Investigation of the reactions of acrylamide during in vitro multistep enzymatic digestion of thermally processed foods
Aytül
Hamzalıoğlu
and
Vural
Gökmen
*
Department of Food Engineering, Hacettepe University, 06800 Beytepe, Ankara, Turkey. E-mail: vgokmen@hacettepe.edu.tr; Fax: +90-312-299-2123; Tel: +90-312-297-7108
First published on 18th November 2014
Abstract
This study investigated the fate of acrylamide in thermally processed foods after ingestion. An in vitro multistep enzymatic digestion system simulating gastric, duodenal and colon phases was used to understand the fate of acrylamide in bakery and fried potato products. Acrylamide levels gradually decreased through gastric, duodenal and colon phases during in vitro digestion of biscuits. At the end of digestion, acrylamide reduction was between 49.2% and 73.4% in biscuits. Binary model systems composed of acrylamide and amino acids were used to understand the mechanism of acrylamide reduction. High-resolution mass spectrometry analyses confirmed Michael addition of amino acids to acrylamide during digestion. In contrast to bakery products, acrylamide levels increased significantly during gastric digestion of fried potatoes. The Schiff base formed between reducing sugars and asparagine disappeared rapidly, whereas the acrylamide level increased during the gastric phase. This suggests that intermediates like the Schiff base that accumulate in potatoes during frying are potential precursors of acrylamide under gastric conditions.
Introduction
Ingestion of food is considered as the major route of exposure to many contaminants in human health risk assessment. Acrylamide is one of the process contaminants formed in foods as a result of thermal processing, such as roasting, frying or baking, at elevated temperatures.1 Bakery products, fried potatoes and roasted coffee are the main dietary sources of acrylamide.2The total amount of a contaminant found in the ingested food does not always reflect the amount that is available to the body. Ingested food enters the digestive tract, which significantly alters its structure and chemical composition by varying the pH and the action of several enzymes in the mouth, stomach and intestine. Thus, bioaccessibility is used to describe the proportion of the ingested contaminants in food that are released from the matrix into digestive juice in the gastrointestinal tract.3 Therefore, determination of the bioaccessibility of a contaminant from the matrix, and the fate of the ingested contaminant during digestion is important for human health. Consequently, in vivo methods, including animal and human subjects, are better for obtaining more accurate results, although they are also time consuming and expensive.4In vitro models that simulate body conditions well are a useful alternative to in vivo studies and allow rapid screening of target compounds.
Several studies have been carried out to determine the bioaccessibility of certain contaminants. Brandon et al.5 used in vitro digestion models to test the bioaccessibilities of lead, phthalates, and benzoic acid in some commercial products. Yang et al.6 tested the bioaccessibility of cadmium in uncooked rice from mining areas using an in vitro digestion model. De Angelis et al.7 studied the bioaccessibility of Fusarium toxins, T-2 and HT-2, in two types of contaminated bread using a digestion model simulating the upper intestinal tract.
Acrylamide is one of the most widely encountered thermal process contaminants in foods. However, information about its fate during the digestion of processed foods is lacking. Owing to its potential reactivity, acrylamide present in foods may interact with certain components released from the food matrix under the varying conditions in the gastrointestinal tract. Moreover, these conditions may favor the conversion of precursors into acrylamide. Therefore, in this study we investigated the fate of acrylamide present in thermally processed foods after ingestion using an in vitro multistep enzymatic digestion system. The system simulating mainly gastric, duodenal and colon phases was used to digest actual foods (bakery and fried potato products). Binary models were also digested to understand better the reactions between acrylamide and the amino acids cysteine and lysine under digestion conditions. The digested samples were analyzed by HRMS to confirm the Michael addition of amino acids to acrylamide, and the conversion of Schiff bases to acrylamide.
Results and discussion
Bakery and fried potato products, which are the main sources of dietary acrylamide, were selected as typical examples of thermally processed foods. A total of four types of commercial biscuits (two sweet and two savory), one laboratory-made biscuit, two types of potato chips, and one type of fried potato were used for the in vitro multistep enzymatic digestion test. Initial acrylamide concentrations were measured as 232 ± 4 and 348 ± 18 ng g−1 for savory biscuits, 270 ± 5 and 318 ± 2 ng g−1 for sweet biscuits, and 173 ± 3 ng g−1 for a laboratory-made sweet biscuit. Acrylamide concentrations of potato chips and fried potato samples were measured as 178 ± 8, 239 ± 18 and 263 ± 18 ng g−1, respectively.Table 1 gives the amounts of acrylamide remaining in the digests of biscuit samples after the gastric and duodenal phases of the digestion process. There were significant reductions in the amounts of acrylamide in the digests of all sweet and savory biscuits at the end of the gastric and duodenal phases. Taking the entire enzymatic digestion process into account, the acrylamide reduction ratio ranged between 49.2% and 73.4% for biscuit samples. The reduction was significantly higher in the gastric and duodenal phases than in the colon phase. For biscuits, the ratio of acrylamide reduction ranged between 17.4% and 49.9% in the gastric phase, and between 23.9% and 58.1% in the duodenal phase.
| Acrylamide per 5 g sample (μmol) | ||||
|---|---|---|---|---|
| Initial | Gastric phase | Duodenal phase | Colon phase | |
a Laboratory-made biscuit sample. a–d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Values marked with different letters in each row are significantly different (P < 0.05). Values marked with different letters in each row are significantly different (P < 0.05).
|
||||
| Non-sweet biscuits | ||||
| Sample 1 | 16.32 ± 0.40a | 10.90 ± 0.10b | 6.68 ± 1.39c | 5.73 ± 1.09c |
| Sample 2 | 24.48 ± 1.79a | 12.27 ± 0.45b | 7.95 ± 0.50c | 7.60 ± 0.56c |
| Sweet biscuits | ||||
| Sample 1 | 18.96 ± 0.45a | 13.33 ± 2.44b | 5.59 ± 0.05c | 5.24 ± 0.35c |
| Sample 2 | 22.33 ± 0.15a | 11.57 ± 2.44b | 7.28 ± 0.25c | 5.94 ± 0.35c |
| Sample 3a | 12.13 ± 0.25a | 10.02 ± 0.25b | 7.63 ± 0.45c | 6.16 ± 0.25d |
The gastric phase was the simulation of stomach where pepsin hydrolyzed proteins into smaller peptides or amino acids under low pH conditions. The duodenal phase included the addition of bile salts capable of promoting the digestion and absorption of lipids by pancreatin, which contains amylase, lipase and trypsin. In the colon phase, proteolytic enzymes of the microbial flora of the colon continued to favor the hydrolysis of proteins and peptides. Thus, the simulated digestion process created a pool of amino acids that may interact with acrylamide. It has been previously reported that Michael addition of amino acids to acrylamide, which is a potential way of decreasing the acrylamide content of foods, can take place under certain conditions.8,9 Because of their highly electrophilic properties, each amino acid molecule could form adducts with one or two acrylamide molecules.10
Three model systems, namely acrylamide (ACR), acrylamide–lysine (ACR–LYS), and acrylamide–cysteine (ACR–CYS), were used to understand the mechanism of acrylamide reduction. Table 2 gives the amounts of acrylamide remaining in the digests of these model systems after the gastric, duodenal and colon phases. At the end of the gastric phase, there were slight but statistically significant (p < 0.05) reductions in the amounts of acrylamide for both the ACR–CYS and ACR–LYS model systems. The amount of acrylamide remained relatively stable after the duodenal and colon phases in the digest of the ACR–LYS model system. However, it tended to decrease significantly (p < 0.05) after the duodenal and colon phases in the digest of the ACR–CYS model system. These findings indicated the potential of acrylamide to react with the nucleophilic groups (–SH, –NH2) of amino acid side chains under the digestion conditions. Hidalgo et al.10,11 reported a rapid decrease in the amount of acrylamide upon heating in the presence of N-acetyl-cysteine or lysine as a consequence of the Michael addition of the nucleophilic groups to the carbon–carbon double bond of acrylamide.11 Owing to its highly nucleophilic –SH group, cysteine was thought to react more readily with acrylamide.12
| Model system | Acrylamide per model system (μmol) | |||
|---|---|---|---|---|
| Initial | Gastric phase | Duodenal phase | Colon phase | |
a–e![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Values marked with different letters in each row are significantly different (P < 0.05). Values marked with different letters in each row are significantly different (P < 0.05). |
||||
| ACR | 10.25 ± 0.56a | 9.29 ± 0.19a,b | 8.10 ± 0.07b,c | 7.79 ± 0.05c |
| Binary Models | ||||
| ACR–LYS | 10.25 ± 0.56a | 8.22 ± 0.01b,c | 8.27 ± 1.25b,c | 8.36 ± 0.43b,c |
| ACR–CYS | 10.25 ± 0.56a | 8.31 ± 0.13b,c | 5.32 ± 0.77d | 4.02 ± 0.41e |
Fig. 1 shows the proposed mechanism of acrylamide elimination through Michael addition of cysteine during the digestion process. According to this mechanism, cysteine may react with one or two moles of acrylamide from both nucleophilic groups (–SH or NH2) forming 1, 1′ or 2 Michael adducts. Scan HRMS analyses of the digests of the ACR–CYS model system confirmed the formation of these adducts with very high mass accuracy (Δ < 2 ppm) under the simulated digestion conditions. Only the formation of Michael adduct 1 was observed in the digests of the ACR–LYS model system under the same conditions. These results suggest that cysteine released through the proteolytic activity of enzymes in the gastrointestinal tract may be responsible for the elimination of acrylamide during digestion.
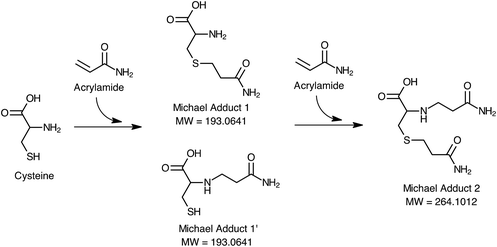 | ||
| Fig. 1 Proposed mechanism for the reduction of acrylamide during in vitro enzymatic digestion through the formation of Michael adducts with cysteine. | ||
Table 3 gives the amounts of acrylamide remaining in the digests of fried potato samples after gastric, duodenal and colon phases of the digestion process. In contrast to biscuits, the amounts of acrylamide increased significantly (p < 0.05) during the gastric digestion of fried potatoes. At the end of the gastric phase, acrylamide levels increased by 3.95, 1.20 and 1.45 times in the fried potato and potato chip digests, respectively. Acrylamide levels tended to decrease significantly (p < 0.05) in the digests of fried potato samples after the duodenal and colon phases. The ratio of acrylamide reduction ranged between 78.2% and 96.8% in the duodenal phase, and between 48.3% and 90.2% in the colon phase.
| Acrylamide per 5 g sample (μmol) | ||||
|---|---|---|---|---|
| Initial | Gastric phase | Duodenal phase | Colon phase | |
a–d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Values marked with different letters in each row are significantly different (P < 0.05). Values marked with different letters in each row are significantly different (P < 0.05). |
||||
| Potato fry | ||||
| Sample 1 | 18.47 ± 1.74a | 73.02 ± 6.17b | 2.32 ± 0.40c | 0.34 ± 0.18d |
| Potato chips | ||||
| Sample 1 | 12.49 ± 0.75a | 14.98 ± 1.29b | 1.20 ± 0.70c | 0.62 ± 0.10c |
| Sample 2 | 16.81 ± 1.79a | 24.41 ± 0.10b | 5.31 ± 1.04c | 0.52 ± 0.03d |
Eriksson et al.13 determined the effects of pH and different enzymes on the extraction of acrylamide from foods. According to their results, the extraction yield of acrylamide from foods at pH values ranging from 2.0 to 7.5, or with pepsin was similar to the yield obtained with water. Therefore, the increase in acrylamide levels in the digests of fried potato products during gastric digestion cannot be attributed to its increased extractability under acidic conditions. Asparagine and reducing sugars are the main precursors of acrylamide.14 According to the formation of acrylamide, Schiff bases, decarboxylated Schiff bases, and 3-aminopropionamide are critical intermediates upon heating a mixture containing asparagine and glucose.15–17 Because raw potato is relatively rich in asparagine and reducing sugars, frying may form high quantities of the intermediates together with acrylamide in fried potatoes. Therefore, these intermediates may be considered as potential precursors of acrylamide during the gastric digestion phase. To confirm this, a model system composed of asparagine and glucose (ASN–GLC) was prepared and heated at 180 °C for 10 min. Then, a heated model system of ASN–GLC was subjected to in vitro multistep digestion. As in fried potato samples, the amount of acrylamide significantly increased (41.3%) in the digest of the ASN–GLC model system during the gastric phase, whereas it significantly decreased (95.7%) after the duodenal and colon phases. Prior to gastric digestion, scan HRMS analyses confirmed the presence of a Schiff base formed between asparagine and glucose in a heated ASN–GLC model system, as well as in fried potato samples with very high mass accuracy (Δ < 1 ppm). In the gastric phase, a decrease in the signal response of the parent [M + H]+ ion of the Schiff base was compatible with the increase in the acrylamide level. More than 90% of the Schiff base present initially in the fried potatoes disappeared in their digests after the gastric phase. The results suggest that intermediates like Schiff bases formed between asparagine and the reducing sugars that accumulated in the fried potatoes during frying are converted to acrylamide under gastric conditions as proposed in Fig. 2.
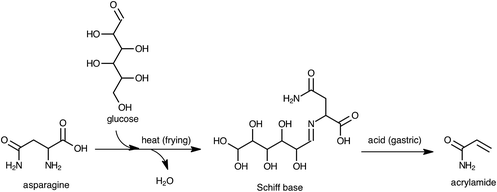 | ||
| Fig. 2 Proposed mechanism for the formation of acrylamide during in vitro gastric digestion from the precursors in fried potato. | ||
Experimental
Chemicals and consumables
Acrylamide (99%), L-cysteine (>99%), L-lysine, and L-asparagine (98%), were purchased from Sigma (Deisenhofen, Germany). Potassium chloride, sodium chloride, magnesium chloride, ammonium bicarbonate, and potassium dihydrogen phosphate were purchased from Merck (Darmstadt, Germany). Potassium hexacyanoferrate(II) trihydrate and zinc sulfate heptahydrate were purchased from Merck (Darmstadt, Germany). Carrez I and Carrez II solutions were prepared by dissolving potassium hexacyanoferrate (15 g) in water (100 mL), and zinc sulfate (30 g) in water (100 mL), respectively. The enzymes pepsin (≥250 U mg−1 solid) from porcine gastric mucosa, pancreatin (4 × USP) from porcine pancreas, protease from Streptomyces griseus (≥3.5 U mg−1 solid), and Viscozyme L were purchased from Sigma-Aldrich (Deisenhofen, Germany). Porcine bile extract was also purchased from Sigma Aldrich (Deisenhofen, Germany). Formic acid (98%), acetonitrile and methanol (HPLC grade) were purchased from J. T. Baker (Deventer, Holland). Oasis MCX solid phase extraction cartridges (1 mL, 30 mg), UPLC HSS T3 column (150 mm × 4.6 mm i.d.; 3 μm), Atlantis T3 column (250 mm × 4.6 mm i.d.; 5 μm), and 0.45 μm nylon syringe filters were supplied by Waters (Millford, MA, USA).Preparation of foods
Both commercial and laboratory-made food samples were used to determine the fate of acrylamide during in vitro digestion. Commercial biscuits and potato chips were obtained from a local market, and fried potato samples were obtained from a fast food restaurant in Ankara.Laboratory-made biscuits were prepared using a recipe adapted from the American Association of Cereal Chemists (AACC) method 10–54. The ingredients were as follows: wheat flour (80 g), shortening (32 g), sucrose (35 g), glucose (0.6 g), fructose (0.6 g), nonfat milk powder (0.8 g), NaHCO3 (0.8 g), NH4HCO3 (0.4 g), NaCl (1 g), and water (17.6 g). All ingredients were thoroughly mixed in accordance with the procedure described in AACC method 10–54 using a dough mixer Artisan Kitchen Aid 5KSM150 (MI, USA). Dough was rolled to a thickness of 4 mm and cut into discs with a diameter of 6 cm. The discs were baked in a conventional oven (Memmert, UNE 400, Germany) at 200 °C for 12 min. All food samples were ground and frozen dried prior to the digestion process. In addition, they were analyzed to determine their initial acrylamide content.
Preparation of model systems
Different model systems composed of acrylamide alone (ACR), acrylamide–cysteine (ACR–CYS), and acrylamide–lysine (ACR–LYS) were prepared to determine both the fate of acrylamide and its interaction with amino acids during in vitro digestion. For these model systems, the reactants (10 μmol) were dissolved in deionized water (10 mL), and directly subjected to the in vitro multistep digestion process.Another model system composed of asparagine (10 μmol) and glucose (ASN–GLC) was also prepared. It was heated at 180 °C for 10 min to form the Maillard reaction products, including acrylamide and intermediate compounds like the Schiff base formed between asparagine and glucose. Then, the heated model system was dissolved in deionized water (10 mL) and subjected to in vitro multistep digestion to determine changes in the levels of acrylamide and the Schiff base formed between asparagine and glucose.
In vitro digestion
Digestion fluids simulating the saliva, gastric juice and duodenal juice were used to mimic the gastrointestinal tract conditions. Simulated salivary fluid (SSF), simulated gastric fluid (SGF), and simulated duodenal fluid (SDF) were prepared according to the procedure described by Minekus et al.18 The in vitro digestion procedure was adapted from procedure reported by Papillo et al.19 Dry ground food (5 g) or the model system (10 mL) was transferred to a glass flask with a screw cap. For food samples, SSF (5 mL) was added and the flask was shaken for 2 min to simulate the oral passage. Liquid model system samples were not exposed to the oral phase, and they were put directly into the gastric phase. After pepsin solution (5 mL, 12.5 mg mL−1 in 0.1 M HCl) and SGF (10 mL) were added, the mixture was adjusted to pH 2.0. Then, the acidified mixture was incubated at 37 °C for 2 h with shaking at an agitation speed of 60 strokes per min to simulate the gastric phase. Bile salts were dissolved in the SDF solution to a concentration of 10 mg mL−1. The pH was adjusted to 7.5 after the gastric phase. After that, the mixture of SDF with bile salts (20 mL) and pancreatin solution (5 mL, 10 mg mL−1 in water) were added to the flask. The mixture was incubated at 37 °C for 2 h with shaking at an agitation speed of 60 strokes per min to simulate the duodenal phase. The colon phase was simulated by the addition of bacterial enzymes of the flora found in the colon. The consecutive hydrolysis of proteins and polysaccharides should occur in the sample during the colon phase. For this, protease solution (5 mL, 1 mg mL−1, pH 8.0) was added, and the mixture was incubated at 37 °C with shaking for 1 h. Then, Viscozyme L (150 μL) was added, and the mixture was incubated at 37 °C for 16 h with shaking at an agitation speed of 30 strokes per min. Aliquots of samples were withdrawn from the flask at the end of the simulated gastric, duodenal and colon phases for the analyses of acrylamide, its precursors and reaction products. All samples were digested with three parallel samples as described above.Analysis of acrylamide in the digests by LC-MS/MS
Aliquots of the digests withdrawn from the samples of the model systems were centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180g for 5 min. The supernatant was filtered through a 0.45 μm filter into an autosampler vial, and analyzed for acrylamide using LC-MS/MS. Aliquots from food samples were transferred to petri dishes and dried in an oven at 37 °C. Dried powder (100 mg) was double extracted with 10 mM formic acid (2 × 1 mL) by vortexing for 3 min. The combined extract was clarified by adding Carrez I and Carrez II (0.125 mL each) solutions. The mixture was centrifuged at 10
180g for 5 min. The supernatant was filtered through a 0.45 μm filter into an autosampler vial, and analyzed for acrylamide using LC-MS/MS. Aliquots from food samples were transferred to petri dishes and dried in an oven at 37 °C. Dried powder (100 mg) was double extracted with 10 mM formic acid (2 × 1 mL) by vortexing for 3 min. The combined extract was clarified by adding Carrez I and Carrez II (0.125 mL each) solutions. The mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min. The supernatant was passed through a preconditioned Oasis MCX solid phase extraction cartridge to clean up the extract further. The first 8 drops of the eluent were discarded. The rest was filtered through a 0.45 μm filter into an autosampler vial, and analyzed for acrylamide using LC-MS/MS.
000g for 5 min. The supernatant was passed through a preconditioned Oasis MCX solid phase extraction cartridge to clean up the extract further. The first 8 drops of the eluent were discarded. The rest was filtered through a 0.45 μm filter into an autosampler vial, and analyzed for acrylamide using LC-MS/MS.
A Waters Acquity H Class UPLC system (Millford, MA) coupled to a TQ detector with electrospray ionization operated in positive mode was used to analyze the extracts for acrylamide. The chromatographic separations were performed on an Acquity UPLC HSS T3 column (150 mm × 4.6 mm i.d.; 3 μm) using 0.1% formic acid in water with 0.1% formic acid in acetonitrile as the mobile phase at a flow rate of 0. 5 mL min−1. The column equilibrated at 40 °C and Waters Acquity FTN autosampler was held at 10 °C during the analysis. The electrospray source had the following settings: capillary voltage of 0.80 kV; cone voltage of 22 V; extractor voltage of 4 V; source temperature of 120 °C; desolvation temperature of 350 °C; and desolvation gas (nitrogen) flow of 900 L h−1. The flow rate of the collision gas (argon) was set to 100 L h−1. Acrylamide was identified by multiple reaction monitoring (MRM) of two channels. The precursor ion [M + H]+ 72 was fragmented and the product ions at m/z 55 (collision energy of 9 V) and 44 (collision energy of 12 V) were monitored. The dwell time was 0.2 s for all MRM transitions. Stock solution of acrylamide was prepared in water to a concentration of 1 mg mL−1. Working solutions were prepared by diluting the stock solution with water. The concentration of acrylamide in samples was calculated by means of a calibration curve built in the range between 1 and 20 ng mL−1 (1, 2, 5, 10, 20 ng mL−1).
Analysis of reaction products and precursors of acrylamide in the digests by high-resolution mass spectrometry (HRMS)
Aliquots of the digests withdrawn from model systems and food samples were transferred to petri dishes and dried in an oven at 37 °C. Dried powder (100 mg) was extracted with water (10 mL) by vortexing for 2 min. Then, the extract was centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180g for 5 min. The supernatant (2 mL) was passed through a 0.45 μm filter into an autosampler vial prior to the analysis of reaction products and precursors of acrylamide. HRMS was used to confirm Michael addition of amino acids (cysteine and lysine) to acrylamide in the digests of model systems. In addition, it was used to confirm the presence of acrylamide precursors, specifically the Schiff base formed between asparagine and glucose in the digests of food samples.
180g for 5 min. The supernatant (2 mL) was passed through a 0.45 μm filter into an autosampler vial prior to the analysis of reaction products and precursors of acrylamide. HRMS was used to confirm Michael addition of amino acids (cysteine and lysine) to acrylamide in the digests of model systems. In addition, it was used to confirm the presence of acrylamide precursors, specifically the Schiff base formed between asparagine and glucose in the digests of food samples.
A Thermo Scientific Accela UHPLC system (San Jose, CA) coupled to a Thermo Scientific Exactive Orbitrap HRMS was used to analyze the extracts. The HRMS system was operated in positive electrospray ionization mode. The chromatographic separations were performed on an Atlantis T3 column (250 mm × 4.6 mm i.d.; 5 μm) using a gradient mixture of 0.05% aqueous formic acid and methanol as the mobile phase at a flow rate of 0.5 mL min−1 (30 °C). The mobile phase gradient was programmed as follows: 70% methanol for 8 min, linear increase to 95% methanol over 4 min, 95% methanol for 4 min, and linear decrease to 70% methanol over 4 min. The scan analyses were performed in a m/z range of 50–400 at ultra-high resolving power (R = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The data acquisition rate, the automatic gain control target and maximum injection time were set to 1 Hz, 1 × 106 and 100 ms, respectively. The source parameters were as follows: sheath gas flow rate of 30 (arbitrary units), auxiliary gas flow rate of 10 (arbitrary units), discharge voltage of 4.5 kV, discharge current of 5 μA, capillary temperature of 330 °C, capillary voltage of 47.5 V, tube lens voltage of 115 V, and vaporizer temperature of 330 °C. The corresponding ions were extracted from the total ion chromatograms to confirm the presence of the Michael addition products of cysteine and lysine to acrylamide, and of the Schiff base of asparagine in the digests.
000). The data acquisition rate, the automatic gain control target and maximum injection time were set to 1 Hz, 1 × 106 and 100 ms, respectively. The source parameters were as follows: sheath gas flow rate of 30 (arbitrary units), auxiliary gas flow rate of 10 (arbitrary units), discharge voltage of 4.5 kV, discharge current of 5 μA, capillary temperature of 330 °C, capillary voltage of 47.5 V, tube lens voltage of 115 V, and vaporizer temperature of 330 °C. The corresponding ions were extracted from the total ion chromatograms to confirm the presence of the Michael addition products of cysteine and lysine to acrylamide, and of the Schiff base of asparagine in the digests.
Statistical analysis
The data were subjected to analysis of variance (one-way ANOVA). The SPSS 17.0 statistical package was used to evaluate the statistical significance of the differences between mean values by the Duncan test. P < 0.05 was considered to be statistically significant for the results.Conclusions
The simulated digestion conditions favored the Michael addition of amino acids to acrylamide. Owing to its two nucleophilic groups (–SH, –NH2), cysteine becomes highly reactive toward acrylamide, especially under the simulated duodenal conditions. As a result, acrylamide levels of baked or fried products decrease significantly during in vitro enzymatic digestion process. However, the intermediates present in fried potatoes act as precursors increasing acrylamide levels under gastric conditions. The balance between the elimination and potential formation of acrylamide during the in vitro digestion process suggests that levels of acrylamide ingested with foods may not directly indicate its absorption rate through gastric, duodenal and colonic routes. Gastrointestinal conditions and the ingested food composition affect the levels of bioavailable acrylamide, and should be taken into consideration.References
- E. Tareke, P. Rydberg, P. Karlsson, S. Eriksson and M. Tornqvist, J. Agric. Food Chem., 2002, 50, 4998–5006 CrossRef CAS PubMed.
- European Food Safety Authority, http://www.efsa.europa.eu/en/press/news/datex110420.htm, published 2005.
- M. V. Ruby, R. Schoof, W. Brattin, M. Goldade, G. Post, M. Harnois, D. E. Mosby, S. W. Casteel, W. Berti, M. Carpenter, D. Edwards, D. Cragin and W. Chappell, Environ. Sci. Technol., 1999, 33, 3697–3705 CrossRef CAS.
- S. Boisen and B. O. Eggum, Nutr. Res. Rev., 1991, 4, 141–162 CrossRef CAS PubMed.
- E. F. A. Brandon, A. G. Oomen, C. J. M. Rompelberg, C. H. M. Versantvoort, J. G. M. van Engelen and A. Sips, Regul. Toxicol. Pharmacol., 2006, 44, 161–171 CrossRef CAS PubMed.
- L. Yang, X. Zhang, Y. Li, H. Li, Y. Wang and W. Wang, Biol. Trace Elem. Res., 2012, 145, 81–86 CrossRef CAS PubMed.
- E. De Angelis, L. Monaci, A. Mackie, L. Salt and A. Visconti, Innovative Food Sci. Emerging Technol., 2014, 22, 248–256 CrossRef CAS PubMed.
- M. Friedman and C. E. Levin, J. Agric. Food Chem., 2008, 56, 6113–6140 CrossRef CAS PubMed.
- G. Koutsidis, S. P. J. Simons, Y. H. Thong, Y. Haldoupis, J. Mojica-Lazaro, B. L. Wedzicha and D. S. Mottram, J. Agric. Food Chem., 2009, 57, 9011–9015 CrossRef CAS PubMed.
- R. Zamora, R. M. Delgado and F. J. Hidalgo, J. Agric. Food Chem., 2010, 58, 1708–1713 CrossRef CAS PubMed.
- F. J. Hidalgo, R. M. Delgado and R. Zamora, Food Chem., 2010, 122, 596–601 CrossRef CAS PubMed.
- F. J. Hidalgo, R. M. Delgado and R. Zamora, Food Res. Int., 2011, 44, 1083–1087 CrossRef CAS PubMed.
- S. Eriksson and P. Karlsson, LWT – Food Sci. and Technol., 2006, 39, 393–399 CrossRef CAS PubMed.
- A. Becalski, B. P. Y. Lau, D. Lewis and S. W. Seaman, J. Agric. Food Chem., 2003, 51, 802–808 CrossRef CAS PubMed.
- V. A. Yaylayan, J. Food Nutri. Res., 2009, 48, 1–7 CAS.
- D. V. Zyzak, R. A. Sanders, M. Stojanovic, D. H. Tallmadge, B. L. Eberhart, D. K. Ewald, D. C. Gruber, T. R. Morsch, M. A. Strothers, G. P. Rizzi and M. D. Villagran, J. Agric. Food Chem., 2003, 51, 4782–4787 CrossRef CAS PubMed.
- M. Granvogl and P. Schieberle, J. Agric. Food Chem., 2006, 54, 5933–5938 CrossRef CAS PubMed.
- M. Minekus, M. Alminger, P. Alvito, S. Balance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mazkie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, Food & Func., 2014, 5, 1113–1124 CAS.
- V. A. Papillo, P. Vitaglione, G. Graziani, V. Gokmen and V. Fogliano, J. Agric. Food Chem., 2014, 62, 4119–4126 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |
