132.72 (t, Ar–C, 2JCP = 20 Hz), 134.06 (N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe), 136.49 (t, Ar–C, 1JCP = 80 Hz), 136.90 (t, Ar–C, 1JCP = 80 Hz). 31P{1H} NMR (CDCl3, 298 K): δP 97.9. FTIR ν/cm−1 (Nujol): 2266 (s), 1432 (s), 1175 (s), 1157 (s), 1073 (s), 998 (s), 951 (s), 919 (s), 873 (s), 859 (s), 836 (s), 812 (s), 748 (s), 712 (s), 699 (s), 672 (s), 648 (s), 617 (s), 532 (s), 520 (s), 496 (s) 454 (s), 440 (s) and 429 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 16
CMe), 136.49 (t, Ar–C, 1JCP = 80 Hz), 136.90 (t, Ar–C, 1JCP = 80 Hz). 31P{1H} NMR (CDCl3, 298 K): δP 97.9. FTIR ν/cm−1 (Nujol): 2266 (s), 1432 (s), 1175 (s), 1157 (s), 1073 (s), 998 (s), 951 (s), 919 (s), 873 (s), 859 (s), 836 (s), 812 (s), 748 (s), 712 (s), 699 (s), 672 (s), 648 (s), 617 (s), 532 (s), 520 (s), 496 (s) 454 (s), 440 (s) and 429 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (95.4), 20
000 (95.4), 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 202 (392), 24
202 (392), 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (553), 40
500 (553), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (22
323 (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). Mössbauer (80 K, mm s−1) I.S. = 0.39, Q.S. = 2.01. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.26 V.
900). Mössbauer (80 K, mm s−1) I.S. = 0.39, Q.S. = 2.01. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.26 V.
2SIP: 2Cl (0.62 g, 1.0 mmol) and TMSI (0.14 mL, 0.8 mmol), yield: 0.57 g, 75%. Anal. calc'd for C38H42FeINP2: C, 60.26; H, 5.59; N, 1.85%. Found: C, 60.36; H, 5.46; N, 1.86%. 1H NMR (CDCl3, 298 K): δH 1.32 (s, 15H, CH3), 2.17 (s, 3H, CH3), 2.18 (m, 4H, CH2) 7.39–7.61 (m, 20H, Ar–H). 13C{1H} NMR (CDCl3, 298 K): δC 7.13 (CH3), 9.89 (CH3) 28.57 (t, CH2, 1JCP = 78 Hz), 87.21 (C5Me5), 128.56 (t, Ar–C, JCP = 18 Hz), 129.09 (t, Ar–C, JCP = 18 Hz), 130.66 (Ar–C), 130.87 (Ar–C) 132.34 (t, Ar–C, JCP = 18 Hz), 133.34 (t, Ar–C, JCP = 21 Hz). 31P{1H} NMR (CDCl3, 298 K): δP 89.9. FTIR ν/cm−1 (Nujol): 2248 (s), 1403 (s), 1156 (s), 949 (s), 890 (s), 861 (s), 786 (s), 759 (s), 742 (s), 695 (s), 661 (s), 640 (s), 528 (s), 516 (s), 483 (s) and 456 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244 (161), 18
244 (161), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 553 (369), 22
553 (369), 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 (810), 40
750 (810), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (32
323 (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700). Mössbauer (80 K, mm s−1) I.S. = 0.42, Q.S. = 2.00. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.06 V.
700). Mössbauer (80 K, mm s−1) I.S. = 0.42, Q.S. = 2.00. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.06 V.
3SIP: 3Cl (1.14 g, 1.8 mmol), and TMSI (0.25 mL, 1.8 mmol), yield: 0.47 g, 36%. Anal. calc'd for C36H40FeINP2Si: C, 56.93; H, 5.31; N, 1.84%. Found: C, 54.54; H, 5.30; N, 2.08%. 1H NMR (CDCl3, 298 K): δH 0.25 (s, 9H, CH3), 2.03 (s, 3H, CH3), 2.61 (m, 4H, CH2), 4.31 (d, 2H, CH) 4.85 (s, 2H, CH), 7.38–7.86 (m, 20H, Ar–H). 13C{1H} NMR (CDCl3, 298 K): δC −0.53 (CH3) 6.81 (CH3), 28.45 (t, CH2, 1JCP = 80 Hz), 71.06 (CH), 72.77 (CH), 74.79 (CH), 88.29 (CH) 129.08 (t, Ar–C, 2JCP = 20 Hz), 129.30 (t, Ar–C, 2JCP = 20 Hz), 130.70 (Ar–C), 130.80 (Ar–C), 131.72 (t, Ar–C, 2JCP = 20 Hz), 132.72 (t, Ar–C, 2JCP = 18 Hz), 134.61 (NCMe), 136.24 (t, Ar–C, 1JCP = 76 Hz), 136.65 (t, Ar–C, 1JCP = 88 Hz). 31P{1H} NMR (CDCl3, 298 K): δP 95.3. 29Si{1H} (CDCl3, 298 K): δSi −3.12. FTIR ν/cm−1 (Nujol): 2268 (s), 1165 (s), 1065 (s) 1031 (s), 950 (s), 899 (s), 871 (s), 832 (s), 746 (s) 698 (s), 674 (s), 616 (s) 527 (s), 517 (s), 490 (s), 455 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 (658), 40
920 (658), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 323 (25
323 (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300). Mössbauer (80 K, mm s−1) I.S. = 0.39, Q.S. = 1.91. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.25 V.
300). Mössbauer (80 K, mm s−1) I.S. = 0.39, Q.S. = 1.91. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.25 V.
4SIP: 4Cl (0.35 g, 0.5 mmol) and TMSI (0.14 mL, 1.0 mmol), yield: 0.27 g, 64%. Anal. calc'd for C39H48FeINP2Si2: C, 56.32; H, 5.82; N, 1.68%. Found: C, 56.66; H, 5.69; N, 1.63%. 1H NMR (CDCl3, 298 K): δH −0.13 (s, 18H, CH3), 1.77 (s, 3H, CH3), 2.51 (m, 4H, CH2), 4.46 (d, 2H, CH) 4.85 (s, 1H, CH), 7.46–7.84 (m, 20H, Ar–H). 13C{1H} NMR (CDCl3, 298 K): δC 0.03 (CH3) 7.79 (CH3), 29.18 (t, CH2, 1JCP = 72 Hz), 83.91 (CH), 84.41 (CH), 98.48 (CSiMe3), 129.13 (t, Ar–C, 2JCP = 18 Hz), 129.36 (t, Ar–C, 2JCP = 18 Hz), 130.77 (Ar–C), 130.83 (Ar–C) 130.83 (Ar–C), 132.36 (t, Ar–C, 2JCP = 18 Hz), 133.29 (t, Ar–C, 2JCP = 18 Hz), 135.67 (NCMe), 136.58 (Ar–C). 31P{1H} NMR (CDCl3, 298 K): δP 89.1. 29Si{1H} (CDCl3, 298 K): δSi −2.76. FTIR ν/cm−1 (Nujol): 2273 (s), 1432 (s), 1193 (s), 1178 (s), 1079 (s), 944 (s), 914 (s), 896 (s), 873 (s), 832 (s), 751 (s), 700 (s), 675 (s), 649 (s), 632 (s), 526 (s), 513 (s), 493 (s) and 436 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 (124), 19
015 (124), 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 (392), 23
120 (392), 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (458), 40
000 (458), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (26
000 (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700). Mössbauer (80 K, mm s−1) I.S. = 0.44, Q.S. = 1.86. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.26 V.
700). Mössbauer (80 K, mm s−1) I.S. = 0.44, Q.S. = 1.86. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.26 V.
5SIP: 5Cl (0.30 g, 1.0 mmol) and TMSI (0.14 Ml, 1.0 mmol), yield: 0.55 g, 69%. Anal. calc'd for C41H48FeINP2: C, 61.59; H, 6.05; N, 1.75%. Found: C, 61.42; H, 5.84; N, 1.73%. 1H NMR (CDCl3, 298 K): δH 0.80 (s, 18H, CH3), 2.02 (s, 3H, CH3), 2.18 (m, 4H, CH2), 4.11 (s, 2H, CH), 4.78 (s, 1H, CH), 7.28–7.80 (m, 20H, Ar–H). 13C{1H} NMR (CDCl3, 298 K): δC 2.65 (CH3), 8.29 (CH3), 31.21 (t, CH2, 1JCP = 88 Hz), 70.31 (CH), 74.89 (CH), 109.72 (CtBu), 129.10 (Ar–C), 129.35 (Ar–C), 130.51 (Ar–C), 130.64 (Ar–C) 132.29 (Ar–C), 133.14 (Ar–C), 136.43 (NCMe), 137.43 (t, Ar–C, JCP = 60 Hz), 137.80 (t, Ar–C, JCP = 60 Hz). 31P{1H} NMR (CDCl3, 298 K): δP 84.6. FTIR ν/cm−1 (Nujol): 2267 (s), 1432 (s), 1411 (s), 1364 (s), 1293 (s), 1248 (s) 1179 (s), 1164 (s), 1127 (s), 998 (s), 951 (s), 923 (s), 892 (s), 866 (s), 758 (s), 741 (s), 716 (s), 701 (s), 678 (s), 654 (s), 520 (s), 505 (s), 471 (s) and 438 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 (221), 18
514 (221), 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 (491), 22
083 (491), 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (716), 40
500 (716), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (28
000 (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). Mössbauer (80 K, mm s−1) I.S. = 0.45, Q.S. = 1.97. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.20 V.
100). Mössbauer (80 K, mm s−1) I.S. = 0.45, Q.S. = 1.97. CV (298 K, MeCN, [NBu4n][[BF4], 1 mM) E1/2 = 0.20 V.
General preparative method for 1H–5H
THF (10 ml) was added dropwise to a mixture of 1Cl–2Cl and LiAlH4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio) at −78 °C with stirring, the suspension was allowed to warm to 0 °C and stirred for a further 3 hours. At 0 °C degassed deionised water was added dropwise until the evolution of gas ceased and the volatiles were removed in vacuo to yield orange solids which were extracted and recrystallized from hexanes to afford orange crystals. The crystals were collected by filtration and dried in vacuo.
5 molar ratio) at −78 °C with stirring, the suspension was allowed to warm to 0 °C and stirred for a further 3 hours. At 0 °C degassed deionised water was added dropwise until the evolution of gas ceased and the volatiles were removed in vacuo to yield orange solids which were extracted and recrystallized from hexanes to afford orange crystals. The crystals were collected by filtration and dried in vacuo.
1H: 1Cl (0.56 g, 1.0 mmol) and LiAlH4 (0.19 g, 5.0 mmol), yield: 0.41 g, 73%. 1H NMR (C6D6, 298 K): δH −15.94 (t, 1H, FeH, 2JPH = 72 Hz) 1.87 (2H, CH2), 2.10 (m, 4H, CH2), 4.31 (s, 5H, C5H5), 7.16–7.97 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 33.02 (t, CH2, 1JCP = 60 Hz), 75.47 (CH), 127.21 (Ar–C), 128.48 (Ar–C), 133.27 (Ar–C), 132.90 (t, Ar–C, 2JCP = 40 Hz), 133.27 (t, Ar–C, 2JCP = 16 Hz), 133.92 (t, Ar–C, 2JCP = 20 Hz), 139.33 (t, Ar–C, 2JCP = 36 Hz), 140.11 (t, Ar–C, 2JCP = 28 Hz), 141.85 (Ar–C), 142.23 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 111.6. FTIR ν/cm−1 (Nujol) 1845 (s), 1621 (w), 1584 (w), 1301 (w), 1260 (w), 1087 (s), 1065 (w), 1027 (w), 812 (w), 740 (w), 695 (vs), 672 (s), 530 (vs), 493 (s), 474 (w) and 439 (w). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (27
683 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). Mössbauer (80 K, mm s−1) I.S. = 0.26, Q.S. = 1.91. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.50 V.
900). Mössbauer (80 K, mm s−1) I.S. = 0.26, Q.S. = 1.91. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.50 V.
2H: 2Cl (0.62 g, 1.0 mmol) and LiAlH4 (0.19 g, 5.0 mmol), yield: 0.40 g, 68%. Anal. calc'd for C36H40FeP2: C, 73.22; H, 6.83%. Found: C, 73.34; H, 6.85%. 1H NMR (C6D6, 298 K): δH −16.66 (t, 1H, FeH, 2JPH = 80 Hz) 1.73 (15H, CH3), 1.84 (m, 4H, CH2), 7.08–7.91 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 11.35 (CH3), 33.02 (t, CH2, 1JCP = 64 Hz) 84.88 (CMe3), 127.21 (Ar–C), 127.43 (Ar–C), 132.89 (t, Ar–C, 2JCP = 36 Hz), 133.27 (t, Ar–C, 2JCP = 16 Hz), 133.92 (t, Ar–C, 2JCP = 20 Hz), 138.89 (t, Ar–C, 2JCP = 35 Hz), 139.93 (t, Ar–C, 2JCP = 32 Hz) 140.11 (t, Ar–C, 2JCP = 35 Hz), 141.85 (t, Ar–C, 1JCP = 68 Hz), 142.23 (t, Ar–C, 1JCP = 68 Hz). 31P{1H} NMR (C6D6, 298 K): δP 107.7. FTIR ν/cm−1 (Nujol): 1844 (s), 1153 (s), 1064 (s), 876 (s), 740 (s), 695 (s), 672 (s), 529 (s), 493 (s) and 474 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 011 (2760), 39
011 (2760), 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (27
683 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900). Mössbauer (80 K, mm s−1) I.S. = 0.25, Q.S. = 1.92. CV (298 K, THF, [NBu4n][[BF4], 1 mM) Eap = −0.67 V, E1/2 = −0.71 V.
900). Mössbauer (80 K, mm s−1) I.S. = 0.25, Q.S. = 1.92. CV (298 K, THF, [NBu4n][[BF4], 1 mM) Eap = −0.67 V, E1/2 = −0.71 V.
3H: 3Cl (1.01 g, 1.5 mmol) and LiAlH4 (0.28 g, 7.5 mmol), yield: 0.31 g, 35%. No satisfactory elemental analysis could be obtained for 3H due to contamination of samples with free dppe ligand. 1H NMR (C6D6, 298 K): δH −15.31 (t, 1H, FeH, 2JPH = 72 Hz), 0.35 (s, 9H, CH3), 1.92 (m, 4H, CH2), 4.22 (m, 4H, CH), 7.21–7.89 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 0.34 (CH3), 31.01 (t, CH2, 1JCP = 92 Hz), 71.27 (CH), 72.91 (CH), 80.84 (CSiMe3), 127.69 (Ar–C), 128.32 (Ar–C), 132.14 (t, Ar–C, 2JCP = 16 Hz), 132.82 (t, Ar–C, 2JCP = 16 Hz), 143.00 (Ar–C), 143.52 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 110.1. 29Si{1H} (C6D6, 298 K): δSi −4.77. FTIR ν/cm−1 (Nujol): 1245 (s), 1160 (s), 1066 (s), 1036 (s), 969 (s), 918 (s), 903 (s), 872 (s), 862 (s), 834 (s), 813 (s), 743 (s), 693 (s), 675 (s), 646 (s), 627 (s), 525 (s), 499 (s), 468 (s), 437 (s) and 422 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (805), 40
000 (805), 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 161 (8730). Mössbauer (80 K, mm s−1) I.S. = 0.26, Q.S. = 1.70. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.50 V.
161 (8730). Mössbauer (80 K, mm s−1) I.S. = 0.26, Q.S. = 1.70. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.50 V.
4H: 4Cl (1.37 g, 2.0 mmol) and LiAlH4 (0.38 g, 10.0 mmol), yield: 0.54 g, 41%. Anal. calc'd for C37H46FeP2: C, 66.86; H, 6.98%. Found: C, 66.98; H, 7.05%. 1H NMR (C6D6, 298 K): δH −16.54 (t, 1H, FeH, 2JPH = 72 Hz), −0.11 (s, 18H, CH3), 2.60 (m, 4H, CH2), 4.45 (d, 2H, CH), 4.88 (s, 1H, CH), 7.49–7.89 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 0.43 (CH3), 31.25 (t, CH2, 1JCP = 88 Hz), 82.16 (CSiMe3), 83.71 (CH), 87.01 (CH), 127.51 (t, Ar–C, 2JCP = 16 Hz), 127.73 (t, Ar–C, 2JCP = 16 Hz), 128.40 (Ar–C), 128.50 (Ar–C), 132.31 (t, Ar–C, 2JCP = 18 Hz) 132.77 (t, Ar–C, 2JCP = 20 Hz), 146.94 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δp 104.8. 29Si{1H} (C6D6, 298 K): δSi −5.01. FTIR ν/cm−1 (Nujol): 1245 (s), 1184 (s), 1155 (s), 1074 (s), 968 (s), 924 (s), 911 (s), 864 (s), 832 (s), 742 (s), 696 (s), 669 (s), 634 (s), 522 (s), 494 (s), 479 (s), 444 (s) and 427 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096 (1660), 39
096 (1660), 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (14
683 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200). Mössbauer (80 K, mm s−1) I.S. = 0.28, Q.S. = 1.71. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.53 V.
200). Mössbauer (80 K, mm s−1) I.S. = 0.28, Q.S. = 1.71. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.53 V.
5H: 5Cl (0.63 g, 1.0 mmol) and LiAlH4 (0.19 g, 5.0 mmol), yield: 0.46 g, 53%. Anal. calc'd for C39H46FeP2: C, 74.05; H, 7.33%. Found: C, 72.03; H, 7.15%. 1H NMR (C6D6, 298 K): δH −15.65 (t, 1H, FeH, 2JPH = 84 Hz) 1.19 (s, 18H, CH3), 1.98 (m, 4H, CH2), 4.05 (d, 2H, CH), 4.09 (s, 1H, CH), 7.17–8.02 (m, 20H, Ar–H). 13C{1H} NMR (C6D6, 298 K): δC 22.54 (CMe3), 30.72 (CH3), 32.06 (CH2) 69.93 (CH), 71.17 (CH), 108.73 (CtBu), 127.46 (Ar–C), 127.66 (Ar–C), 128.20 (Ar–C), 128.26 (Ar–C), 132.26 (t, Ar–C, 2JCP = 24 Hz) 132.65 (t, Ar–C, 2JCP = 16 Hz), 144.19 (Ar–C), 144.39 (Ar–C). 31P{1H} NMR (C6D6, 298 K): δP 104.5. FTIR ν/cm−1 (Nujol): 1431 (s), 1360 (s), 1198 (s), 1162 (s), 1047 (2), 939 (s), 918 (s), 911 (s), 874 (s), 842 (s), 743 (s), 696 (s), 676 (s), 657 (s), 617 (s), 588 (s), 524 (s), 514 (s), 496 (s) and 475 (s). UV-vis (THF): λmax/cm−1 (ε/mol−1 cm−1) 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 474 (2610), 39
474 (2610), 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 (20
683 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500). Mössbauer (80 K, mm s−1) I.S. = 0.27, Q.S. = 1.96. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.62 V.
500). Mössbauer (80 K, mm s−1) I.S. = 0.27, Q.S. = 1.96. CV (298 K, THF, [NBu4n][[BF4], 1 mM) E1/2 = −0.62 V.
X-ray crystallographic details
Crystallographic data can be found in the ESI in cif format.† Crystals were examined variously on: (a) a Bruker AXS CCD area detector diffractometer operating at 90 K/150 K using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å), with intensities integrated from a sphere of data recorded on 0.3° frames by rotation of ω, or (b) an Oxford Diffraction CCD area detector diffractometer operating at 90 K using mirror-monochromated Cu Kα radiation (λ = 1.5418 Å) with intensities integrated from a sphere of data recorded on 1° frames by rotation of ώ. Cell parameters were refined from the observed positions of all strong reflections in the data set. The structures were solved by either direct methods or heavy atom method and refined by least-squares methods on all unique F2 values, with all non-H non solvent atoms being anisotropic. The largest residual electron densities in final difference syntheses were close to heavy atoms unless stated otherwise. Absorption correction was performed by multi-scan methods. Criterion for observed reflections is I > 2σ(I) and weighting scheme used is W = 1/[σ2(Fo2) + (xP)2 + yP] where P = (Fo2 + 2Fc2)/3. Programs used for Bruker AXS were SMART (control), SAINT (integration) and SHELXTL for structure solution and refinement. Programs used for Oxford diffraction were CrysalisPro CCD (control), CrysalisPro RED (integration), SHELXTL for structure solution and refinement.56Cyclic voltammetry experimental details and ESI†
Electrochemical measurements were performed using an Autolab PGSTAT20 potentiostat with a three-electrode configuration consisting of a saturated calomel electrode (SCE) reference electrode, Pt wire secondary electrode and a glassy carbon working electrode. All voltammograms were recorded at ambient temperatures for solutions of the sample at ca. 1 mM in tetrahydrofuran (THF) or acetonitrile (MeCN) containing 0.5 M or 0.1 M [NBun4][BF4], respectively, as the supporting electrolyte. Samples were prepared in a glove box and maintained under an argon atmosphere using Schlenk line techniques during the experiment. All electrochemical potentials were measured relative to SCE and were corrected for liquid-junction potentials via the use of the [(η5-C5H5)2Fe]+/[(η5-C5H5)2Fe] ([Cp2Fe]+/[Cp2Fe]) couple as an internal redox standard. When the potential of a redox process of the compound of interest overlapped with that of the ([Cp2Fe]+/[Cp2Fe]) couple, either the [(η55-Me5)2Fe]+/[(η5-C5Me5)2Fe] or ([Cp2Co]+/[Cp2Co]) couples was used as a standard. Under these circumstances, the redox processes of the compound were referenced to the [Cp2Fe]+/[Cp2Fe] couple by an independent calibration under identical conditions; E1/2 [Cp2Co]+/[Cp2Co] was −1.358 V vs. [Cp2Fe]+/[Cp2Fe] in THF at 1 V s−1 (used for 4H only) and E1/2 [(η5-C5Me5)2Fe]+/[(η5-C5Me5)2Fe] was −0.505 V vs. [Cp2Fe]+/[Cp2Fe] in MeCN at 0.1 V s−1 (used for 1SIP–5SIP).DFT experimental and ESI†
Restricted gas-phase DFT calculations were performed using the Amsterdam Density Functional (ADF) suite version 2012.01 with initial models of were derived from the X-ray crystal structures of 1Cl–5Cl, 1Br–5Br, 1I–5I, 1SIP–5SIP and 1H–5H.57–59 The DFT calculations employed a Slater-type orbital (STO) all-electron triple-ζ-plus one polarization function basis set from the ZORA/TZP database of the ADF suite for all atoms. The scalar relativistic (SR) approach was used within the ZORA Hamiltonian for the inclusion of relativistic effects. The calculations employed the local density approximation (LDA) with the correlation potential due to Vosko et al.60 Gradient corrections were performed using the functionals of Becke61 and Perdew.62 Pictorial representations of the MOs were generated using MOLEKEL63 and the orbital compositions were calculated using AOMIX.64Acknowledgements
We thank the Royal Society, EPSRC, ERC, and University of Nottingham for supporting this work.References
- R. D. Theys, M. E. Dudley and M. M. Hossain, Coord. Chem. Rev., 2009, 253, 180 CrossRef CAS PubMed
.
- A search of the Cambridge Strucutral Database v1.17, accessed on 15–01–2015, reveals over six hundred complexes containing a CpFe(CO)2 fragment bonded to a d- or f-block metal.
- M. P. Blake, N. Kaltsoyannis and P. Mountford, Chem. Commun., 2013, 49, 3315 RSC
; B. Oelkers, M. V. Butovskii and R. Kempe, Chem. – Eur. J., 2012, 18, 13566 CrossRef CAS PubMed
; S. T. Liddle and D. P. Mills, Dalton Trans., 2009, 5569 Search PubMed
; S. T. Liddle, Philos. Trans. R. Soc. London, Ser A, 2009, 465, 1673 CrossRef
; P. Beletskaya, A. Z. Voskoboynikov, E. B. Chuklanova, N. I. Kirillova, A. K. Shestakova, I. N. Parshina, A. I. Gusev and G. K.-I. Magomedov, J. Am. Chem. Soc., 1993, 115, 3156 CrossRef
.
- B. M. Gardner, J. McMaster, F. Moro, W. Lewis, A. J. Blake and S. T. Liddle, Chem. – Eur. J., 2011, 17, 6909 CrossRef CAS PubMed
; B. M. Gardner, W. Lewis, A. J. Blake and S. T. Liddle, Inorg. Chem., 2011, 50, 9631 CrossRef PubMed
; D. Patel, D. M. King, B. M. Gardner, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Chem. Commun., 2011, 47, 295 RSC
; S. T. Liddle, D. P. Mills, B. M. Gardner, J. McMaster, C. Jones and W. D. Woodul, Inorg. Chem., 2009, 48, 3520 CrossRef PubMed
; B. M. Gardner, J. McMaster, W. Lewis and S. T. Liddle, Chem. Commun., 2009, 2851 RSC
; S. T. Liddle, J. McMaster, D. P. Mills, A. J. Blake, C. Jones and W. D. Woodul, Angew. Chem., Int. Ed., 2009, 48, 1077 CrossRef PubMed
.
- A. P. Sobaczynski, T. Bauer and R. Kempe, Organometallics, 2013, 32, 1363 CrossRef CAS
.
- B. M. Gardner, D. Patel, A. D. Cornish, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Chem. – Eur. J., 2011, 17, 11266 CrossRef CAS PubMed
.
- M. J. Mays and P. L. Sears, J. Chem. Soc., Dalton Trans., 1973, 1873 RSC
; G. Hata, H. Kondo and A. Miyake, J. Am. Chem. Soc., 1968, 90(9), 2278–2281 CrossRef CAS
; J. E. Barclay, G. J. Leigh, A. Houlton and J. Silver, J. Chem. Soc., Dalton Trans., 1988, 2865–2870 RSC
; S. Pohl, U. Opitz, D. Hasse and W. Saak, Z. Anorg. Allg. Chem., 1995, 621, 1140–1146 CrossRef PubMed
; R. Langer, F. Bönisch, L. Maser, C. Pietzonka, L. Vondung and T. P. Zimmermann, Eur. J. Inorg. Chem., 2015, 141–148 CrossRef PubMed
.
- J. Niemeyer, D. A. Addy, I. Riddlestone, M. Kelly, A. L. Thompson, D. Vidovic and S. Aldridge, Angew. Chem., Int. Ed., 2011, 50, 8908 CrossRef CAS PubMed
; J. A. van Rijn, E. Gouré, M. A. Siegler, A. L. Spek, E. Drent and E. Bouwman, J. Organomet. Chem., 2011, 696, 1899 CrossRef PubMed
; S. G. Davies, J. Organomet. Chem., 1979, 179, C5 CrossRef
.
- E. C. Fitzgerald, A. Ladjarafi, N. J. Brown, D. Collison, K. Costuas, R. Edge, J.-F. Halet, F. Justaud, P. J. Low, H. Meghezzi, T. Roisnel, M. W. Whiteley and C. Lapinte, Organometallics, 2011, 30, 4180 CrossRef CAS
; Y. Tanaka, J. A. Shaw-Taberlet, F. D. R. Justaud, O. Cador, T. Roisnel, M. Akita, J.-R. Hamon and C. Lapinte, Organometallics, 2009, 28, 4656 CrossRef
; L. Medei, L. Orian, O. V. Semeikin, M. G. Peterleitner, N. A. Ustynyuk, S. Santi, C. Durante, A. Ricci and C. Lo Sterzo, Eur. J. Inorg. Chem., 2006, 2582 CrossRef PubMed
; M. I. Bruce, K. Costuas, T. Davin, B. G. Ellis, J.-F. Halet, C. Lapinte, P. J. Low, M. E. Smith, B. W. Skelton, L. Toupet and A. H. White, Organometallics, 2005, 24, 3864 CrossRef
; F. de Montigny, G. Argouarch, K. Costuas, J.-F. Halet, T. Roisnel, L. Toupet and C. Lapinte, Organometallics, 2005, 24, 4558 CrossRef
; N. Le Narvor and C. Lapinte, C. R. l'Académie. Sci., Ser. IIC Chem., 1998, 1, 745 Search PubMed
; T. Weyland, C. Lapinte, G. Frapper, M. J. Calhorda, J.-F. Halet and L. Toupet, Organometallics, 1997, 16, 2024 CrossRef
; F. Coat and C. Lapinte, Organometallics, 1996, 15, 477 CrossRef
; N. Le Narvor and C. Lapinte, Organometallics, 1995, 14, 634 CrossRef
.
- D. Patel, F. Moro, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2011, 50, 10388 CrossRef CAS PubMed
.
- S. El-Tarhuni, M. Ho, M. H. Kawser, S. Shi and M. W. Whiteley, J. Organomet. Chem., 2014, 752, 30 CrossRef CAS PubMed
.
- P. Hamon, L. Toupet, J.-R. Hamon and C. Lapinte, Organometallics, 1996, 15, 10 CrossRef CAS
.
- M. J. Mays and P. L. Sears, J. Chem. Soc., Dalton Trans., 1973, 1873 RSC
.
- C. Roger, P. Hamon, L. Toupet, H. Rabaa, J. Y. Saillard, J. R. Hamon and C. Lapinte, Organometallics, 1991, 10, 1045 CrossRef CAS
; Y.-P. Ou, D. Feng and J.-J. Yuan, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2010, 66, m921 Search PubMed
.
- D. H. Hill, M. Parvez and A. Sen, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1990, 46, 133 CrossRef
.
- As determined by a search of the Cambridge Structural Database v1.17, accessed on the 15-01-2015.
- J.-R. Hamon, P. Hamon, L. Toupet, K. Costuas and J.-Y. Saillard, C. R. Chim., 2002, 5, 89 CrossRef CAS
.
- M. Jimenez-Tenorio, M. C. Puerta and P. Valerga, Organometallics, 1994, 13, 3330 CrossRef CAS
.
- E. B. Lobkovskii, M. Y. Antipin, A. P. Borisov, V. D. Makhaev, K. N. Semenenko and Y. T. Struchkov, Koord. Khim., 1980, 6, 1267 CAS
.
- R. H. Morris, J. F. Sawyer, M. Shiralian and J. Zubkowski, J. Am. Chem. Soc., 1985, 107, 5581 CrossRef CAS
.
- M. Knorr, J. Mueller and U. Schubert, Chem. Ber., 1987, 120, 879 CrossRef CAS PubMed
.
- J. S. Ricci, T. F. Koetzle, M. T. Bautista, T. M. Hofstede, R. H. Morris and J. F. Sawyer, J. Am. Chem. Soc., 1989, 111, 8823 CrossRef CAS
.
- P. B. Hitchcock, M. A. N. D. A. Lemos, M. F. Meidine, J. F. Nixon and A. J. L. Pombeiro, J. Organomet. Chem., 1991, 402, C23 CrossRef CAS
.
- M. F. Meidine, M. A. N. D. A. Lemos, A. J. L. Pombeiro, J. F. Nixon and P. B. Hitchcock, J. Chem. Soc., Dalton Trans., 1998, 3319 RSC
.
- J.-G. Lee, G.-S. Jung and S. W. Lee, Bull. Korean Chem. Soc., 1998, 19, 267 CrossRef CAS
.
- S. S. P. R. Almeida, M. F. C. G. da Silva, J. J. R. Fraustoda Silva and A. J. L. Pombeiro, J. Chem. Soc., Dalton Trans., 1999, 467 RSC
.
- J. G. Lee, B. S. Yoo, N. S. Choi, K. I. Park, S. I. Cho, C. Lee and S. W. Lee, J. Organomet. Chem., 1999, 589, 138 CrossRef CAS
.
- L. M. D. R. S. Martins, J. J. R. Frausto Da Silva, A. J. L. Pombeiro, R. A. Henderson, D. J. Evans, F. Benetollo, G. Bombieri and R. A. Michelin, Inorg. Chim. Acta, 1999, 291, 39 CrossRef CAS
.
- J. Y. Baeg, W. S. Han and S. W. Lee, J. Korean Chem. Soc., 2002, 46, 427 CrossRef CAS
.
- B.-S. Yoo, N.-S. Choi, C. Y. Shim, Y. Son and S. W. Lee, Inorg. Chim. Acta, 2000, 309, 137 CrossRef CAS
.
- J. H. Lee, B. S. Yoo and S. W. Lee, Inorg. Chim. Acta, 2001, 321, 75 CrossRef CAS
.
- S. S. P. R. Almeida, M. F. C. Guedes da Silva, L. B. Jerzykiewicz, P. Sobota and A. J. L. Pombeiro, Inorg. Chim. Acta, 2003, 356, 259 CrossRef CAS
.
- H. V. Nanishankar, M. Nethaji and B. R. Jagirdar, Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2003, 42A, 2332 CAS
.
- M. P. Rabalo, A. P. S. Teixeira, M. H. Garcia, M. F. Minas da Piedade, M. T. Duarte, A. R. Dias, J. Campo, W. Wenseleers and E. Goovaerts, Eur. J. Inorg. Chem., 2006, 2175 CrossRef CAS PubMed
.
- J. G. Cordaro, D. Stein and H. Gruetzmacher, J. Am. Chem. Soc., 2006, 128, 14962 CrossRef CAS PubMed
.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS
.
- G. Boschloo and A. Hagfeldt, Acc. Chem. Res., 2009, 42, 1819 CrossRef CAS PubMed
.
- M. Tilset, I. Fjeldahl, J.-R. Hamon, P. Hamon, L. Toupet, J.-Y. Saillard, K. Costuas and A. Haynes, J. Am. Chem. Soc., 2001, 123, 9984 CrossRef CAS PubMed
.
- F. Paul and C. Lapinte, Coord. Chem. Rev., 1998, 178–180, 431 CrossRef CAS
.
-
N. N. Greenwood and T. C. Gibb, Mössbauer Spectroscopy, Chapman and Hall Ltd, London, 1971 Search PubMed
.
- V. Guillaume, P. Thominot, F. Coat, A. Mari and C. Lapinte, J. Organomet. Chem., 1998, 565, 75 CrossRef CAS
.
- P. Hamon, L. Toupet, J. R. Hamon and C. Lapinte, Organometallics, 1992, 11, 1429 CrossRef CAS
.
-
T. A. Albright, J. K. Burdett and M.-H. Wangbo, Orbital Interactions in Chemistry, John Wiley, New York, 1985 Search PubMed
.
- G. M. Aston, S. Badriya, R. D. Farley, R. W. Grime, S. J. Ledger, F. E. Mabbs, E. J. L. McInnes, H. W. Morris, A. Ricalton, C. C. Rowlands, K. Wagner and M. W. Whiteley, J. Chem. Soc., Dalton Trans., 1999, 4379 RSC
.
- I. M. Bartlett, S. Carlton, N. G. Connelly, D. J. Harding, O. D. Hayward, A. G. Orpen, C. D. Ray and P. H. Reiger, Chem. Commun., 1999, 2403 RSC
.
- S. T. Krueger, R. Poli, A. L. Rheingold and D. L. Staley, Inorg. Chem., 1989, 28, 4599 CrossRef CAS
.
- T. C. Zietlow, M. D. Hopkins and H. B. Gray, J. Am. Chem. Soc., 1986, 108, 8266 CrossRef CAS
.
- T. Hascall, D. Rabinovich, V. J. Murphy, M. D. Beachy, R. A. Friesner and G. Parkin, J. Am. Chem. Soc., 1999, 121, 11402 CrossRef CAS
.
- S. T. Krueger, B. E. Owens and R. Poli, Inorg. Chem., 1990, 29, 2001 CrossRef CAS
.
- C. M. Fendrick, L. D. Schertz, E. A. Mintz, T. J. Marks, T. E. Bitterwolf, P. A. Horine, T. L. Hubler, J. A. Sheldon and D. D. Belin, Inorg. Synth., 1992, 29, 193 CrossRef CAS PubMed
.
- F. X. Kohl and P. Jutzi, J. Organomet. Chem., 1983, 243, 119 CrossRef CAS
.
- H. Schumann, I. Albrecht, J. Loebel, E. Hahn, M. B. Hossain and D. Van der Helm, Organometallics, 1986, 5, 1296 CrossRef CAS
.
-
C. Ting, J.-C. Tsai, Y.-C. Chen, S.-S. Hua, T.-T. Su and Y.-S. Chao, Pat. NoUS6207773B1, 2001, Metallocene catalyst for preparing olefin polymer Search PubMed
.
- M. D. Walter, C. D. Sofield, C. H. Booth and R. A. Andersen, Organometallics, 2009, 28, 2005 CrossRef CAS
.
- D. J. Evans, P. B. Hitchcock, G. J. Leigh, B. K. Nicholson, A. C. Niedwieski, F. S. Nunes and J. F. Soares, Inorg. Chim. Acta, 2001, 319, 147 CrossRef CAS
.
-
Bruker SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, 2001
; G. M. Sheldrick, SHELXTL, version 5, Bruker AXS Inc., Madison, WI Search PubMed
; SHELXTL, version 5, Bruker AXS Inc., Madison, WI, 2001 Search PubMed
.
- G. t. Velde, F. M. Bickelhaupt, S. J. A. v. Gisbergen, C. F. Guerra, E. J. Baerends and S. J. G. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef PubMed
.
- C. F. Guerra, J. G. Snijders, G. t. Velde and E. J. Baerends, Theor. Chem. Acc., 1998, 99, 391 CAS
.
-
ADF2014, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, http://www.scm.com Search PubMed
.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS
.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS
.
- J. P. Perdew, Phys. Rev. B: Condens. Matter, 1986, 33, 8822 CrossRef
.
- S. Portmann and H. P. Lüthi, Chimia, 2000, 54, 766 CAS
.
- S. I. Gorelsky and A. B. P. Lever, J. Organomet. Chem., 2001, 635, 187 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Electrochemical, DFT, and Mössbauer data for 1Cl–5Cl, 1Br–5Br, 1I–5I, 1SIP–5SIP, and 1H–5H, and X-ray data for I–III, 1Cl, 3Cl–5Cl, 1Br, 3Br–5Br, 2I–5I, 1SIP–5SIP, and 1H–5H. CCDC 1049712–1049737. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5dt00704f |
| This journal is © The Royal Society of Chemistry 2015 |