Correlating the structure and in vitro digestion viscosities of different pectin fibers to in vivo human satiety
Kirstyn
Logan
a,
Amanda J.
Wright
b and
H. Douglas
Goff
*a
aDepartment of Food Science, University of Guelph, Guelph, ON, Canada N1G 2W. E-mail: dgoff@uoguelph.ca
bDepartment of Human Health and Nutritional Sciences, University of Guelph, Guelph, ON, Canada N1G 2W1
First published on 13th October 2014
Abstract
The effects of a simulated in vitro digestion on the viscosity of orange juice with added high methoxyl (HM), low methoxyl (LM), and low methoxyl amidated (LMA) pectins were examined in conjunction with a human satiety study with healthy men (n = 10) and women (n = 15). Orange juice solutions were formulated to be either low (0.039 ± 0.007 Pa s) or high viscosity (0.14 ± 0.035 Pa s). The apparent viscosities after an in vitro digestion simulating the gastric and small intestinal phases in the presence of hydrolytic enzymes and bile salts were recorded at 10 and 50 s−1. The viscosity induced by LM pectin increased considerably after the gastric phase whereas samples with all pectin types showed considerable reductions in viscosity, compared to initial apparent viscosity, after the small intestinal phase. For satiety testing, the orange juice solutions were consumed with a standardized breakfast meal after a 12 h overnight fast. Self-reported visual analogue scale (VAS) measurements of Hunger, Fullness, Satisfaction and Prospective Food Intake were obtained at fasting, after consumption of the breakfast and for the subsequent 3 h. The LM low and high viscosity pectin beverages were associated with the greatest effects on subjective ratings of satiety. The HM low and high viscosity pectin beverages had lower but significant effects on satiety, while LMA pectin had no effect. There was not a strong correlation between apparent viscosity of in vitro digested beverages and in vivo satiety scores. Thus, in this study, fiber-induced satiety could not be fully explained by digestate viscosity alone although gastric-phase viscosity may have played a significant role.
1 Introduction
Obesity is a global epidemic that increases the risk of developing many life-threatening diseases such as cardiovascular disease, hypertension, type II diabetes, and some types of cancer.1 The most recent definition of dietary fiber given by the Codex Alimentarius Commission states that dietary fibers are “carbohydrate polymers with 10 or more monomeric units which are not hydrolyzed by the endogenous enzymes in the small intestine of humans”.2 Epidemiological studies have indicated an inverse relationship between dietary fiber consumption and body weight.3–5 Despite the strong evidence of numerous health benefits,6 regular consumption of dietary fiber in North America is low7 and thus the inclusion of more fiber into diets is justified. Hence, the food industry has begun to add functional fibers into existing food products, with one potential benefit being that this could help to address the rising incidence of overweight and obesity through enhanced satiety.Numerous scientific reviews have evaluated the ability of different dietary fibers to increase satiety and it is evident that not all fibers are equally satiating.8–11 Previous research has shown that soluble, viscous and/or gel forming fibers are more capable of inducing satiety and reducing subsequent energy intake through various mechanisms related to increases in the viscosity of digesta. An increase in the viscosity of the food matrix could prolong mastication, increase stomach distention and delay nutrient absorption.12 The viscosity induced within the gastrointestinal tract is the result of fiber structure, concentration and chemical composition.13,14 Importantly, in many studies, the physicochemical properties such as the viscosity of the food matrix or the viscosity of the digested food matrix developed along the gastrointestinal tract have not been adequately quantified.15 Analyzing the physicochemical properties of soluble dietary fibers under in vitro human gastrointestinal conditions offers the opportunity to rapidly and economically predict these properties when ingested by humans. However, establishing correlations between in vitro viscosity and in vivo satiety is essential to validate the results and support the development of foods with credible claims related to physiological function.
Pectin is a type of soluble fiber available in several forms. Depending on processing, the structure of the pectin molecule can have different ratios of methyl ester groups and/or amide groups, imparting different viscous or gelling properties. Previous literature relating pectin and appetite has yielded inconsistent results.10,11,16–21 In previous satiety studies, there has been a wide range of fiber doses, food matrices, lengths of study, viscosity comparisons and fiber analogues used, making comparisons and conclusions difficult. Therefore, the primary aim of the present study was to investigate the effect of different types of soluble, viscous pectin on human appetite sensations. The pectin types selected were high methoxyl (HM) pectin with a degree of esterification (DE) of 72%, low methoxyl (LM) pectin with a DE of 34% and low methoxyl amidated (LMA) pectin with a DE of 33% and a degree of amidation (DA) of 18%. Each of these is known to induce varying viscosity and/or gelation effects in solution.22 The different pectin types were hydrated in orange juice at concentrations designed to produce equal viscosities at two levels, low and high. Viscosities attained during an in vitro gastrointestinal digestion were evaluated at two physiologically-relevant shear rates (10 and 50 s−1) for the appropriate human digestive stages, which should cover the physiological range of interest to satiety notably the gastric and small intestinal phases, and correlated to appetite sensations to determine if there was a relationship between viscosity at various stages of digestion and satiety parameters.
2 Materials and methods
2.1 Materials
HM pectin DE 72% was sourced from CEAMSA (Vigo, Spain) and LM pectin DE 34% and LMA pectin DE 33% and DA 18% were sourced from Herbstreith and Fox (Neuenbürg, Germany). Tropicana Orange Juice (No Pulp) was purchased from a local grocery store. Simulated gastric fluid (Ricca Chemical Company, Arlington, Texas), purified pepsin (Fisher Scientific, Fair Lawn, New Jersey, USA), pancreatin U.S.P. (MP Biomedicals, Solon, Ohio, USA), bile salts (sodium salts of glycocholic and taurocholic acids from Ox bile, Ox Gall Powder), 2 N hydrochloric acid solution, sodium phosphate monobasic monohydrate, sodium phosphate dibasic anhydrous, and calcium chloride anhydrous were purchased from Fisher Scientific, Fair Lawn, New Jersey, USA, and were used to create the fluids in the simulated gastrointestinal digestion.2.2 Treatment solution preparation
Six pectin treatment and two control solutions were formulated and tested using both in vitro and in vivo tests. The treatments consisted of each of the three types of pectin at different concentrations to achieve high viscosity (HV) and low viscosity (LV) solutions, each at equal viscosities (Table 1). The apparent viscosities for the HV and LV pectin fortified beverages were matched to evaluate the effects of initial viscosity and to control for sensory cues during consumption. The viscosities of HM pectin at the LV concentration of 1% (w/w), providing 5 g of HM pectin per treatment, and HV concentration of 2% (w/w), providing 10 g of HM pectin per treatment, were used as standards to which the apparent viscosities of LM pectin and LMA pectin were matched at a shear rate of 50 s−1. Therefore, LM and LMA treatments provided 3 g (LV) or 6 g (HV) per treatment (Table 1). The HV and LV HM pectin concentrations were determined from the study conducted by Tiwary et al.17 who demonstrated that HM pectin in concentrations as low as 5 g of pectin mixed with 448 mL orange juice could increase human satiety. The pectin was dissolved in commercial orange juice at 4 °C to a final volume of 500 mL in 2 L beakers using a hand blender for up to 10 min until the sample was homogenous. The pectin-fortified orange juice was served immediately after blending at 4 °C.| Low viscosity solutions | High viscosity solutions | |||
|---|---|---|---|---|
| Pectin | Concentration (%) | Apparent viscosity (Pa s) | Concentration (%) | Apparent viscosity (Pa s) |
| High methoxyl | 1.0 | 0.042 ± 0.006 | 2.0 | 0.14 ± 0.025 |
| Low methoxyl | 0.6 | 0.042 ± 0.001 | 1.2 | 0.14 ± 0.012 |
| Low methoxyl amidated | 0.6 | 0.043 ± 0.003 | 1.2 | 0.13 ± 0.003 |
2.3 Viscosity measurement
Pectin is characteristically non-Newtonian in nature, and its viscosity is thus shear rate dependent. Therefore, viscosity should be reported across various shear rates.23 This study reports the apparent viscosity at two physiologically-relevant shear rates (10 and 50 s−1) calculated after a shear rate sweep from 10 to 200 s−1. Viscosity measurements at 37 °C were obtained in triplicate using cone and plate geometry (6 cm diameter acrylic cone, 2°, 50 μm truncation gap) on a controlled-stress rheometer (AR 2000, TA Instruments, Delaware, USA).2.4 In vitro gastrointestinal digestion
The in vitro digestion protocol was as previously described.24 The digestive solutions were prepared fresh each day. To mimic the gastric phase of digestion, 15 g of orange juice containing pectin was placed in a 125 mL Erlenmeyer flask along with four 1 cm glass beads and was incubated for 2 h in a 37 °C shaking water bath (Thermo Scientific, Marietta, OH, USA) at 175 rpm. The gastric solution consisted of 7 mL of SGF [0.2% NaCl (w/w) in 0.7% HCl (w/v)] and 3.2 mg mL−1 of pepsin. The chyme mixture was adjusted to pH 2.0 by the dropwise addition of 1.0 M HCl with stirring. Samples were withdrawn for rheological characterization after 1 h and at the end of the 2 h incubation period.To mimic the intestinal stage of digestion, the solutions that had undergone the simulated gastric phase were then incubated for 2 h with the addition of 2 mL CaCl2 (750 mM), 4.6 mL simulated bile fluid (SBF), which contained 8 mg mL−1 bile salts, and 12 mL simulated intestinal fluid (SIF), which contained 5 mg mL−1 pancreatin dissolved in 0.5 M sodium phosphate buffer, in a shaking water bath at 175 rpm at a temperature of 37 °C and pH of 7.6. Samples were taken for rheological characterization after 1 and 2 h.
2.5 Human clinical trial design
The University of Guelph Human Research Ethics Board approved the human study (REB #13FE009) and all participants provided written informed consent. This study utilized a double-blind randomized crossover design and consisted of 8 morning study sessions, each of 3 h duration and separated by 1 week. Participants consumed the treatment and control beverages (completed in duplicate) in random order allocated based on order of enrollment using a computer generated Latin Square design. The sample size was based on previous studies in which a difference of 8 mm on a 100 mm scale was considered to be meaningful.25 Participants arrived following an overnight fast of 12 h at the Human Nutraceutical Research Unit (HNRU) at the University of Guelph at their designated time (between 6:30–9:00 AM). They completed baseline (time = 0 min) appetite measurements on anchored 100 mm visual analogue scales (VAS) pertaining to Hunger, Satisfaction, Fullness, and Prospective Consumption.26 The questions were as follows:1. How hungry do you feel? Not hungry at all (0 mm) vs. I have never been more hungry (100 mm).
2. How satisfied do you feel? I am completely empty (0 mm) vs. I cannot eat another bite 9 (100 mm).
3. How full do you feel? Not at all full (0 mm) vs. Totally full (100 mm).
4. How much do you think you can eat? Nothing at all (0 mm) vs. A lot (100 mm).
Participants were also asked to rate their liking of each test beverage using VAS for the following questions:
1. How did you find the texture of the beverage? Dislike extremely (0 mm) vs. Like extremely (100 mm).
2. How did you find the taste of the beverage? Dislike extremely (0 mm) vs. Like extremely (100 mm).
3. Overall, how did you find the palatability of the beverage? Dislike extremely (0 mm) vs. Like extremely (100 mm).
The test beverages (500 mL, 1.0 MJ) were served with a commercially prepared breakfast sandwich (Tim Horton's ham, egg, and cheese sandwich on an English muffin, 1.3 MJ), purchased fresh each morning. The participants ate the breakfast meal in an assigned sensory panelist booth within 10 min. Thereafter, participants were required to sit in a classroom between ratings and work quietly, listen to music, and use personal computers. They were only allowed to leave the classroom to use the restroom. VAS ratings were completed in the panelist booths at 0 (fasting), 10, 30, 45, 60, 120 and 180 min. Participants completed a 24 hour food record for the day prior to their first study visit and were asked to adhere to it on days prior to all study visits. Food records were reviewed by a study coordinator and analyzed for average energy, carbohydrate, fat, protein, and fiber intake using The Food Processor Nutritional software program (ESHA, Salem, OR).
2.6 Participants
Twenty-six healthy males (n = 11) and females (n = 15) aged 18–30 were recruited from the University of Guelph community through flyers and email advertisements. Participants completed an email or telephone screening to determine initial eligibility and were then invited to attend a screening visit at the HNRU. The study coordinator administered the in-person screening questionnaire, which included a gastrointestinal upset questionnaire and the Three Factor Eating Questionnaire R-21 (TFEQ-R21).27 Participants’ height was measured to the nearest cm using a stadiometer (Model 217, SECA®, Hanover, USA) and non-fasted body weight was determined to the nearest 0.1 kg using a digital scale (SVI-200F, Acculab®, Barrie, Canada).Eligible participants were English-speaking, non-smokers, not taking any medication other than dietary supplements not anticipated to affect appetite or birth control, had a stable body weight over the previous 2 months, and consumed over 7 g of fiber daily. Participants were excluded if they had a BMI lower than 18.6 or greater than 30 kg m−2, were pregnant or lactating, consumed more than 5 alcoholic beverages a day or were considered restrained, uncontrolled or emotional eaters as determined by TFEQ-R21. Persons who did not like the test foods, did not consume breakfast regularly, had a history of disease or acute gastrointestinal dysfunction were also excluded. One male participant did not complete the study due to non-compliance with the guidelines of the study.
2.7 Statistical analysis
In vitro viscosity data was analyzed by calculating the mean values of the data recorded in triplicate along with the standard error of mean (SEM). One-way analysis of variance (ANOVA) and post-hoc Bonferroni–Dunn tests were carried out to determine significant differences between the results (p < 0.05). Differences in the satiety parameters of Hunger, Fullness, Satisfaction and Prospective Food Intake were assessed by calculating total area under the curve (AUC) using the trapezoid rule. AUC result for the two control treatments were pooled after comparisons using Student's t-test showed no statistical differences. Treatments were then compared using analysis of covariance (ANCOVA) treating baseline rating and study week as covariates. Analytical models included participant, treatment, week and week*treatment interaction. Post-hoc Bonferroni–Dunn tests were used to compare means for the treatment and the weeks. Data are reported as the mean ± SEM. Two-tailed Spearman correlations were conducted to explore relationships between viscosity and AUC for each satiety parameter as well as taste, texture and palatability. Statistical analysis was performed with SPSS version 21.0 (IBM Corp., Armonk, NY, USA) for the AUC data and GraphPad Prism 5.0™ (GraphPad Prism Software Inc., La Jolla, CA, USA) was used for the viscosity data.3 Results and discussion
3.1 Participant characteristics
Twenty-five participants (women n = 15, men n = 10) completed all eight study visits. The mean age ± SD for females was 23.1 ± 3.0 years and 24.8 ± 2.4 years for males. The mean BMI ± SD was 22.1 ± 1 and 23.4 ± 2.1 kg m−2 for females and males, respectively. The TFEQ cognitive restraint, emotional and uncontrolled eating scores for females were 2.1 ± 0.4, 1.6 ± 0.5 and 1.9 ± 0.4, respectively. For males, these scores were 1.9 ± 0.5, 1.9 ± 0.4 and 2.1 ± 0.4, respectively. Participants who were taking dietary supplements maintained typical supplement use throughout the study. The supplements taken were multivitamin (n = 6) and omega 3 fatty acids (n = 2).3.2 Pectin solution viscosity and effect of digestive processes
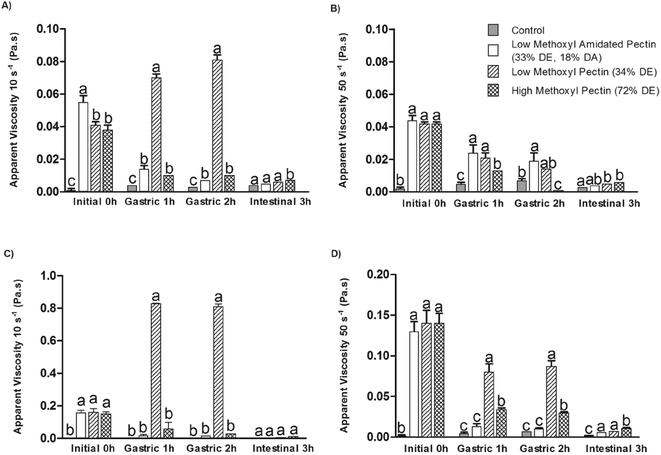
The apparent viscosities at 10 and 50 s−1 of orange juice and LV and HV orange juice/pectin solutions initially (0 h) and after 1 h gastric, 2 h gastric and 1 h intestinal human digestion are shown in Fig. 1. All pectin types increased the viscosity of orange juice and these were standardized to be the same at 50 s−1. All samples demonstrated pseudoplastic behavior, as evidenced by the reductions in viscosity from 10 to 50 s−1. Also, overall, there were reductions in apparent viscosity for all samples comparing the 3 h gastrointestinal digestion samples to those at 0 h. However, during the different phases of the gastrointestinal digestion, the pectin-fortified beverages demonstrated different rheological behaviors. The control, LMA and HM pectin at both viscosity levels exhibited gradual declines in apparent viscosity (both at 10 and 50 s−1) after each step of the digestion. However, the LM pectin at both viscosity levels showed a large increase in apparent viscosity at 10 s−1, but not 50 s−1, after the gastric 1 h and 2 h digestions compared to the initial viscosity. Thus this fiber appears to have formed a weak network easily disrupted by shear. It should be noted that there was insufficient calcium in the sandwich plus beverage to induce gelation at the concentrations of LM pectin used, so the weak network may have been induced by pH alone, rather than by calcium. The LV-HM pectin had a significantly higher apparent viscosity after the small intestinal phase compared to the control as did the HV-HM pectin at 50 s−1 whereas the LMA pectin was only significantly higher than the control after the intestinal phase at HV and 50 s−1.The differences in fiber-induced in vitro viscosity can be attributed to the variability of substituents (methyl-esters or amide groups) and of the charge distribution along the galacturonan backbone. The degree of charge is determined by the DE and DA and imparts different physical properties. HM pectin has a high DE and thus a low overall charge, while LM pectin has a low DE and high overall charge such that it reacts differently to the changes in pH between the gastric and intestinal phases.28,29 The reduction in pH reduces the electrostatic repulsion between the pectin chains, which promotes hydrogen bonding by the protonated carboxyl groups and leads to aggregation. With LMA pectin, the added amide groups can interfere with gelation, reducing the gelation kinetics and the calcium sensitivity.30 Dilution effects during the in vitro digestion process can partly explain the large drop in apparent viscosity observed.31 Besides the effect of dilution from the gastric and intestinal fluids, polymer degradation may also have occurred due to the hydrolysis of the glycosidic linkages in addition to the cleavage of neutral sugar side chains as the pH is lowered and temperature is increased.22,32 At low pH values, de-esterification reactions are also favored.33
3.3 Change in participant VAS scores
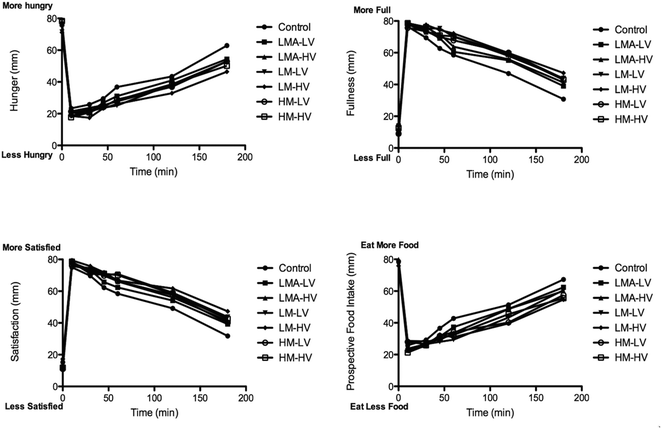
VAS scores over 180 min for all treatments and controls are shown in Fig. 2. Each appetite sensation varied as a function of time in a predictable fashion, with participants reporting high ratings for Hunger and Prospective Food Intake and low ratings for Satisfaction and Fullness at the baseline, followed by rapid changes after breakfast consumption and gradual reversals thereafter. No scores returned fully to baseline within 3 h. Area under the curve (AUC) values were calculated and interpreted for treatment-related effects, which will be discussed in the next section.3.4 AUC of participant satiety ratings
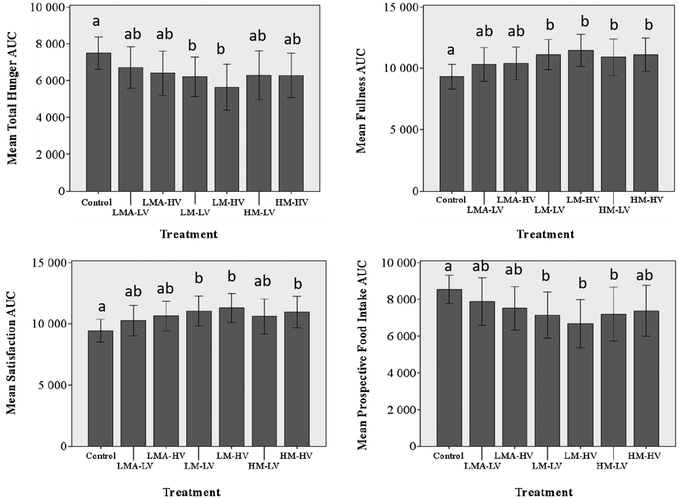
Satiety scores were decreased by the HM and LM pectin treatments, relative to the fiber-free control (Fig. 3). This is in accordance with the body of literature affirming that pectin fibers have the ability to increase feelings of satiety.10,17,19–21 Mean AUC Hunger scores were significantly lower for the LM-LV and LM-HV treatments when compared to the control (p < 0.05). Mean Fullness AUC was significantly increased compared to the control for the LM-LV, LM-HV, HM-LV, and HM-HV treatments. Satisfaction mean AUC was significantly higher for the LM-LV, LM-HV, and HM-HV treatments compared to the control. Mean values of Prospective Food Intake AUC were significantly lower as compared to the control for the LM-LV, LM-HV and HM-LV treatments indicating participants felt they would be able to eat less after consuming those treatments.Overall, the LM pectin fortified beverages were consistently more satiating compared to the control. The HM pectin fortified beverage exhibited satiating effects for the majority of the questions asked compared to the control but less than the LM pectin, despite the fact that HM pectin was present in higher absolute quantity and at equal viscosity to the LM pectin. Overall, there were no significant differences between the LV and HV treatments. Therefore there was no dose–response relationship observed at the two concentrations investigated. The LMA pectin fortified beverage did not have any significant impacts on sensations of satiety when compared to the control.
3.5 Effect of pectin solutions on male and female populations
Sub-group analyses revealed that males and females differed in their mean total AUC for each of the four satiety sensations evaluated (data not shown). Overall, male participants had higher Hunger and Prospective Food Intake scores and lower Fullness and Satisfaction scores compared to the female participants. This may relate to the fact that male participants had higher BMI and higher calculated Estimated Energy Requirements compared to the females, but all ate the same test meals which were isocaloric. There is also evidence that males and females differ in terms of their satiety responses,34 which may be based on the gender-specific release of CCK.35 In the male participants, LM and HM treatments at high viscosities yielded significantly higher Fullness and Satisfaction scores and lower Prospective Food Intake scores compared to the control (p < 0.05). None of the low viscosity treatments were significantly different from the control. For females, the LM-LV and LM-HV had scores which were significantly different from the control for all appetite ratings. The gender-specific response to the LV treatment was interesting, suggesting dose may have more of an influence on satiety in females compared to males.3.6 Effect of treatment week
Despite randomization, there was a significant effect of study week on mean total AUC for Hunger (p < 0.05) and Prospective Food Intake (p < 0.05), with values tending to decrease during the testing period. Similarly, effects of mean total AUC for Fullness (p < 0.05) and Satisfaction (p < 0.05) were observed with values tending to increase during the testing period. A number of factors may have contributed to this, including adjustment to the study protocol or seasonal effects (the study ran from May to August). To account for this, the effect of study week was included in the statistical model. Despite the effect of treatment week, there were still significant results. Regardless, the potential for participant fatigue or acclimatization to study protocols warrants further investigation.3.7 Correlations between in vitro digestate apparent viscosity and in vivo satiety scores
To determine if there was a relationship between apparent viscosity after in vitro gastric and intestinal digestion and participant-rated satiety sensations, nonparametric Spearman's rank order correlation coefficients were calculated. This testing was selected after investigation of the data indicated it was not normally distributed. The Spearman's correlation value revealed a statistically significant positive relationship between apparent viscosity at 50 s−1 and mean AUC for Fullness and Satisfaction (Table 2). Apparent viscosity was negatively correlated to total mean AUC of Hunger and Prospective Food Intake, but was not significant at either shear rate. Additionally, there were no significant correlations observed between apparent viscosity and total mean AUC of Fullness and Satisfaction at a shear rate of 10 s−1. However, when apparent viscosities at various stages of the in vitro digestion were considered, there was a strong significant correlation between gastric viscosity at 10 s−1 and all satiety parameters, with correlation coefficients of 0.878 and 0.976 observed for the LV and HV treatments, respectively. These correlations are strongly driven by the high apparent viscosities observed for the LM pectin treatments at low shear after in vitro gastric digestion and the significant effects of LM pectin on all of the human satiety scales, although satiety may also be related to some aspect of LM pectin other than viscosity, since correlation does not necessarily demonstrate causality.The orange juice was formulated to have equal viscosity for the three pectins but the digestion process had varying effects on the viscosity after in vitro digestion. This study did not reveal a strong correlation between digesta apparent viscosity and total mean AUC subjective satiety ratings. The results of the correlation could be due to the challenges in mimicking the human digestive system in vitro. Additionally, the sandwich was not included in the in vitro digestion in order to maintain sample homogeneity. This is not to say that there is no relationship between viscosity and satiety, only that satiety cannot be explained in full by viscosity alone. It has been suggested that soluble, viscous or gel forming fibers positively impact satiety in different ways, such as increasing the time required to swallow, increasing gastric distention,36,37 delaying gastric emptying times16,21 or impacting nutrient absorption in the small intestine.38,39 These parameters were not examined in the present study, but the present results indicate that viscosity might only be a contributing factor. The significant correlation between high viscosity at low shear after gastric digestion for LM pectins and satiety suggest that delayed gastric emptying may be a factor in its physiological functionality, whereas the effects of HM pectin may have been more related to the intestinal viscosity and its potential effects on nutrient absorption, in other words, different pectins may affect different aspects of the satiety cascade. The fact that LMA pectin at a similar apparent viscosity to LM or HM pectin had no impact on satiety scores, whereas LM and HM pectin did, is also notable in suggesting that satiety cannot be fully explained by viscosity alone.
3.8 Meal palatability
Unpalatable foods might serve as a deterrent to the return of hunger and meal palatability could have had an effect on reported appetite ratings. Therefore, this study also examined potential relationships between participants beverage liking and subsequent appetite ratings. Accordingly, VAS ratings for texture (p < 0.05), taste (p < 0.05), and palatability (p < 0.05) differed significantly among treatments (data not shown). The ratings for texture were significantly lower for all treatments compared to the control, indicating a decrease in liking. The control had significantly higher taste ratings than all treatments, except the low viscosity LM and LMA pectins. Palatability of the control was rated significantly higher than all other treatments. Despite these differences, the total mean AUC for all satiety responses was not related to preferences of texture (p > 0.05), taste (p > 0.05), or palatability (p > 0.05), according to Spearman correlation analysis. Therefore, although differences were observed in terms of meal palatability, these differences were not correlated with the subjective appetite ratings.4 Conclusion
The viscosities of HM, LM and LMA pectin fortified orange juice beverages following in vitro simulated digestion were measured and compared to the satiety-inducing effects of the beverages in human participants. The orange juice beverages were formulated to have equivalent viscosities initially, but viscosity values changed after in vitro human gastrointestinal digestion. During the simulated gastric digestion, HM pectin showed the greatest decline in gastric viscosity, but retained the highest intestinal viscosity overall. The apparent viscosity of LM pectin at the end the gastric phase increased by approximately 50% compared to 0 h at 10 s−1 and decreased by approximately 50% compared to 0 h at 50 s−1 and was then greatly reduced by intestinal digestion. Overall, the viscosity of LMA pectin decreased over the course of the digestion and showed the lowest values of any of the three pectins.The different types of pectin had differing effects on human satiety. The LM pectin fortified treatments consistently induced satiety at high and low concentrations compared to the control. HM pectin treatments had an effect on only some satiety parameters, despite being present at a higher concentration and same initial viscosity as the LM pectin. LMA pectin treatments had no significant effects compared to the control beverage.
The apparent viscosity of the digesta at the two low shear rates studied here did not correlate strongly with human participant satiety ratings. The most significant correlation was seen with LM pectin, where a high apparent viscosity at low shear rate observed after gastric digestion was associated with significant inductions of satiety. The differences in effects both in vivo and in vitro can be attributed to the dissimilar physicochemical properties of the pectin molecules. Therefore, fiber-induced viscosity of orange juice served with a breakfast meal was not the only contributing factor to human sensations of satiety. Further research is needed to examine how molecular aspects of pectins and other dietary fibers impact gastrointestinal processes and ultimately satiety.
Acknowledgements
Thank you to our study participants and to Melissa McNeil for technical support. Funding was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC).References
- S. D. Malnick and H. Knobler, The medical complications of obesity, Quar. J. Med., 2006, 99(9), 565–579 CrossRef CAS PubMed.
- G. O. Phillips and S. W. Cui, Evolution and finalisation of the regulatory definition of dietary fibre, Food Hydrocolloids, 2011, 25, 139–143 CrossRef CAS PubMed.
- J. N. Davis, V. A. Hodges and M. B. Gillham, Normal-weight adults consume more fiber and fruit than their age and height-matched overweight/obese counterparts, J. Am. Diet. Assoc., 2006, 106, 833–840 CrossRef PubMed.
- L. A. Tucker and K. S. Thomas, Increasing total fiber intake reduces risk of weight and fat gains in women, J. Nutr., 2009, 139(3), 576–581 CrossRef CAS PubMed.
- H. Du, D. L. van der A, H. C. Boshuizen, N. G. Forouhi, N. J. Wareham, J. Halkjaer, A. Tjonneland, K. Overvad, M. U. Jakobsen, H. Boeing, B. Buijsse, G. Masala, D. Palli, T. I. Sorensen, W. H. Saris and E. J. Feskens, Dietary fiber and subsequent changes in body weight and waist circumference in European men and women, Am. J. Clin. Nutr., 2010, 91(2), 329–336 CrossRef CAS PubMed.
- J. W. Anderson, P. Baird, R. H. Davis Jr., S. Ferreri, M. Knudtson, A. Koraym, V. Waters and C. L. Williams, Health benefits of dietary fiber, Nutr. Rev., 2009, 67(4), 188–205 CrossRef PubMed.
- C. W. C. Kendall, A. Esfajhani and D. J. A. Jenkins, The link between dietary fibre and human health, Food Hydrocolloids, 2010, 24, 42–48 CrossRef CAS PubMed.
- H. J. Willis, A. L. Eldridge, J. Beiseigel, W. Thomas and J. L. Slavin, Greater satiety response with resistant starch and corn bran in human subjects, Nutr. Residency, 2009, 29, 100–105 CrossRef CAS PubMed.
- P. Monsivais, B. E. Carter, M. Christiansen, M. M. Perrigue and A. Drewnows, Soluble fiber dextrin enhances the satiating power of beverages, Appetite, 2011, 56(1), 9–14 CrossRef CAS PubMed.
- A. J. Wanders, J. J. van de Borne, C. de Graaf, T. Hulshof, M. C. Jonathan, M. Kristensen, M. Mars, H. A. Schiks and E. J. M. Feskens, Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials, Obes. Rev., 2011, 12(9), 724–739 CAS.
- M. Clark and J. L. Slavin, Fiber and prebiotics: mechanisms and health benefits, Nutrients, 2013, 5, 1417–1435 CrossRef PubMed.
- M. Kristensen and M. G. Jensen, Dietary fibres in the regulation of appetite and food intake. Importance of viscosity, Appetite, 2011, 56, 65–70 CrossRef CAS PubMed.
- C. L. Dikeman, M. R. Murphy and G. C. Fahey Jr., Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta, J. Nutr., 2006, 136(4), 913–919 CAS.
- C. L. Dikeman and G. C. Fahey Jr., Viscosity as related to dietary fiber: a review, Crit. Rev. Food Sci. Nutr., 2006, 46(8), 649–663 CrossRef CAS PubMed.
- S. Fiszman and P. Varela, The role of gums in satiety/satiation. A review, Food Hydrocolloids, 2013, 32, 147–154 CrossRef CAS PubMed.
- C. Di Lorenzo, C. M. Williams, F. Hajnal and J. E. Valenzuela, Pectin delays gastric emptying and increases satiety in obese subjects, Gastroenterology, 1988, 95, 1211–1215 CAS.
- C. M. Tiwary, J. A. Ward and B. A. Jackson, Effect of pectin on satiety in healthy US Army adults, J. Am. Coll. Nutr., 1997, 16, 423–428 CrossRef CAS.
- M. Perrigue, B. Carter, S. A. Roberts and A. Drewnowski, A low-calorie beverage supplemented with low-viscosity pectin reduces energy intake at a subsequent meal, J. Food Sci., 2010, 75, H300–H305 CrossRef CAS PubMed.
- A. J. Wanders, M. C. Jonathan, J. J. van de Borne, M. Mars, H. A. Schols, E. J. M. Feskens and C. de Graaf, The effects of bulking, viscous and gel-forming dietary fibres on satiation, Br. J. Nutr., 2013, 109, 1330–1337 CrossRef CAS PubMed.
- A. J. Wanders, M. Mars, K. J. Borgonjen-van den Berg, C. de Graaf and E. J. M. Feskens, Satiety and energy intake after single and repeated exposure to gel forming dietary fiber: post ingestive effects, Int. J. Obes., 2014, 38, 794–800 CrossRef CAS PubMed.
- A. J. Wanders, E. J. M. Feskens, M. C. Jonathan, H. A. Schols, C. de Graaf and M. Mars, Pectin is not pectin: A randomized trial on the effect of different physicochemical properties of dietary fiber on appetite and energy intake, Physiol. Behav., 2014, 128, 212–219 CrossRef CAS PubMed.
- M. A. V. Axelos and M. Branger, The effect of the degree of esterification on the thermal-stability and chain conformation of pectins, Food Hydrocolloids, 1993, 7(2), 91–102 CrossRef CAS.
- F. Kong and R. P. Singh, A Human Gastric Simulator (HGS) to study food digestion in human stomach, J. Food Sci., 2011, 76(5), E627–E632 CrossRef PubMed.
- H. Fabek, S. Messerschmidt, V. Brulport and H. D. Goff, The effect of in vitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion, Food Hydrocolloids, 2014, 35, 718–726 CrossRef CAS PubMed.
- J. E. Blundell, S. Green and V. Burley, Carbohydrates and human appetite, Am. J. Clin. Nutr., 1994, 59, 728–734 Search PubMed.
- A. Flint, A. Raben, J. E. Blundell and A. Astrup, Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies, Int. J. Obes. Relat. Metab. Disord., 2000, 24, 38–48 CrossRef CAS.
- J. C. Capellerie, A. G. Bushmakin, R. A. Gerber, N. K. Leidy, C. C. Sexton, M. R. Lowe and J. Karlsson, Psychometric analysis of the Three-Factor Eating Questionnaire-R21: results from a large diverse sample of obese and non-obese participants, Int. J. Obes., 2009, 33(6), 611–620 CrossRef PubMed.
- C. Lofgren, S. Guillotin and A. M. Hermansson, Microstructure and kinetic rheological behavior of amidated and nonamidated LM pectin gels, Biomacromolecules, 2006, 7(1), 114–121 CrossRef PubMed.
- B. L. Sperber, M. A. Cohen Stuart, H. A. Schols, A. G. Voragen and W. Norde, Overall charge and local charge density of pectin determines the enthalpic and entropic contributions to complexation with β-lactoglobulin, Biomacromolecules, 2010, 13(11), 3578–3583 CrossRef PubMed.
- F. Capel, T. Nicolai, D. Durand, P. Boulenguer and V. Langendorff, Calcium and acid induced gelation of (amidated) low methoxyl pectin, Food Hydrocolloids, 2006, 6(20), 901–907 CrossRef PubMed.
- D. Saito, S. Nakaji, S. Fukuda, T. Shimoyama, J. Sakamoto and K. Sugawara, Comparison of the amount of pectin in the human terminal ileum with the amount of orally administered pectin, Nutrition, 2005, 21, 914–919 CrossRef CAS PubMed.
- G. A. Morris, T. J. Foster and S. E. Harding, A hydrodynamic study of the depolymerisation of a high methoxyl pectin at elevated tempratures, Carbohydr. Polym., 2002, 48(4), 361–367 CrossRef CAS.
- C. M. G. C. Renard and J. F. Thibault, Degradation of pectins in alkaline conditions: kinetics of demethylaton, Carbohydr. Res., 1996, 286, 139–150 CrossRef CAS.
- G. J. Wang, N. D. Wolkow and J. S. Fowler, Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(4), 1249–1254 CrossRef CAS PubMed.
- B. Burton-Freeman, Dietary fiber and energy regulation, J. Nutr., 2000, 130, 272S–275S CAS.
- L. Marciani, P. A. Gowland, R. C. Spiller, P. Manoj, R. J. Moore and P. Young, et al., Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI, Am. J. Physiol. – Gastrointest. Liver Physiol., 2001, 280(6), G1227–G1233 CAS.
- C. L. Hoad, P. Rayment, R. C. Spiller, L. Marciani, B. C. Alonso and C. Traynor, In vivo imaging of intragastric gelation and its effect on satiety in humans, J. Nutr., 2004, 134, 2293–2300 CAS.
- B. Flourie, Effects of pectin on jejunal glucose absorption and unstirred layer thickness in normal man, Gut, 1984, 25, 936–937 CrossRef CAS PubMed.
- D. J. A. Jenkins, A. Marchie, L. S. A. Augustin, E. Ros and C. W. C. Kendall, Viscous dietary fibre and metabolic effects, Clin. Nutr., 2004, 1(2), 39–49 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |