Air-processed organic tandem solar cells on glass: toward competitive operating lifetimes†
Jens
Adams‡
a,
George D.
Spyropoulos‡
*b,
Michael
Salvador
*b,
Ning
Li
b,
Sebastian
Strohm
a,
Luca
Lucera
a,
Stefan
Langner
b,
Florian
Machui
b,
Hong
Zhang
b,
Tayebeh
Ameri
b,
Monika M.
Voigt
ab,
Frederik C.
Krebs
c and
Christoph J.
Brabec
ab
aBavarian Center for Applied Energy Research (ZAE Bayern), Haberstraße 2a, 91058 Erlangen, Germany
bInstitute of Materials for Electronics and Energy Technology (i-MEET), Friedrich-Alexander-University Erlangen-Nuremberg, Martensstraße 7, 91058 Erlangen, Germany. E-mail: michael.salvador@fau.de; George.Spyropoulos@zae-bayern.de
cDepartment of Energy Conversion and Storage, Technical University of Denmark, Frederiksborgvej 399, DK-4000 Roskilde, Denmark
First published on 12th September 2014
Abstract
Photovoltaic devices based on organic semiconductors (OPVs) hold great promise as a cost-effective renewable energy platform because they can be processed from solution and deposited on flexible plastics using roll-to-roll processing. Despite important progress and reported power conversion efficiencies of more than 10% the rather limited stability of this type of devices raises concerns towards future commercialization. The tandem concept allows for both absorbing a broader range of the solar spectrum and reducing thermalization losses. We designed an organic tandem solar cell with an inverted device geometry comprising environmentally stable active and charge-selecting layers. Under continuous white light irradiation, we demonstrate an extrapolated, operating lifetime in excess of one decade. We elucidate that for the current generation of organic tandem cells one critical requirement for long operating lifetimes consists of periodic UV light treatment. These results suggest that new material approaches towards UV-resilient active and interfacial layers may enable efficient organic tandem solar cells with lifetimes competitive with traditional inorganic photovoltaics.
Broader contextLow-cost, thin-film solar cells represent a potentially sustainable pathway towards replacing fossil fuel-based technologies. In this context, organic semiconductor based solar cells (OPVs) are particularly attractive because they can be deposited from solution on plastic substrates, making them compatible with large-scale industry standards. One possible setback when evaluating the commercial potential of organic photovoltaics is related to the limited lifetime that is typically observed for this type of devices. Most device performance reported in the literature is based on photovoltaic devices that have remained in an inert environment from the initial fabrication steps up to the final characterization process. However, it is clear that commercialization will only be possible under the premise of long-term air and UV stable materials. We adopted an innovative combination of device geometry and choice of active as well as charge selecting materials that allowed us to fabricate efficient organic tandem solar cells in air. The tandem OPVs feature stable performance over 2000 h of operation and an extrapolated lifetime in excess of one decade. Furthermore, we show that periodic UV light treatment during operation is an essential requirement for attaining long operating lifetimes. This progress suggests that operating lifetimes closer to what is known from traditional inorganic thin-film photovoltaics are within reach. |
Introduction
Organic photovoltaics (OPVs) bears the potential for highly efficient, low-cost solar energy conversion. The emergence of new device architectures and material concepts led to a fast development in the recent past with reported efficiencies >10%.1,2 Yet, all the advantages of organic materials, which provide OPVs with the well-known versatility of flexible, light weight and transparent devices, are accompanied with challenges when considering the next level of device efficiency towards commercialization. For instance, the photon harvesting efficiency and open circuit voltage (Voc) are affected by the relatively narrow absorption spectrum of the vast majority of organic donor materials and by thermalization losses due to electronic mismatching, respectively.3,4 The tandem solar cell design is one of the most promising concepts to mitigate these limitations.5–8 Theoretical predictions based on already proven device parameters of short circuit current density (Jsc), fill factor (FF) and Voc point to possible power conversion efficiencies (PCEs) of >20% using multi-layer tandem stacks.9 Recently, triple-junction devices with 11.5% and 12% conversion efficiency have been announced by Yang Yang's group10 and by Heliatek,11 respectively, which clearly states the potential of this technology.However, while considerable, world-wide effort has been directed towards increasing the light-to-energy conversion efficiencies, reaching a point where long-term sustainability becomes potentially viable, both the operating lifetimes and reliability of OPV devices have received only limited attention.12 In fact, a common metric for estimating the viability of a PV technology is the levelized cost of energy (LCOE), which evaluates the lifetime cost of an energy system divided by its lifetime energy production.13 The lifetime, therefore, represents a key factor when assessing market competitiveness.
Despite substantial effort to reduce the energy payback time by developing low-cost device fabrication strategies with high PCE outcomes, the requirement for long operating lifetimes requires in-depth investigation. In general terms, a lifetime for OPVs above 20 years, comparable to the lifetimes often reported for inorganic thin film solar cell technologies such as CIGS or amorphous silicon,14,15 would be highly desirable to further increase the competitiveness of this technology. A common statement is that OPVs with 10% PCE and up to 10 years lifetime would need to have very low fabrication costs to compete with the inorganic counterpart.16 With reports on accelerated lifetimes for encapsulated OPVs exceeding 7 years17 and more than one year of outdoor lifetime for solar modules on flexible polymer substrates,18 it is likely that OPVs can reach more than 10 years of operating lifetimes.
There are many aspects that can limit the lifetime of OPVs.19,20 Most organic semiconducting materials are highly reactive in the presence of oxygen, water, high temperatures and light, which can induce thermal and photooxidation reactions as well as local trap formation and structure variations in the active layer.21 In a device, adverse environmental conditions can lead to morphological changes, compositional gradients, and electronic defects at interfaces and electrodes.22,23 Although a systematic investigation of all possible degradation channels is desirable, the effects of oxygen, water and temperature can be considerably minimized through encapsulation. In the best scenario, this would reduce degradation to the effects of photoinduced aging of the active materials, restructuring or degradation at interfaces, and changes in the morphology.24
In the case of single-cell OPVs, prolonged exposure to simulated sunlight has been shown to accelerate photooxidation, trap formation and oligomerization of the fullerene derivative PCBM.24–28 Particularly the case of P3HT/fullerene blends has been well documented and revealed extrapolated lifetimes of about 3–4 years for encapsulated solar cells and more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h under outdoor operation for roll-to-roll coated OPV modules.18,29 For this model system, it has been shown that water, presumably in the presence of oxygen, is likely to be the strongest promoter of performance loss.30,31 Lifetime studies on organic tandem cells are still underrepresented in the literature. First studies have revealed very short lifetimes of about 25 h for continuous irradiation with 1000 W m−2 for tandem modules consisting of 8 cells. However, the focus of those studies was primarily to demonstrate the feasibility of the technology and encapsulation was not optimized.32
000 h under outdoor operation for roll-to-roll coated OPV modules.18,29 For this model system, it has been shown that water, presumably in the presence of oxygen, is likely to be the strongest promoter of performance loss.30,31 Lifetime studies on organic tandem cells are still underrepresented in the literature. First studies have revealed very short lifetimes of about 25 h for continuous irradiation with 1000 W m−2 for tandem modules consisting of 8 cells. However, the focus of those studies was primarily to demonstrate the feasibility of the technology and encapsulation was not optimized.32
Herein, we describe one of the first and longest photo-degradation studies (duty cycle of 100%) of solution processed organic tandem solar cells with concrete measures for improving the device lifetime. We present evidence for the photostability of encapsulated, air-processed organic tandem solar cells with a loss in PCE of only ≈11% within the first 2000 h of continuous irradiation under ambient conditions when exposed to 1000 W m−2 of incident white light. When extrapolating to 80% of the initial PCE, which is a common evaluation metric,33 we find an accelerated lifetime of >10 years under open-circuit conditions, which represents the longest reported lifetime for organic tandem cells to date. We elucidate the need for UV light soaking for increasing the lifetime of current generation ZnO-based organic tandem solar cells and sub-cells by recording the photostability of photovoltaic parameters after initial UV light treatment.
Experimental methods
Materials
P3HT was purchased from Merck. pDPP5T-2 (batch no. GKS1-001) was provided by BASF. Al-doped ZnO (AZO) was synthesized according to previous reports.34 A ZnO nanoparticle dispersion in acetone, used in the intermediate layer, was synthesized at the Technical University of Denmark (DTU).35 PC[60]BM (99.5%) and PC[70]BM (99%) were obtained from Solenne BV. Ba(OH)2 was purchased from Sigma-Aldrich. For encapsulation, we used the adhesive Katiobond LP655 (DELO GmbH & Co KGaA), an UV curable epoxy resin with a specified water vapor transmission rate (WVTR) of 6.1 g per m2 per day.Single and tandem device fabrication
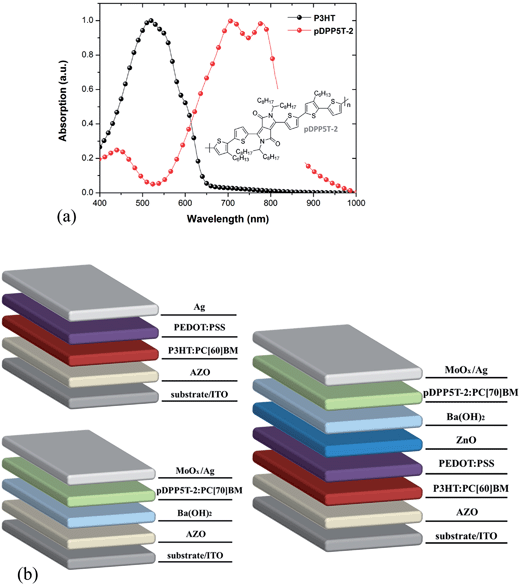
All photovoltaic devices were fabricated by doctor blading under ambient conditions using an inverted device structure (Fig. 1a). Laser-patterned ITO coated glass substrates (area of 2.5 × 2.5 cm2) were successively cleaned in an ultrasonic cleaner using acetone and isopropanol. After drying, the substrates were coated with a ≈ 40 nm thick AZO layer and annealed for 10 minutes on a hot plate at 140 °C. For the tandem solar cells, a chlorobenzene based solution of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC[60]BM (1
PC[60]BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt%, 32 mg mL−1 in total) was coated on top of the AZO layer to form a ≈ 130 nm thick bottom active layer. Subsequently, the intermediate layer was deposited by successively blading a ≈ 40 nm thick PEDOT HIL 3.3 and ≈30 nm thick ZnO layer. These layers were dried at 70 °C for 5 min in air. We modified the ZnO layer by coating a very thin (≈20 nm) Ba(OH)2 film on top (7 mg mL−1 in 2-methoxyethanol). Afterwards, we deposited an ≈80 nm thick layer of pDPP5T-2–PC[70]BM (1
1 wt%, 32 mg mL−1 in total) was coated on top of the AZO layer to form a ≈ 130 nm thick bottom active layer. Subsequently, the intermediate layer was deposited by successively blading a ≈ 40 nm thick PEDOT HIL 3.3 and ≈30 nm thick ZnO layer. These layers were dried at 70 °C for 5 min in air. We modified the ZnO layer by coating a very thin (≈20 nm) Ba(OH)2 film on top (7 mg mL−1 in 2-methoxyethanol). Afterwards, we deposited an ≈80 nm thick layer of pDPP5T-2–PC[70]BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 wt%, dissolved in a solvent consisting of 90% chloroform and 10% dichlorobenzene with a total concentration of 24 mg mL−1) as the top active layer. Finally, a 10 nm MoOx layer and 100 nm Ag layer were evaporated to form the top electrode. The solar cells had an active area of 10.4 mm2. The active layer thicknesses in the tandem structure were tuned to give satisfactory current balancing between the sub-cells. The single solar cell devices were prepared in a way equivalent to the tandem solar cell device fabrication protocol described above. For intimate comparison, the thicknesses of the active layers were chosen to be the same as in the tandem structure, i.e., ≈130 nm for P3HT–PCBM and ≈80 nm for pDPP5T-2–PC[70]BM single cell devices. Optical simulation results for the tandem solar cell geometry based on the transfer matrix approach are depicted in the ESI (Fig. S1†).
2 wt%, dissolved in a solvent consisting of 90% chloroform and 10% dichlorobenzene with a total concentration of 24 mg mL−1) as the top active layer. Finally, a 10 nm MoOx layer and 100 nm Ag layer were evaporated to form the top electrode. The solar cells had an active area of 10.4 mm2. The active layer thicknesses in the tandem structure were tuned to give satisfactory current balancing between the sub-cells. The single solar cell devices were prepared in a way equivalent to the tandem solar cell device fabrication protocol described above. For intimate comparison, the thicknesses of the active layers were chosen to be the same as in the tandem structure, i.e., ≈130 nm for P3HT–PCBM and ≈80 nm for pDPP5T-2–PC[70]BM single cell devices. Optical simulation results for the tandem solar cell geometry based on the transfer matrix approach are depicted in the ESI (Fig. S1†).
Device encapsulation
A dispenser robot I&J 4100-LF from I&J Fisnar Inc. was used to distribute the adhesive Katiobond LP655 from DELO GmbH & Co KGaA on top of the completed devices, which were overcoated with a 1.5 mm glass barrier. The epoxy was cured for one minute inside a UVACUBE 100 from Hönle AG equipped with an iron doped lamp.Characterization
Current–voltage measurements of the solar cells were performed in ambient atmosphere at room temperature using the source measure unit 2900 A from Agilent and a light emitting diode (LED) array featuring an AM1.5G spectrum at 1000 W m−2 (FUTURELED GmbH, Berlin, Germany). For photoaging of the solar cells, we used high power LEDs with a spectral emission between 400 nm and 750 nm and an intensity of ≈1000 W m−2. The intensity of the high power LEDs was set by adjusting the distance between the solar cell and the light source to give the same photocurrent as first measured with the solar simulator. The intensity of the solar simulator was calibrated using a standard silicon photodiode certificated by ISE Fraunhofer. UV light soaking was carried out using a UV exposure unit from isel automation (λc = 365 nm, 32 W).Results and discussion
The solar cells for this study are based on an inverted tandem structure comprising sub-cells of the blends poly(3-hexylthiophene)–phenyl-C60-butyric acid methyl ester (P3HT–PC[60]BM) and diketopyrrolopyrrole-quinquethiophene–phenyl-C70-butyric acid methyl ester (pDPP5T-2–PC[70]BM) as active layers (Fig. 1a). We used the model P3HT–PC[60]BM blend as the active layer of the bottom sub-cell because of its known stability17 and its absorption being complementary to that of the low bandgap polymer pDPP5T-2. We used zinc oxide (ZnO) in combination with poly(3,4-ethylenedioxythiophene) (PEDOT) as the recombination layer and aluminum-doped ZnO (AZO) as electron extraction layer.34 The interface between ZnO and pDPP5T-2–PCBM was modified by coating a thin barium hydroxide (Ba(OH)2) layer on top of ZnO to enhance the photovoltaic performance.36,37 For a better understanding, we also fabricated and studied in parallel inverted single OPV cells based on the polymer blends used in the tandem structure. A detailed description of the device geometry of the three types of solar cells is depicted in Fig. 1b. Each single cell was made in the same way as the corresponding sub-cell of the tandem device for intimate comparison. For obtaining representative variations of device performance vs. time we prepared and encapsulated five devices of each solar cell type. We used a glass-on-glass encapsulation geometry to ensure reliable and reproducible protection as well as for minimizing extrinsic effects induced by water and oxygen.17 We refer the reader to the Experimental methods section for details regarding device fabrication and encapsulation. The quality of the encapsulation was probed using electroluminescence lock-in (ELLI) imaging before and after the photoaging process (see ESI Fig. S2†).The tandem cells and the corresponding single sub-cells were aged under continuous white light irradiation without UV component at ≈1000 W m−2 (ESI Fig. S3†). The absence of UV light during photodegradation was essential for studying the effect of UV light treatment on the lifetime of the devices. The cells were maintained under open circuit during photodegradation. The initial device performance of the tandem and single cells under AM1.5 conditions was 4.4% ± 0.2% (tandem), 2.8% ± 0.1% (P3HT), and 4.1% ± 0.2% (pDPP5T-2), respectively (see ESI Fig. S4 and S5;† the errors represents the standard deviation).
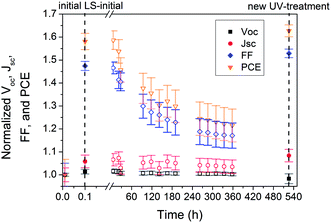
First, to eliminate the double diode effect that is intrinsic to devices incorporating ZnO and/or AZO38,39 (see ESI Fig. S6†) we applied a single light soaking (LS) treatment by irradiating the solar cells with UV light (365 nm) for the time of 10 s (prior to starting J–V characterization) and studied the subsequent decay of the UV light soaking state under continuous photoaging using white light. Fig. 2 shows two consecutive 60 h photodegradation cycles for elucidating the effect of the initial light-soaking step. These cycles lie within the burn-in period of photodegradation, which typically follows an exponential decay of the initial device efficiency (see also Fig. 4).17 Within the first cycle of continuous illumination, the PCE of the tandem and the P3HT based single cells decreased to 60% and 50% of the initial value, respectively. In both cases, the loss in PCE is mainly dominated by losses in Jsc (≈ 15–20%) and FF (25–40%), which can be most likely related to a reduction in charge carrier extraction and the accumulation of carriers in the device, respectively.40,41 The latter leads to the formation of an S-shape in the J–V-characteristics (see ESI Fig. S6†).39 The Voc, on the other hand, remains fairly stable. Interestingly, under the same conditions the solar cell parameters and the overall PCE of the pDPP5T-2 based single cells remained almost intact throughout the same period of time. Note that after 60 h we repeated the UV treatment. Notably, all solar cells were almost fully restored and followed the same degradation behavior as in the first cycle. This behavior suggests that the burn-in period that is typically observed in the first hours of degradation is triggered by a reversible reaction in these types of devices. A plausible explanation would be that the conductivity of ZnO degrades with time due to the presence of traces of oxygen, while UV treatment can release oxygen and restore its electronic properties.42,43
 | ||
| Fig. 2 Lifetime of the UV light soaking state under continuous photoaging. The plots show the device parameters Voc, Isc, FF and PCE over time for the different types of single and tandem solar cells (cf.Fig. 1) upon initial UV light soaking (365 nm, 10 s) and under continuous photoaging using white light (400–750 nm) without UV component at 1000 W m−2. UV light treatment was repeated after 60 hours. Each data point represents the average values of 5 solar cells and is normalized to the initial value at t = 0 hours. | ||
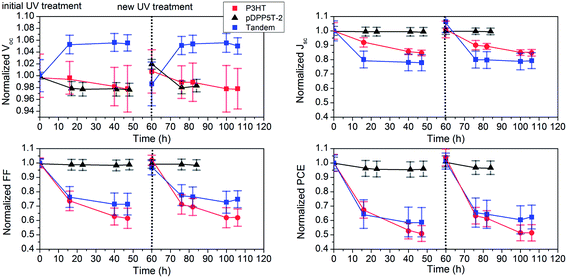
To gain additional insight into the effect of the UV light-soaking process, we further looked into the transient behavior of the UV treatment in the dark. Fig. 3 shows the periodically measured change of the photovoltaic parameters for tandem cells upon a single UV light-soaking step. In this case, the cells were stored in the dark between J–V characterization. Upon UV light soaking, the FF increases dramatically (≈45%) while Jsc increases by about 5% and Voc barely changes. It is well documented in the literature that UV radiation can improve the electronic properties (conductivity) of the ZnO layer as well as the contact at the ZnO interface.39,42,43 This is most likely the reason for the J–V characteristics translating from a double-diode type behavior (S-shape) to a diode behavior with high FF (see ESI Fig. S6†).
The long-term behavior of UV treatment is much less documented. Fig. 3 shows that the light soaking state remains constant for about 10 h after which the photovoltaic performance decays sharply. Moreover, Fig. 3 reveals that the light soaking state features a half-time of about 200 h and is, therefore, expected to contribute decisively to the burn-in period of OPVs containing ZnO.
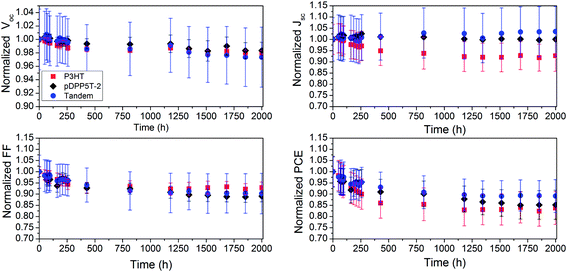
From the previous result, we can infer the importance of UV light exposure during continuous 1 sun irradiation. We, therefore, designed a long-term photoaging test, in which the solar cells were exposed to UV light for 10 s prior to each J–V measurement. Fig. 4 shows the average long-term temporal evolution of Voc, Jsc, FF, and PCE for the single and tandem cells under continuous white light illumination with intermittent UV treatment. The burn-in period extends to ≈800 h, after which the decay of the PCE follows a close to linear trend (Fig. 4d). Remarkably, in the long-term measurements the tandem cells showed the most stable behavior by losing only 11% of the initial value after 2000 h. The PCE of P3HT and pDPP5T-2 based single cells followed a similar decay with losses of 16% and 15%, respectively (Fig. 5). Overall, the loss in PCE is mainly determined by a loss in FF (5–11%) and a modest decay in Jsc (0–8%). Impressively, in the case of the tandem and pDPP5T-2-based single cells we observe almost no current losses throughout 2000 h of light exposure. To put our results into perspective, PCE drops in the range of ≈10–20% for P3HT single cells and ≈25% for PCDTBT-based single cell devices under 1 sun exposure and within similar periods of time have been reported in the past.17,44,45
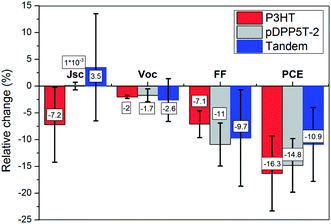 | ||
| Fig. 5 Representation of the relative change of device performance after 2000 h of continuous white light illumination. The relative change is calculated based on the performance of the single and tandem devices shown in Fig. 4. The tandem cells showed better overall device stability. | ||
We imagine that the reasons for the enhanced long-term stability of our solar cells are manifold. Specifically, we used an inverted device structure, thereby eliminating reactive metal interfaces.46 Additionally, we replaced the widely used but moisture sensitive and reactive PEDOT–PSS with MoOx as the top buffer layer. The benefit of using the chemically more inert MoOx for better device stability has been shown before.47,48 Moreover, we chose to modify the ETL/pDPP5T-2 (ETL: electron transporting layer) interface with a hole blocking Ba(OH)2 layer in the case of the more stable tandem and pDPP5T-2 single cells. Barium and Ba(OH)2 interlayers have been proven to reduce exciton quenching and trap induced recombination at cathode interfaces as well as improve the overall device efficiency in the case of OLEDs and OPVs.36,37,49 Based on our findings, we hypothesize that Ba(OH)2 could also contribute to stabilizing the ETL/polymer interface by reducing electronic trap formation and oxygen adsorption. Indeed, comparison of the temporal evolution of photovoltaic device performance in the case of P3HT–PCBM with and without Ba(OH)2 suggests that Ba(OH)2 may increase the operating lifetime (ESI Fig. S8 and S9†). A more elaborate mechanistic study in this direction is currently underway. Furthermore, periodic UV light soaking during prolonged operation is expected to desorb oxygen trapped at the surface of ZnO and AZO. This step is likely to prevent significant conductivity losses of the ETLs ZnO and AZO, contributing to a larger FF throughout the lifetime measurements.42,50
For an estimation of the lifetime of the tandem solar cells, we applied a linear regression to the slowly, linearly decreasing PCE data points and extended this line up to 80% of the initial value (ESI Fig. S7†). In doing so, an extrapolated operating lifetime of ≈27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h can be extracted. Considering an average 1500 hours of sunshine per year in central Europe, this represents, under the current conditions, a best case lifetime of ≈18 years. We further derived a more conservative lifetime for our cells by accounting for the error bars of our measurement, which still resulted in a lifetime of ≈8 years (ESI Fig. S7†). It is important to note that the presented operating lifetime was extrapolated from cells, which were aged under open circuit and under indoor conditions using a LED based solar simulator that does not emit radiation in the 180–400 nm wavelength range. We recognize that the absence of UV light, which has been shown to accelerate degradation through bond scission and free radical formation in OPV semiconducting polymers,20 may artificially increase the lifetime of our cells. Furthermore, for outdoor conditions in the field, there are influences from other sources such as natural thermal cycling, shading, and humidity cycling, which need to be taken into account.
000 h can be extracted. Considering an average 1500 hours of sunshine per year in central Europe, this represents, under the current conditions, a best case lifetime of ≈18 years. We further derived a more conservative lifetime for our cells by accounting for the error bars of our measurement, which still resulted in a lifetime of ≈8 years (ESI Fig. S7†). It is important to note that the presented operating lifetime was extrapolated from cells, which were aged under open circuit and under indoor conditions using a LED based solar simulator that does not emit radiation in the 180–400 nm wavelength range. We recognize that the absence of UV light, which has been shown to accelerate degradation through bond scission and free radical formation in OPV semiconducting polymers,20 may artificially increase the lifetime of our cells. Furthermore, for outdoor conditions in the field, there are influences from other sources such as natural thermal cycling, shading, and humidity cycling, which need to be taken into account.
Given that we used a glass-on-glass encapsulation architecture and an evaporated top electrode, the lifetime presented here certainly reflects an upper bound for the lifetime of this type of organic tandem solar cells. However, if high quality packaging is used, including UV filters, combined with better interface materials, e.g., towards blue light soaking, similar lifetimes are perhaps not impossible but need to be documented by thorough outdoor studies.
Conclusion
In summary, we demonstrated an organic tandem cell with a PCE loss of only 11% within the first 2000 h of operation. The stable tandem operation was possible by choosing active layer materials that can be processed in air and by adopting an inverted device geometry, in which we used MoOx as a replacement for PEDOT and Ba(OH)2 as hole blocking layer at the ETL/blend interface. Moreover, we confirmed that the well-characterized importance of UV light treatment for devices based on ZnO is also an essential requirement for attaining long-term device stability, which requires periodic light soaking by UV-photons. This procedure mainly prevents the formation of S-shaped J–V performance.While the current generation of organic tandem devices does still require some UV light soaking, we anticipate that the final OPV product will not rely on UV light treatment. As such, future studies should primarily foster materials development of new absorbers with enhanced photo and structural stability as well as alternative electron transport layers that are not subject to the requirement of photodoping.51–54 Moreover, fully solution processed tandem OPVs on flexible plastics with state-of-the-art encapsulation need to be demonstrated for certifying market readiness.
Assuming that the slow degradation rate observed in this work can be further improved, organic tandem solar cells with operating lifetimes comparable to traditional PV are thought possible in the forthcoming future.
Author contributions
M. M. V. and C. J. B. conceived of the experiments. G. D. S., N. L., L. L., T. A. fabricated and optimized the single and tandem solar cell geometry and verified its reproducibility. H. Z. developed the Ba(OH)2 interface layer. J. A. and S. S. measured the photovoltaic performance while photoaging the devices and investigated the UV light soaking behavior. S. L. and F. M. designed and optimized the encapsulation procedure. F. C. K. prepared the ZnO formulation. M. S., J. A. and G. D. S. analyzed the data and wrote the manuscript. All authors reviewed the final manuscript.Acknowledgements
The authors gratefully acknowledge the support of the European commission as part of the Framework 7 ICT 2009 collaborative projects ROTROT (grant no. 288565) and X10D (grant no. 287818). M. S. acknowledges primary support from a fellowship by the Portuguese Fundação para a Ciência e a Tecnologia (SFRH/BPD/71816/2010).Notes and references
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C. C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446–1455 CrossRef PubMed.
- R. A. J. Janssen and J. Nelson, Adv. Mater., 2013, 25, 1847–1858 CrossRef CAS PubMed.
- T. Kirchartz, K. Taretto and U. Rau, J. Phys. Chem. C, 2009, 113, 17958–17966 CAS.
- T. Ameri, G. Dennler, C. Lungenschmied and C. J. Brabec, Energy Environ. Sci., 2009, 2, 347–363 CAS.
- T. Ameri, N. Li and C. J. Brabec, Energy Environ. Sci., 2013, 6, 2390–2413 CAS.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T.-Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222–225 CrossRef CAS PubMed.
- G. D. Spyropoulos, P. Kubis, N. Li, D. Baran, L. Lucera, M. Salvador, T. Ameri, M. Voigt, F. C. Krebs and C. J. Brabec, Energy Environ. Sci., 2014 10.1039/c4ee02003k.
- N. Li, D. Baran, G. D. Spyropoulos, H. Zhang, S. Berny, M. Turbiez, T. Ameri, F. C. Krebs and C. J. Brabec, Adv. Energy Mater., 2014, 4, 1400084 Search PubMed.
- C.-C. Chen, W.-H. Chang, K. Yoshimura, K. Ohya, J. You, J. Gao, Z. Hong and Y. Yang, Adv. Mater., 2014, 26, 5670–5677 CrossRef CAS PubMed.
- Heliatek consolidates its technology leadership by establishing a new world record for organic solar technology with a cell efficiency of 12%, Heliatek GmbH, 2013.
- C. J. Brabec, Sol. Energy Mater. Sol. Cells, 2004, 83, 273–292 CrossRef CAS PubMed.
- S. B. Darling, F. You, T. Veselka and A. Velosa, Energy Environ. Sci., 2011, 4, 3133–3139 Search PubMed.
- M. A. Green, P. A. Basore, N. Chang, D. Clugston, R. Egan, R. Evans, D. Hogg, S. Jarnason, M. Keevers, P. Lasswell, J. O'Sullivan, U. Schubert, A. Turner, S. R. Wenham and T. Young, Sol. Energy, 2004, 77, 857–863 CrossRef CAS PubMed.
- T. Ott, T. Walter, D. Hariskos, O. Kiowski and R. Schaffler, IEEE Journal of Photovoltaics, 2013, 3, 514–519 CrossRef.
- S. B. Darling and F. You, RSC Adv., 2013, 3, 17633–17648 RSC.
- C. H. Peters, I. T. Sachs-Quintana, J. P. Kastrop, S. Beaupré, M. Leclerc and M. D. McGehee, Adv. Energy Mater., 2011, 1, 491–494 CrossRef CAS.
- J. A. Hauch, P. Schilinsky, S. A. Choulis, R. Childers, M. Biele and C. J. Brabec, Sol. Energy Mater. Sol. Cells, 2008, 92, 727–731 CrossRef CAS PubMed.
- A. Aguirre, S. C. J. Meskers, R. A. J. Janssen and H. J. Egelhaaf, Org. Electron., 2011, 12, 1657–1662 CrossRef CAS PubMed.
- J. U. Lee, J. W. Jung, J. W. Jo and W. H. Jo, J. Mater. Chem., 2012, 22, 24265–24283 RSC.
- G. Shao, G. E. Rayermann, E. M. Smith and D. S. Ginger, J. Phys. Chem. B, 2012, 117, 4654–4660 CrossRef PubMed.
- N. Grossiord, J. M. Kroon, R. Andriessen and P. W. M. Blom, Org. Electron., 2012, 13, 432–456 CrossRef CAS PubMed.
- A. Seemann, T. Sauermann, C. Lungenschmied, O. Armbruster, S. Bauer, H. J. Egelhaaf and J. Hauch, Sol. Energy, 2011, 85, 1238–1249 CrossRef CAS PubMed.
- M. O. Reese, A. M. Nardes, B. L. Rupert, R. E. Larsen, D. C. Olson, M. T. Lloyd, S. E. Shaheen, D. S. Ginley, G. Rumbles and N. Kopidakis, Adv. Funct. Mater., 2010, 20, 3476–3483 CrossRef CAS.
- N. Sai, K. Leung, J. Zador and G. Henkelman, Phys. Chem. Chem. Phys., 2014, 16, 8092–8099 RSC.
- K. Kawano and C. Adachi, Adv. Funct. Mater., 2009, 19, 3934–3940 CrossRef CAS.
- A. Distler, T. Sauermann, H.-J. Egelhaaf, S. Rodman, D. Waller, K.-S. Cheon, M. Lee and D. M. Guldi, Adv. Energy Mater., 2014, 4, 1370018 Search PubMed.
- T. Heumueller, W. R. Mateker, I. T. Sachs-Quintana, K. Vandewal, J. A. Bartelt, T. M. Burke, T. Ameri, C. J. Brabec and M. D. McGehee, Energy Environ. Sci., 2014, 7, 2974–2980 CAS.
- S. A. Gevorgyan, M. V. Madsen, H. F. Dam, M. Jørgensen, C. J. Fell, K. F. Anderson, B. C. Duck, A. Mescheloff, E. A. Katz, A. Elschner, R. Roesch, H. Hoppe, M. Hermenau, M. Riede and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2013, 116, 187–196 CrossRef CAS PubMed.
- M. P. Nikiforov, J. Strzalka and S. B. Darling, Sol. Energy Mater. Sol. Cells, 2013, 111, 36–42 CrossRef PubMed.
- H. Hintz, H.-J. Egelhaaf, L. Lüer, J. Hauch, H. Peisert and T. Chassé, Chem. Mater., 2011, 23, 145–154 CrossRef CAS.
- T. R. Andersen, H. F. Dam, M. Hosel, M. Helgesen, J. E. Carle, T. T. Larsen-Olsen, S. A. Gevorgyan, J. W. Andreasen, J. Adams, N. Li, F. Machui, G. D. Spyropoulos, T. Ameri, N. Lemaitre, M. Legros, A. Scheel, D. Gaiser, K. Kreul, S. Berny, O. R. Lozman, S. Nordman, M. Valimaki, M. Vilkman, R. R. Sondergaard, M. Jorgensen, C. J. Brabec and F. C. Krebs, Energy Environ. Sci., 2014, 7, 2925–2933 CAS.
- M. O. Reese, S. A. Gevorgyan, M. Jørgensen, E. Bundgaard, S. R. Kurtz, D. S. Ginley, D. C. Olson, M. T. Lloyd, P. Morvillo, E. A. Katz, A. Elschner, O. Haillant, T. R. Currier, V. Shrotriya, M. Hermenau, M. Riede, K. R. Kirov, G. Trimmel, T. Rath, O. Inganäs, F. Zhang, M. Andersson, K. Tvingstedt, M. Lira-Cantu, D. Laird, C. McGuiness, S. Gowrisanker, M. Pannone, M. Xiao, J. Hauch, R. Steim, D. M. DeLongchamp, R. Rösch, H. Hoppe, N. Espinosa, A. Urbina, G. Yaman-Uzunoglu, J.-B. Bonekamp, A. J. J. M. van Breemen, C. Girotto, E. Voroshazi and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2011, 95, 1253–1267 CrossRef CAS PubMed.
- T. Stubhan, H. Oh, L. Pinna, J. Krantz, I. Litzov and C. J. Brabec, Org. Electron., 2011, 12, 1539–1543 CrossRef CAS PubMed.
- F. C. Krebs, Org. Electron., 2009, 10, 761–768 CrossRef CAS PubMed.
- L. P. Lu, D. Kabra and R. H. Friend, Adv. Funct. Mater., 2012, 22, 4165–4171 CrossRef CAS.
- V. Gupta, A. K. K. Kyaw, D. H. Wang, S. Chand, G. C. Bazan and A. J. Heeger, Sci. Rep., 2013, 3, 1965 Search PubMed.
- J. Gilot, M. M. Wienk and R. A. J. Janssen, Appl. Phys. Lett., 2007, 90, 143512 CrossRef PubMed.
- M. R. Lilliedal, A. J. Medford, M. V. Madsen, K. Norrman and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2010, 94, 2018–2031 CrossRef CAS PubMed.
- B. Y. Finck and B. J. Schwartz, Appl. Phys. Lett., 2013, 103, 053306 CrossRef PubMed.
- A. Wagenpfahl, D. Rauh, M. Binder, C. Deibel and V. Dyakonov, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 115306 CrossRef.
- F. Verbakel, S. C. J. Meskers and R. A. J. Janssen, J. Appl. Phys., 2007, 102, 083701 CrossRef PubMed.
- A. Manor, E. A. Katz, T. Tromholt and F. C. Krebs, Adv. Energy Mater., 2011, 1, 836–843 CrossRef CAS.
- M. Jørgensen, K. Norrman and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2008, 92, 686–714 CrossRef PubMed.
- E. Voroshazi, B. Verreet, T. Aernouts and P. Heremans, Sol. Energy Mater. Sol. Cells, 2011, 95, 1303–1307 CrossRef CAS PubMed.
- S. K. Hau, H.-L. Yip, N. S. Baek, J. Zou, K. O'Malley and A. K.-Y. Jen, Appl. Phys. Lett., 2008, 92, 253301 CrossRef PubMed.
- Y. Sun, C. J. Takacs, S. R. Cowan, J. H. Seo, X. Gong, A. Roy and A. J. Heeger, Adv. Mater., 2011, 23, 2226–2230 CrossRef CAS PubMed.
- Yu-C. Tseng, A. U. Mane, J. W. Elam and S. B. Darling, Sol. Energy Mater. Sol. Cells, 2012, 99, 235–239 CrossRef CAS PubMed.
- H. Zhang, T. Stubhan, N. Li, M. Turbiez, G. J. Matt, T. Ameri and C. J. Brabec, J. Mater. Chem. A , 2014 10.1039/c4ta03421j.
- C. H. Peters, I. T. Sachs-Quintana, W. R. Mateker, T. Heumueller, J. Rivnay, R. Noriega, Z. M. Beiley, E. T. Hoke, A. Salleo and M. D. McGehee, Adv. Mater., 2012, 24, 663–668 CrossRef CAS PubMed.
- B. Paci, A. Generosi, V. R. Albertini, G. D. Spyropoulos, E. Stratakis and E. Kymakis, Nanoscale, 2012, 4, 7452–7459 RSC.
- B. Paci, G. D. Spyropoulos, A. Generosi, D. Bailo, V. R. Albertini, E. Stratakis and E. Kymakis, Adv. Funct. Mater., 2011, 21, 3573–3582 CrossRef CAS.
- H.-L. Yip and A. K. Y. Jen, Energy Environ. Sci., 2012, 5, 5994–6011 CAS.
- S. Trost, K. Zilberberg, A. Behrendt, A. Polywka, P. Görrn, P. Reckers, J. Maibach, T. Mayer and T. Riedl, Adv. Energy Mater., 2013, 3, 1437–1444 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ee02582b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |