JAK2 and AMP-kinase inhibition in vitro by food extracts, fractions and purified phytochemicals
Harry
Martin
ab,
Elaine J.
Burgess
bc,
Wendy A.
Smith
bd,
Tony K.
McGhie
a,
Janine M.
Cooney
bd,
Rona C. M.
Lunken
ab,
Erika
de Guzman
e,
Tania
Trower
bd and
Nigel B.
Perry
*bc
aFood Innovation, The New Zealand Institute for Plant & Food Research Ltd, Private Bag 11 600, Palmerston North 4442, New Zealand. E-mail: harry.martin@plantandfood.co.nz
bNutrigenomics New Zealand is a collaboration between The University of Auckland, AgResearch Limited and Plant & Food Research, and is funded by the Ministry of Business, Innovation and Employment, Brisbane, Queensland, Australia
cThe New Zealand Institute for Plant & Food Research Ltd, University of Otago, Dunedin 9016, New Zealand
dThe New Zealand Institute for Plant & Food Research Ltd, Hamilton 3214, New Zealand
eThe University of Queensland Diamantina Institute, Translational Research Institute, The University of Queensland, Brisbane, Queensland, Australia
First published on 25th November 2014
Abstract
We have identified a range of food phytochemicals that inhibit Janus Kinase 2 (JAK2) and Adenosine Monophosphate Kinase (AMPK). A mutated and dysregulated form of JAK2, a tyrosine kinase, is associated with several diseases including Crohn's disease. Using an in vitro, time-resolved fluorescence (TR-FRET) assay, we tested 49 different types of food extracts, plus 10 concentrated fractions of increasing hydrophobicity from each extract, to find foods containing JAK2 inhibitors. The food extracts tested included grains, meat, fish, shellfish, dairy products, herbs, mushrooms, hops, fruits and vegetables. Several fruits were potent inhibitors of JAK2: blackberry, boysenberry, feijoa, pomegranate, rosehip and strawberry, which all contain ellagitannins, known inhibitors of kinases. These fruits are in the Rosales and Myrtales plant orders. No other foods gave >1% of the maximal JAK2 inhibitory activities of these fruits. AMPK, a sensor and regulator of energy metabolism in cells, is a serine-threonine kinase which is reported to be activated by various flavonoid phytochemicals. Using a TR-FRET assay, we tested various fruit extracts for AMPK activation and inhibition. Ellagitannin containing foods scored highly as AMPK inhibitors. Despite several reports of AMPK activation in whole cells by phytochemicals, no extracts or pure compounds activated AMPK in our assay.
Introduction
Natural foods may contain anti-inflammatory molecules. For example the concentration of salicylates in cumin occur at 1.5% by weight1 while oleocanthal, found in olive oil, has ibuprofen-like activity.2 Systemic inflammation is associated with cancer, obesity, aging and diabetes.3,4 Several dietary phytochemicals are reported to alleviate inflammatory diseases. For example, resveratrol and capsaicin reduce obesity associated inflammation in mice,5,6 and orally administered D-limonene was found to reduce inflammation in rat colitis and to lower plasma markers of inflammation in humans.7The inflammatory bowel condition known as Crohn's disease has several genetic susceptibility markers including Nucleotide-binding oligomerization domain-containing protein 2, Autophagy-related protein 16, tumor necrosis factor α and JAK2.8 JAK2 is a protein tyrosine kinase and mediates signal transduction following cell stimulation by inflammatory cytokines including interleukins 2 through 6 and Interferon-γ.9,10 An acquired mutation of JAK2 (V617F) is associated with the bone-marrow diseases polycythemia vera, essential thrombocythemia, idiopathic myelofibrosis11 and also with unregulated lymphocyte activation in Crohn's disease.12 V617F JAK2 lacks the normal autoregulation conferred by the JH2 pseudokinase domain of JAK2.13
Adenosine Monophosphate-activated Protein Kinase (AMPK) is a cellular energy sensor, present in almost all eukaryotes. An increasing AMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ATP ratio leads to allosteric activation of AMPK, which can also be activated by phosphorylation by upstream kinases.14 AMPK plays a fundamental role in controlling metabolic rate, glucose consumption and appetite15 and has therefore attracted a great deal of attention from medicinal chemists and drug designers.
ATP ratio leads to allosteric activation of AMPK, which can also be activated by phosphorylation by upstream kinases.14 AMPK plays a fundamental role in controlling metabolic rate, glucose consumption and appetite15 and has therefore attracted a great deal of attention from medicinal chemists and drug designers.
Several phytochemicals that are JAK2 inhibitors16 are also activators of AMPK, e.g. genistein and resveratrol.17–19 Other phytochemicals reported to activate AMPK in a variety of cell-based and animal models include apigenin,20 anthocyanins21 and ellagic acid.22 Ellagic acid is a known kinase inhibitor due to its affinity for the kinase ATP binding site.23 In a comparison of 12 kinases, this affinity ranged from 40 nM to greater than 40 μM.24 In our recent study of boysenberry ellagitannin inhibition of JAK2 we found that ellagic acid had an IC50 of 92 nM.16
To find additional foods with JAK2 inhibitory activity, we have conducted a large scale screen of 49 common and New Zealand sourced food products. These foods included fruits, vegetables, herbs, shellfish, meats and grains which we tested for JAK2 inhibitory activity as well as 10 fractions of increasing hydrophobicity prepared from each food sample using solid phase extraction. The JAK2 and AMPK studies were performed on different samples, as projects running in parallel. Although JAK2 inhibition and AMPK activation are fundamentally different physiological targets, we found similar activities in selected foods and phytochemicals.
Although several previous reports have identified ellagitannins as inhibitors of kinases, we show that the overriding indicator of whether a food has strong JAK2 inhibitory capacity is the presence of ellagitannins in the food. With 49 different types of food included in this study we believe this is the most comprehensive analysis of its kind. We also show that several phytochemicals that are reported to be AMPK activators in whole cell systems are, in fact, inhibitors of AMPK in a direct biochemical assay of AMPK.
Materials and methods
Materials and reagents
Activated JAK2 (SH1, SH2) was supplied by Invitrogen Inc. AMPK (α1,β1,γ1) was supplied by Promega Inc. ULight™ substrates, Europium-coupled anti-phosphotyrosine antibody, 384-well proxiplates and Lance detection buffer were supplied by Perkin Elmer Inc. The ULight™ substrate sequences for the JAK2 and AMPK assays were CAGAGAIETDKEY![[Y with combining low line]](https://www.rsc.org/images/entities/char_0059_0332.gif) TVKD and CHMRSAM
TVKD and CHMRSAM![[S with combining low line]](https://www.rsc.org/images/entities/char_0053_0332.gif) GLHLVKRR respectively. The phosphorylation target amino acids are underlined. Europium-labelled antibodies Eu-W1024 anti-phosphotyrosine (PT66) and Eu-anti P acetyl CoA Carboxylase ser79 used in the JAK2 and AMPK assays respectively, were supplied by PerkinElmer Inc. The AMPK activating compound A769662 was supplied by Reagents Direct. All other reagents were supplied by Sigma Inc. Foods were purchased from retail outlets or obtained direct from producers.
GLHLVKRR respectively. The phosphorylation target amino acids are underlined. Europium-labelled antibodies Eu-W1024 anti-phosphotyrosine (PT66) and Eu-anti P acetyl CoA Carboxylase ser79 used in the JAK2 and AMPK assays respectively, were supplied by PerkinElmer Inc. The AMPK activating compound A769662 was supplied by Reagents Direct. All other reagents were supplied by Sigma Inc. Foods were purchased from retail outlets or obtained direct from producers.
Food extraction and fractionation procedure (JAK2 assay)
Freeze-dried ground food material (10 g) was extracted overnight by shaking with 96% EtOH, 4% H2O (100 ml), then filtered to give an extract, which was stored at −20 °C. An aliquot of extract (50 ml) was coated onto 2 g C18 (Aldrich octadecyl-functionalized silica gel) by rotary evaporating at 30 °C and applied to a 5 g C18 Isolute SPE cartridge preconditioned with EtOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O, then H2O (10 ml each). Fractions were eluted with 2 × 10 ml of H2O, 1
1 EtOH–H2O, then H2O (10 ml each). Fractions were eluted with 2 × 10 ml of H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 EtOH–H2O, 1
4 EtOH–H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O, 4
1 EtOH–H2O, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O, EtOH, and EtOAc to give 12 10 ml fractions. Aliquots (1 ml) of extract and fractions were dried at 20 °C in Eppendorf Safelock™ tubes and stored at −20 °C until assayed. The samples were dissolved in 250 μl of DMSO then diluted into the JAK2 assay to achieve a final concentration of 0.83% DMSO. To simplify the set up for robotic assays, the first two fractions were pooled after suspension in DMSO, as were the last two fractions, thus reducing the sample number per food to 12 (whole extract, 10 fractions and one control sample e.g. staurosporine). The foods extracted, fractionated and assayed are summarised in Table 1.
1 EtOH–H2O, EtOH, and EtOAc to give 12 10 ml fractions. Aliquots (1 ml) of extract and fractions were dried at 20 °C in Eppendorf Safelock™ tubes and stored at −20 °C until assayed. The samples were dissolved in 250 μl of DMSO then diluted into the JAK2 assay to achieve a final concentration of 0.83% DMSO. To simplify the set up for robotic assays, the first two fractions were pooled after suspension in DMSO, as were the last two fractions, thus reducing the sample number per food to 12 (whole extract, 10 fractions and one control sample e.g. staurosporine). The foods extracted, fractionated and assayed are summarised in Table 1.
| Food type | Sample | Fraction with max. activity | Activity | Food type | Sample | Fraction with max. activity | Activity |
|---|---|---|---|---|---|---|---|
| Fruit | Apple, Cox's Orange | 4 | 1.5* | Grain | Bread, brown, gluten free | 8 | 1.9* |
| Fruit | Apple, Crab apple, flesh | 3 | 2.5 | Grain | Bread, white, gluten free | 8 | 1.3* |
| Fruit | Apple, Crab apple, skin | 4 | 2.7 | Grain | Oats, bran | 8 | 2.1* |
| Fruit | Apple, Merton Russet, flesh | 2 | 1.5 | Grain | Oats, rolled, plus Omega 3 | — | — |
| Fruit | Apple, Merton Russet, skin | 4 | 2.6 | Grain | Oats, rolled | — | — |
| Fruit | Apple, Niagara, flesh | 3 | 1.6 | ||||
| Fruit | Apple, Niagara, skin | 8 | 2.5 | Herbs and sundry | Hops 1 | 3 | 2.7 |
| Fruit | Apple, Royal Gala, flesh | 2 | 1.8* | Herbs and sundry | Hops 2 | 10 | 2.0 |
| Fruit | Apple, Royal Gala, skin | 4 | 1.5* | Herbs and sundry | Mushroom, saffron milk cap | — | — |
| Fruit | Avocado, organic | 6 | 1.5* | Herbs and sundry | Olive, leaf | — | |
| Fruit | Avocado | 5 | 1.3* | Herbs and sundry | Parsley | 1 | 1.9* |
| Fruit | Blackberry | 3 | 5.3 | Herbs and sundry | Rosemary 1 | 7 | 2.6 |
| Fruit | Blackcurrant | 1 | 2.8 | Herbs and sundry | Rosemary 2 | 7 | 2.8* |
| Fruit | Blueberry | E | 2.3 | Herbs and sundry | Sage | 4 | 2.4* |
| Fruit | Boysenberry | 4 | 5.0 | Herbs and sundry | Tea, green 1 | — | — |
| Fruit | Cherry | 7 | 2.8* | Herbs and sundry | Tea, green 2 | 6 | 1.8 |
| Fruit | Feijoa 1 | 3 | 5.1 | ||||
| Fruit | Feijoa 2 | 4 | 5.1 | Meat | Beef | 7 | 1.5 |
| Fruit | Gooseberry | — | — | Meat | Deer velvet | 1 | 2.4 |
| Fruit | Grapefruit | — | — | Meat | Kidney, pig | 8 | 2.2* |
| Fruit | Kiwifruit, gold, crush | 8 | 1.1* | Meat | Lamb | 1 | 1.7* |
| Fruit | Kiwifruit, green, crush | 5 | 1.3* | ||||
| Fruit | Kiwifruit + boysenberry, crush | 1 | 4.9 | Seafood | Arrow Squid, flesh | 8 | 1.7 |
| Fruit | Kiwifruit, Actinidia callosa var henryi | 6 | 1.6 | Seafood | Arrow Squid, intestine | 8 | 3.0 |
| Fruit | Kiwifruit, A. chinensis Hort 16A | 2 | 1.6 | Seafood | Arrow Squid, tentacles 1 | 8 | 3.0 |
| Fruit | Kiwifruit, A. chinensis var rufopulpa | 3 | 1.8 | Seafood | Arrow Squid, tentacles 2 | 8 | 2.7 |
| Fruit | Kiwifruit, A. chrysantha | 2 | 2.5 | Seafood | Crayfish, flesh | 8 | 1.8 |
| Fruit | Kiwifruit, A. deliciosa (DA36-01) | 8 | 1.6 | Seafood | Crayfish, exoskeleton | 8 | 2.1 |
| Fruit | Kiwifruit, A. deliciosa (diploid DA) | 1 | 1.7 | Seafood | Crayfish, intestine | 7 | 2.4 |
| Fruit | Kiwifruit, A. deliciosa (Hayward) | 7 | 1.6 | Seafood | Fish, Alfonsino, flesh | 8 | 1.8* |
| Fruit | Kiwifruit, A. glaucophylla | 7 | 1.4 | Seafood | Fish, Alfonsino skin, skeleton, head | 8 | 2.2* |
| Fruit | Kiwifruit, A. indochinensis | 7 | 1.7 | Seafood | Fish, Eel, flesh | 8 | 1.3* |
| Fruit | Kiwifruit, A. latifolia | 4 | 1.6 | Seafood | Fish, Salmon, flesh 1 | — | — |
| Fruit | Kiwifruit, A. purpurea | 8 | 1.7 | Seafood | Fish, Salmon, flesh 2 | — | — |
| Fruit | Kiwifruit, A. setosa | E | 2.5 | Seafood | Fish, Salmon, flesh, smoked 1 | 8 | 2.7 |
| Fruit | Lemon | 8 | 2.3 | Seafood | Fish, Salmon, flesh, smoked 2 | 8 | 2.6* |
| Fruit | Orange | 8 | 2.3 | Seafood | Fish, Salmon, flesh, smoked 3 | 7 | 1.9 |
| Fruit | Pomegranate | 2 | 6.0 | Seafood | Fish, Salmon, intestine | 8 | 2.4 |
| Fruit | Rosehip 1 | 4 | 3.4 | Seafood | Fish, Salmon, skeleton+head | 7 | 2.5 |
| Fruit | Rosehip 2 | 2 | 3.8 | Seafood | Fish, Snapper – intestine | 7 | — |
| Fruit | Strawberry | 4 | 6.0 | Seafood | Oyster, Bluff | 8 | 2.4 |
| Fruit | Tomato | 8 | 1.8* | ||||
| Fruit | Tomato, paste | 6 | 1.3 | Vegetable | Broccoli | — | — |
| Fruit | Wolfberry | — | — | Vegetable | Carrots | — | — |
| Vegetable | Kumara | — | — | ||||
| Dairy | Colostrum, Cow | — | — | Vegetable | Onions | — | — |
| Dairy | Milk, goat | 1 | 1.5 | Vegetable | Pumpkin | 8 | 2.3 |
| Dairy | Milk, cow, protein powder, casein | 11 | 1.2* | ||||
| Dairy | Milk, sheep | — | — |
Production of fruit extracts for AMPK assay
Polyphenol-rich extracts were prepared for apple, blackcurrant, blueberry, boysenberry, feijoa and kiwifruit as previously described.23 Briefly, fruit were extracted with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EtOH–H2O, the polyphenolic compounds were bound to XAD-7 resin, eluted with EtOH and dried to a powder using a rotary evaporator. The dried extracts were dissolved in DMSO (10 mg mL−1).
1 EtOH–H2O, the polyphenolic compounds were bound to XAD-7 resin, eluted with EtOH and dried to a powder using a rotary evaporator. The dried extracts were dissolved in DMSO (10 mg mL−1).
JAK2 and AMPK assays
The JAK2 and AMPK assays used Perkin-Elmer Lance® Ultra fluorescence resonance energy transfer (TR-FRET).11 Briefly, the phosphorylated amino acid on the fluorescently tagged (Ulight™) JAK2 or AMPK peptide substrate is detected by the proximity of europium chelated to an anti-phospho-amino acid antibody. Standard reaction buffer contained 1 nM kinase; 200 nM ULight substrate; 100 μM Mg ATP; 50 mM HEPES; 9 mM MgCl2; 2 mM dithiothreitol; 1 mM EGTA and 0.01% Tween® 20. The kinase reaction was stopped by the addition of europium antibody to a final concentration of 2 nM in Lance detection buffer and EDTA to a final concentration of 10 mM. Kinase and antibody reaction phases were incubated for 60 min at 20–25 °C. Europium fluorescence was read at excitation and emission wavelengths of 345 nm and 625 nm and the phosphorylated peptide product was read at 665 nm. All statistical analyses (Sigmoidal curve fitting and IC50 estimates) were performed with Origin® software v7.5 (OriginLab, Northampton, MA, USA). IC50 estimates were derived using the logistic equation (y = A2 + (A1 − A2)/(1 + (x/x0)^p)). Each IC50 is produced with a standard error of the IC50 estimate. Although the “time resolved” component of TR-FRET is effective at reducing interference from sample fluorescence, some concentrated samples nonetheless contained components that substantially altered the emission of either the europium or the ULight fluors. To detect these artefacts samples were read for ‘normal’ (i.e. non-time resolved) ULight fluorescence (excitation 625 nm, emission 665 nm) as soon as substrate was added. In addition, interference with Europium emission was detected as soon as the antibody was added by reading at 345 nm excitation and 625 nm emission. Any microplate wells showing more than 15% decrease in fluorescence, compared to DMSO controls, were excluded from TR-FRET analyses.For JAK2 analysis, samples dissolved in DMSO were diluted into assay buffer on a Biomek 2000 liquid handling system and assayed at four ten-fold dilutions ranging from 1/125 to 1/125000. AMPK analysis was on a much smaller scale than JAK2, and therefore carried out manually. Adenosine monophosphate and the thienopyridone compound A769662 are allosteric AMPK activators that bind at different sites on AMPK.25 These compounds served as positive controls in our search for AMPK activating phytochemicals.
Results and discussion
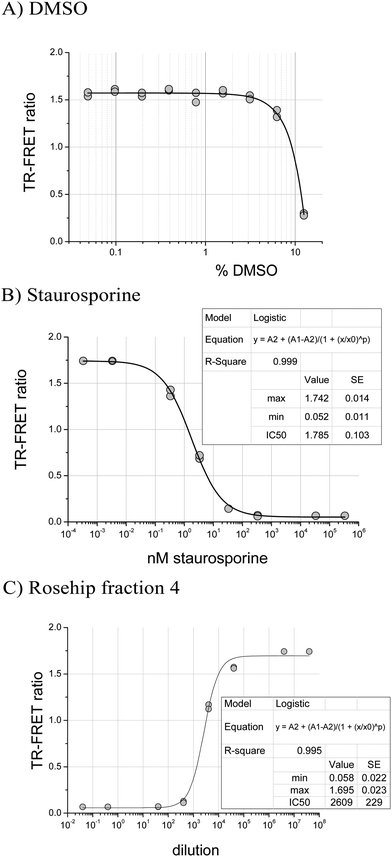
In the JAK2 assay, samples dissolved in DMSO were diluted to a final concentration of 0.83% DMSO in assay buffer. This concentration had no effect on JAK2 activity since DMSO was tolerated up to 2% (Fig. 1A). For JAK2, an IC50 of 1.8 nM for staurosporine was measured using the robotic assay, close to the 0.9 nM determined by the JAK2 suppliers (Fig. 1B). Rosehip samples, which contain ellagic acid,26 a known phytochemical kinase inhibitor,16 were tested to confirm that the automated JAK2 assay was functioning. Fig. 1C shows the dose response curve for a fraction 4 which gave 50% inhibition at a dilution factor of 2609.Once the assay protocol was validated we analysed 49 different types of food extract (Table 1). In some cases several varieties of one type of food were tested. For example, four types of apple were tested and some of these were separated into skin and flesh before extraction and fractionation because of differences in phytochemical contents.27 The crude extracts were assayed alongside 10 reversed-phase fractions designed to concentrate and separate food components ranging from water soluble compounds to lipids.27 This fractionation can potentially separate active inhibitors from masking compounds, and the activity profile assists with dereplication of known inhibitors. The screening results are summarised in Table 1.
Only the most active sample in the set of 11 for each food extract is reported in Table 1. Occasionally the unfractionated whole extract scored as the most active material. During the fractionation procedure the samples are concentrated. Therefore it is possible that during the assay's aqueous dilution steps, the higher concentration of inhibitor in the fractions may cause it to precipitate thereby causing an underestimate of its quantity. The whole extracts contain a complex mixture of hydrophobic and hydrophilic molecules from plant and animal tissues. There is the potential for the formation of an emulsion in the whole extract which artificially increases the ‘apparent solubility’ of the inhibitors during aqueous dilution. In addition, the pure fractions have been subjected to more exposure to light and possibly oxidation by the air or may be chemically labile outside of their original environment. We suppose that insolubility and instability of phytochemicals might sometimes reduce the inhibitory activity of the fractions. In addition, although this study does not look into synergistic mechanisms of inhibition, it is formally possible that the presence of different kinase inhibitors could act in combination with each other in the whole extract and that this synergy is lost in the fractions.
The most potent JAK2 inhibitors were readily detected because they caused inhibition at dilutions ≥1 × 105, presumably well beyond any possible interference from other components. The distribution of highest JAK2 inhibitory activity across extract and fractions summarised in Fig. 2 with IC50 values on a log scale to facilitate comparison. Samples with activities higher than 1 × 106 could not be scored accurately in the high throughput screening process and have been shown in Fig. 2 as having an activity of only 1 × 106. This occurred in some fractions of pomegranate and strawberry samples.
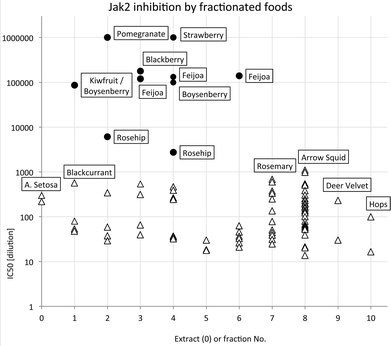 | ||
| Fig. 2 Distribution of highest JAK2 inhibitory activity across extract and fractions (● foods known or suspected to contain ellagitannins). | ||
It is apparent that the data fall into two groups, with several fruit samples two to three orders of magnitude more active than the other foods (Fig. 2). Labelling foods reported in the literature as containing ellagic acid or ellagitannins28–30 with solid black circles showed that all the JAK2 inhibitory activity found in this screening was attributable to these phytochemicals. Note that kiwifruit extracts were not active, but a combined kiwifruit and boysenberry “crush” was active due to the boysenberry ellagitannins (Table 1).16
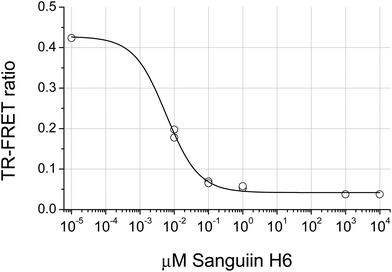
The distribution of the JAK2 inhibitory activity across the fractions was characteristic, peaking around fractions 2–4, but did vary slightly between ellagitannin containing fruits (Fig. 3). The peak of inhibitory activity for pomegranate, rich in punicalagin,29 occurred in fractions 2 and 3 whereas the berry fruits, rich in Sanguiin H-6,29 showed peak activity in slightly less polar fractions (Fig. 3). Sanguiin H-6 purified from boysenberry extracts31 was found to have an IC50 of 58 ± 6.1 nM in this JAK2 assay (Fig. 4).
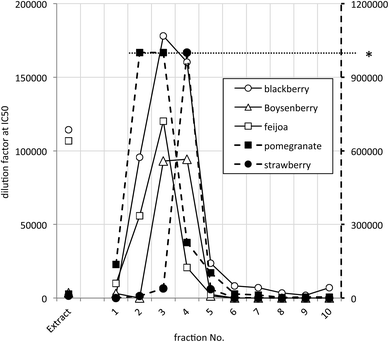 | ||
| Fig. 3 JAK2 inhibitory activity in selected active ellagitannin containing samples. * Dilution factors above 1 × 106 were not scored accurately – shown by the dashed line to the right hand Y-axis. | ||
Ellagitannins are known to be converted by hydrolysis to ellagic acid and subsequently to urolithins in the gut,32 and ellagic acid is known as a strong inhibitor of kinases.23 Healthful properties have been ascribed to ellagic acid including anti-cancer33,34 and anti-inflammatory activity in animal models of gut inflammation.35,36 The concentration of ellagitannins in raspberries and blackberries is reported to be as high as 2 mg kg−1,37 most of which is hydrolysed to ellagic acid by acid hydrolysis in vivo.38 However, ellagic acid bioavailability is reported to be very low. In rats the plasma concentration reached only 0.2 μg ml−1 after an oral dose of 0.8 g kg−1.39 In a human study when volunteers consumed around 350 mg of an ellagic acid/ellagitannin mixture in the form of pomegranate drink, the maximum plasma concentration achieved was around 30 ng ml−1.40 A contributory factor to this poor bioavailability may be that ellagic acid binds non-specifically to enterocyte protein and DNA. In a study using the human enterocyte cells Caco2, 93% of cellular ellagic acid was irreversibly bound to macromolecules.41 However, although ellagic acid has poor bioavailability, the increased permeability of the gut in Crohn's disease42 coupled with the high affinity of ellagic acid for JAK2, may contribute to the beneficial effects of ellagic acid in animal models of digestive tract cancer and inflammation.
AMPK
AMPK assay conditions were validated by confirming inhibition by staurosporine and activation by AMP and the synthetic small molecule activator A769662 (Fig. 5).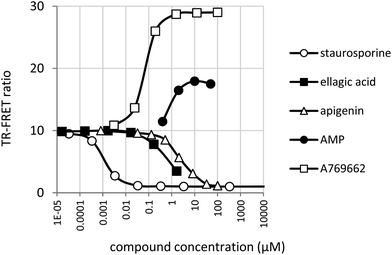 | ||
| Fig. 5 Inhibition of AMPK by staurosporine, apigenin and ellagic acid. Activation by AMP and A769662. | ||
Compared to JAK2, the activity of AMPK was slightly more sensitive to DMSO, but this was well tolerated below a concentration of 1% (data not shown). 50% of maximum activation was achieved at 69 ± 3 nM by A769662 and at 0.9 ± 0.03 μM by AMP. These values are close to the values reported for A76966243 and AMP.44 A769662 was capable of increasing substrate phosphorylation by approximately 3-fold whereas the maximum increase in activity observed with AMP was nearer to 1.5 fold. The greater amplification of AMPK by A769662 compared with AMP and the slight inhibition of AMPK by AMP in the hundred-micromolar range is in agreement with published results.43Fig. 5 also shows the inhibitory effect of ellagic acid (IC50 = 0.67 ± 0.028 μM) and apigenin (IC50 = 2.53 ± 0.22 μM) on AMPK. Due to limited ellagic acid solubility, it was not tested above a concentration of 1.67 μM.
A panel of 14 purified phytochemicals were assayed for AMP activation or inhibition. The panel included resveratrol and genistein which are reported to activate AMPK.17–19 These compounds gave clear inhibition of the TR-FRET signal at concentrations of 100 μM and 10 μM. The results are shown in Table 2 along with the ellagic acid and apigenin IC50 values. Ellagic acid is clearly the most active inhibitor having a sub-micromolar IC50. However, both apigenin and cyanidin-3 glucosyl-rutinoside are potent inhibitors with IC50 values of 2.5 μM and 3.2 μM respectively.
| Phytochemical | μM IC50 | SE |
|---|---|---|
| Apigenin | 2.53 | 0.22 |
| Curcumin | — | — |
| Cyanidin 3-glucosyl-rutinoside | 3.2 | 0.61 |
| Cyanidin 3-rutinoside | 198 | 74.1 |
| Cyanidin chloride | 31.5 | 11.2 |
| Delphinidin 3-rutinoside | 546 | 125 |
| Ellagic acid | 0.67 | 0.028 |
| Genistein | 1474 | 154 |
| Idaein | 47.7 | 5.07 |
| Kaempferol 3-glucoside | 215 | 26.4 |
| Quercetin | 12.3 | 2.82 |
| Quercetin-3 beta-glucoside | 114.4 | 13.8 |
| Quercitrin | 44.9 | 4.45 |
| Resveratrol | 95.1 | 24.2 |
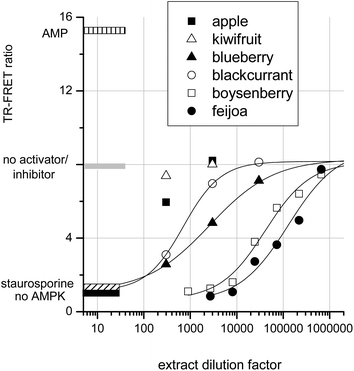
To determine whether crude extracts could activate or inhibit AMPK, a selection of fruit extracts were assayed. Fig. 6 shows that apple and kiwifruit slightly reduced the AMPK rate, but this inhibition was too slight to be quantifiable. Blackcurrant and blueberry extracts gave intermediate AMPK inhibition: IC50s were achieved at extract dilution factors of 700 and 2600 respectively. Boysenberry and feijoa extracts were much more potent inhibitors of AMPK. Boysenberry extract inhibited AMPK by 50% at a dilution of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and feijoa at a dilution of 130
000 and feijoa at a dilution of 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
It should be noted that these extracts were produced differently from the extracts tested in the JAK2 assay. However, as with JAK2, strong AMPK kinase inhibition by a fruit extract correlates with presence of ellagitannins. The inhibition of AMPK by ellagic acid and by ellagitannin containing extracts agrees with published data showing ‘broad-spectrum’ kinase inhibition by ellagic acid.16,24 JAK2, a tyrosine kinase, and casein Kinase II, a serine threonine kinase, are both inhibited in the nanomolar range by ellagic acid. However, the observed inhibition of AMPK by apigenin and resveratrol are somewhat at odds with published data. The data reported here are from a direct assay on purified AMPK, whereas apigenin and resveratrol treatment of keratinocytes20 and adipocytes18 leads to AMPK activation within the cells. It therefore seems likely that, although these compounds are AMPK activators in whole cells, they do not exert their effects directly on AMPK. Conceivably these AMPK activating phytochemicals may exert their influence on AMPK regulators within the cells. For example AMPK activity is suppressed by dephosphorylation of threonine 172 by phosphatase 2A.45 Thus inhibition of an AMPK control protein may lead indirectly to AMPK activation. An alternative explanation may be that apigenin and resveratrol are able to directly inhibit protein kinase A (PKA).46 PKA phosphorylates AMPK at serine-173 and thereby prevents AMPK activation via phospho-threonine-172. Thus inhibition of PKA might conceivably result in AMPK activation depending on the relative affinities of the phytochemicals for AMPK compared with its control proteins phosphatase 2A or PKA.
Ellagitannins have a widespread occurrence in human foods28 including several not tested in this study (Table 3). We predict that these other foods could be JAK2 inhibitors in vitro, although ellagitannin structures vary depending on their source. Sanguiin H-6 is the major ellagitannin in the order Rosales, castalagin is the major ellagitannin component of chestnut and oak (the order Fagales) while punicalagin is a major component of pomegranate ellagitannins (order Myrtales). Many toxic plant species also produce ellagitannins. For example Euphorbia species contain ellagitannins but also highly toxic phorbol esters and the structurally related ingenols.52
| Food/plant material | This study | Species/order | Ellagitannin reference |
|---|---|---|---|
| Blackberry | Yes | Rubus fruticosus/Rosales | 37 |
| Boysenberry | Yes | Rubus ursinus × idaeus/Rosales | 31 |
| Pomegranate | Yes | Punica granatum/Myrtales | 40 |
| Rosehip | Yes | Rosa rugosa/Rosales | 26 |
| Strawberry | Yes | Fragaria × ananassa/Rosales | 47 |
| Muscadine grape | No | Vitis rotundifolia/Vitales | 48 |
| Oak (for wine casks) | No | Quercus petraea/Fagales | 49 |
| Walnut | No | Juglans regia/Fagales | 50 |
| Almond | No | Prunus amygdalus/Rosales | 51 |
Ellagitannins are also known inhibitors of various tyrosine and serine/threonine kinases including JAK2,16 human epidermal growth factor receptor33 Protein Kinase C,53 Protein Kinase A and Casein Kinase II.54 Here we show that ellagitannins are also potent inhibitors of AMPK and we report that the ellagitannin Sanguiin H-6 inhibits JAK2 with an IC50 of 58nM.
Conclusions
We have shown that potent JAK2 and AMPK inhibitory compounds are common in the human diet. JAK2 and AMPK inhibitory activity is strongly associated with the presence of ellagitannins in these foods. Future studies will be required to determine if dietary ellagitannins have sufficient bioavailability to ameliorate inflammatory bowel disease or influence metabolism through effects on AMPK.Abbreviations
| AMP | Adenosine monophosphate |
| AMPK | Adenosine monophosphate kinase |
| ATP | Adenosine triphosphate |
| DMSO | Dimethyl sulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| EGTA | Ethylene-glycol-tetraacetic acid |
| IC50 | Half maximal inhibitory concentration |
| JAK2 | Janus kinase 2 |
| PKA | Protein kinase A |
| TR-FRET | Time resolved, Förster resonance energy transfer |
Acknowledgements
This work was supported by Nutrigenomics New Zealand, funded by the Ministry of Business, Innovation and Employment (contracts C11X1009 and CO2X0403), and by a ‘blue-skies’ grant from Plant & Food Research.References
- J. R. Paterson, R. Srivastava, G. J. Baxter, A. B. Graham and J. R. Lawrence, J. Agric. Food Chem., 2006, 54, 2891–2896 CrossRef CAS PubMed.
- G. K. Beauchamp, R. S. J. Keast, D. Morel, J. M. Lin, J. Pika, Q. Han, C. H. Lee, A. B. Smith and P. A. S. Breslin, Nature, 2005, 437, 45–46 CrossRef CAS PubMed.
- P. G. Morris, X. K. Zhou, G. L. Milne, D. Goldstein, L. C. Hawks, C. T. Dang, S. Modi, M. N. Fornier, C. A. Hudis and A. J. Dannenberg, Cancer Prev. Res., 2013, 6, 428–436 CrossRef CAS PubMed.
- N. Dali-Youcef, M. Mecili, R. Ricci and E. Andres, Ann. Med., 2013, 45, 242–253 CrossRef CAS PubMed.
- B. T. Jeon, E. A. Jeong, H. J. Shin, Y. Lee, D. H. Lee, H. J. Kim, S. S. Kang, G. J. Cho, W. S. Choi and G. S. Roh, Diabetes, 2012, 61, 1444–1454 CrossRef CAS PubMed.
- J.-H. Kang, G. Tsuyoshi, I.-S. Han, T. Kawada and R. Yu, Ann. Nutr. Metab., 2009, 55, 675–675 Search PubMed.
- P. A. d'Alessio, R. Ostan, J.-F. Bisson, J. D. Schulzke, M. V. Ursini and M. C. Bene, Life Sci., 2013, 92, 1151–1156 CrossRef PubMed.
- J. C. Barrett, S. Hansoul, D. L. Nicolae, J. H. Cho, R. H. Duerr, J. D. Rioux, S. R. Brant, M. S. Silverberg, K. D. Taylor, M. M. Barmada, A. Bitton, T. Dassopoulos, L. W. Datta, T. Green, A. M. Griffiths, E. O. Kistner, M. T. Murtha, M. D. Regueiro, J. I. Rotter, L. P. Schumm, A. H. Steinhart, S. R. Targan, R. J. Xavier, C. Libioulle, C. Sandor, M. Lathrop, J. Belaiche, O. Dewit, I. Gut, S. Heath, D. Laukens, M. Mni, P. Rutgeerts, A. Van Gossum, D. Zelenika, D. Franchimont, J. P. Hugot, M. de Vos, S. Vermeire, E. Louis, L. R. Cardon, C. A. Anderson, H. Drummond, E. Nimmo, T. Ahmad, N. J. Prescott, C. M. Onnie, S. A. Fisher, J. Marchini, J. Ghori, S. Bumpstead, R. Gwilliam, M. Tremelling, P. Deloukas, J. Mansfield, D. Jewell, J. Satsangi, C. G. Mathew, M. Parkes, M. Georges and M. J. Daly, Nat. Genet., 2008, 40, 955–962 CrossRef CAS PubMed.
- J. N. Ihle and I. M. Kerr, Trends Genet., 1995, 11, 69–74 CrossRef CAS.
- E. Parganas, D. Wang, D. Stravopodis, D. J. Topham, J. C. Marine, S. Teglund, E. F. Vanin, S. Bodner, O. R. Colamonici, J. M. van Deursen, G. Grosveld and J. N. Ihle, Cell, 1998, 93, 385–395 CrossRef CAS.
- E. J. Baxter, L. M. Scott, P. J. Campbell, C. East, N. Fourouclas, S. Swanton, G. S. Vassiliou, A. J. Bench, E. M. Boyd, N. Curtin, M. A. Scott, W. N. Erber, A. R. Green and P. Canc Genome, Lancet, 2005, 365, 1054–1061 CrossRef CAS.
- L. R. Ferguson, D. Y. Han, A. G. Fraser, C. Huebner, W. J. Lam, A. R. Morgan, H. Duan and N. Karunasinghe, Mutat. Res. Fundam. Mol. Mech. Mutagen., 2010, 690, 108–115 CrossRef CAS PubMed.
- P. Saharinen and O. Silvennoinen, J. Biol. Chem., 2002, 277, 47954–47963 CrossRef CAS PubMed.
- D. G. Hardie, Am. J. Clin. Nutr., 2011, 93, 891S–896S CrossRef CAS PubMed.
- B. Kola, J. Neuroendocrinol., 2008, 20, 942–951 CrossRef CAS PubMed.
- T. K. McGhie, H. Martin and R. C. M. Lunken, Food Funct., 2012, 3, 1170–1175 CAS.
- E. Arunkumar and C. V. Anuradha, Nutr. Res., 2012, 32, 617–625 CrossRef CAS PubMed.
- S. F. Chen, Z. L. Li, W. X. Li, Z. M. Shan and W. Zhu, Can. J. Physiol. Pharmacol., 2011, 89, 793–799 CAS.
- Y. C. Wu, X. Q. Li, J. X. Zhu, W. J. Xie, W. D. Le, Z. Fan, J. Jankovic and T. H. Pan, Neurosignals, 2011, 19, 163–174 CrossRef CAS PubMed.
- X. Tong, K. A. Smith and J. C. Pelling, Mol. Carcinog., 2012, 51, 268–279 CrossRef CAS PubMed.
- Y. P. Hwang, J. H. Choi, E. H. Han, H. G. Kim, J. H. Wee, K. O. Jung, K. H. Jung, K. I. Kwon, T. C. Jeong, Y. C. Chung and H. G. Jeong, Nutr. Res., 2011, 31, 896–906 CrossRef CAS PubMed.
- N. Poulose, C. N. V. Prasad, P. A. N. Haridas and G. Anilkumar, J. Diabetes Metab., 2011, 2 Search PubMed.
- R. L. Dow, T. T. Chou, B. M. Bechle, C. Goddard and E. R. Larson, J. Med. Chem., 1994, 37, 2224–2231 CrossRef CAS.
- G. Cozza, P. Bonvini, E. Zorzi, G. Poletto, M. A. Pagano, S. Sarno, A. Donella-Deana, G. Zagotto, A. Rosolen, L. A. Pinna, F. Meggio and S. Moro, J. Med. Chem., 2006, 49, 2363–2366 CrossRef CAS PubMed.
- J. W. Scott, B. J. W. van Denderen, S. B. Jorgensen, J. E. Honeyman, G. R. Steinberg, J. S. Oakhill, T. J. Iseli, A. Koay, P. R. Gooley, D. Stapleton and B. E. Kemp, Chem. Ind., 2008, 15, 1220–1230 CAS.
- V. T. Tumbas, J. M. Canadanovic-Brunet, D. D. Cetojevic-Simin, G. S. Cetkovic, S. M. Dilas and L. Gille, J. Sci. Food Agric., 2012, 92, 1273–1281 CrossRef CAS PubMed.
- C. M. Andre, J. M. Greenwood, E. G. Walker, M. Rassam, M. Sullivan, D. Evers, N. B. Perry and W. A. Laing, J. Agric. Food Chem., 2012, 60, 10546–10554 CrossRef CAS PubMed.
- J. A. Ascacio-Valdes, J. J. Buenrostro-Figueroa, A. Aguilera-Carbo, A. Prado-Barragan, R. Rodriguez-Herrera and C. N. Aguilar, J. M. Plants Res., 2011, 5, 4696–4703 CAS.
- J. M. Landete, Food Res. Int., 2011, 44, 1150–1160 CrossRef CAS PubMed.
- M. T. Monforte, V. Fimiani, F. Lanuzza, C. Naccari, S. Restuccia and E. M. Galati, J. Med. Food, 2014, 17, 455–461 CrossRef CAS PubMed.
- M. Kool, D. J. Comeskey, J. M. Cooney and T. K. McGhie, Food Chem., 2010, 119, 1535–1534 CrossRef CAS PubMed.
- M. Sharma, L. Y. Li, J. Celver, C. Killian, A. Kovoor and N. P. Seeram, J. Agric. Food Chem., 2010, 58, 3965–3969 CrossRef CAS PubMed.
- D. Fridrich, A. Glabasnia, J. Fritz, M. Esselen, G. Pahlke, T. Hofmann and D. Markor, J. Agric. Food Chem., 2008, 56, 3010–3015 CrossRef CAS PubMed.
- G. D. Stoner, L. S. Wang, C. Seguin, C. Rocha, K. Stoner, S. Chiu and A. D. Kinghorn, Pharm. Res., 2010, 27, 1138–1145 CrossRef CAS PubMed.
- A. Beserra, P. I. Calegari, M. D. Souza, R. A. N. dos Santos, J. C. D. Lima, R. M. Silva, S. O. Balogun and D. T. D. Martins, J. Agric. Food Chem., 2011, 59, 6957–6965 CrossRef CAS PubMed.
- M. A. Rosillo, M. Sanchez-Hidalgo, A. Cardeno and C. A. de la Lastra, Biochem. Pharmacol., 2011, 82, 737–745 CrossRef CAS PubMed.
- M. N. Clifford and A. Scalbert, J. Sci. Food Agric., 2000, 80, 1118–1125 CrossRef CAS.
- U. Vrhovsek, A. Palchetti, F. Reniero, C. Guillou, D. Masuero and F. Mattivi, J. Agric. Food Chem., 2006, 54, 4469–4475 CrossRef CAS PubMed.
- F. Lei, D. M. Xing, L. Xiang, Y. N. Zhao, W. Wang, L. J. Zhang and L. J. Du, J. Chromatogr., B: Biomed. Appl., 2003, 796, 189–194 CrossRef CAS.
- N. P. Seeram, R. Lee and D. Heber, Clin. Chim. Acta, 2004, 348, 63–68 CrossRef CAS PubMed.
- A. C. Whitley, G. D. Stoner, M. V. Darby and T. Walle, Biochem. Pharmacol., 2003, 66, 907–915 CrossRef CAS.
- M. Dastych, M. Dastych, H. Novotna and J. Cihalova, Dig. Dis. Sci., 2008, 53, 2789–2792 CrossRef CAS PubMed.
- O. Goransson, A. McBride, S. A. Hawley, F. A. Ross, N. Shpiro, M. Foretz, B. Viollet, D. G. Hardie and K. Sakamoto, J. Biol. Chem., 2007, 282, 32549–32560 CrossRef PubMed.
- M. Suter, U. Riek, R. Tuerk, U. Schlattner, T. Wallimann and D. Neumann, J. Biol. Chem., 2006, 281, 32207–32216 CrossRef CAS PubMed.
- T. Wang, Q. J. Yu, J. A. Chen, B. Deng, L. H. Qian and Y. Y. Le, PLoS One, 2010, 5 Search PubMed.
- N. Djouder, R. D. Tuerk, M. Suter, P. Salvioni, R. F. Thali, R. Scholz, K. Vaahtomeri, Y. Auchli, H. Rechsteiner, R. A. Brunisholz, B. Viollet, T. P. Makela, T. Wallimann, D. Neumann and W. Krek, EMBO J., 2010, 29, 469–481 CrossRef CAS PubMed.
- J. L. Maas, S. Y. Wang and G. J. Galletta, HortScience, 1991, 26, 66–68 Search PubMed.
- J. A. Boyle and L. Hsu, Am. J. Enol. Vitic., 1990, 41, 43–47 CAS.
- M. K. Quinn and V. L. Singleton, Am. J. Enol. Vitic., 1985, 36, 148–155 CAS.
- K. J. Anderson, S. S. Teuber, A. Gobeille, P. Cremin, A. L. Waterhouse and F. M. Steinberg, J. Nutr., 2001, 131, 2837–2842 CAS.
- L. Y. Xie, A. V. Roto and B. W. Bolling, J. Agric. Food Chem., 2012, 60, 12151–12156 CrossRef CAS PubMed.
- T. Yoshida, K. Yokoyama, O. Namba and T. Okuda, Chem. Pharm. Bull., 1991, 39, 1137–1143 CrossRef CAS.
- T. Ueno, T. Miyanaga, F. Kawakami, M. Okano, T. Tanaka and K. Ohtsuki, Biol. Pharm. Bull., 2002, 25, 1401–1404 CAS.
- S. Kosuge, T. Maekawa, C. Saito, T. Tanaka, I. Kouno and K. Ohtsuki, J. Biochem., 2001, 129, 403–409 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |