Three-component reaction between substituted β-nitrostyrenes, β-dicarbonyl compounds and amines: diversity-oriented synthesis of novel β-enaminones†
Weijian
Ye
,
Yan
Li
,
Lanxiang
Zhou
,
Juanjuan
Liu
and
Cunde
Wang
*
School of Chemistry and Chemical Engineering, Yangzhou University, 180 Siwangting Street, Yangzhou 225002, P. R. China. E-mail: wangcd@yzu.edu.cn; Fax: +86-514-8797-5244; Tel: +86-514-8797-5568
First published on 18th September 2014
Abstract
An efficient and practical method is developed for the diversity-oriented synthesis of β-enaminones via three-component reaction between substituted β-nitrostyrenes, β-dicarbonyl compounds and amines for the generation of a wide range of structurally interesting and pharmacologically significant compounds under mild conditions.
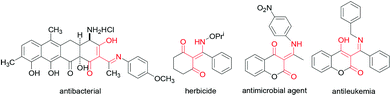
The enamino diketone skeleton is a basic structural feature found in a number of important pharmaceutical and agrochemical molecules, having antimicrobial, antibacterial, anti-inflammatory, antiaggregant, antiischemic, antileukemia1 and other types of physiological activities. β-Enaminones are also known as effective and ecologically relevant herbicides and safe plant protection agents2 (Fig. 1). Additionally, some compounds with the enamino diketone skeleton have great potential for complexation with different metals such as Pd, Ni, and Cu, and the products have different biological activities.3
The variety of chemical reactivities possible with an enamino diketone motif means that they also serve as versatile intermediates in many synthetic routes.4 This combination of significant pharmacological and biological properties and compelling synthetic utility has resulted in the development of a number of methods for enamino diketone preparation. They are most commonly synthesized either by enamination of the corresponding cyclic 1,3-diketone and triethyl orthoformate with aromatic amines,5 or by direct nucleophilic addition of the triketones such as 2-benzoyl-1,3-cyclohexanedione with a primary or secondary amine.6 In addition, the condensation of methylketones or ketones with activated methylene and dimethoxy-N,N-dimethylmethanamine was usually used to prepare the enaminones.7 Copper-promoted aminolysis of β-carbonyl 1,3-dithianes with amines was also described to provide an efficient access to β-enaminones.4d However, many of the previously developed β-enaminone syntheses possess several disadvantages, including the use of the limited availability of triketone reactants or β-carbonyl 1,3-dithianes.
Substituted β-nitrostyrenes are important synthetic intermediates and starting materials for the synthesis of a variety of useful building blocks.8 In addition, due to the strong electron-withdrawing nature of the NO2 group, they play a key role in the Michael addition reaction which is an efficient synthetic tool for the formation of C–C bonds.9 Recently we reported a very straightforward one-pot multicomponent synthesis of polysubstituted piperidines, substituted furo[3,2-c]chromen-4-ones and substituted 4-hydroxybenzofuranes using substituted β-nitrostyrenes as essential building blocks.10 As a part of our continuous interest directed towards the green and sustainable development of new methodologies using substituted β-nitrostyrenes as essential building blocks for the synthesis of highly functionalized molecules, we report the results of our recent efforts devoted to the efficient three-component reaction between substituted β-nitrostyrenes, β-dicarbonyl compounds and amines for the direct formation of β-enaminones under mild conditions.
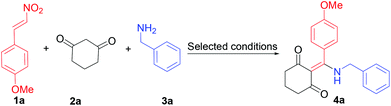
Initially, we examined the reaction of 1-methoxy-4-(2-nitrovinyl)benzene (1a), and cyclohexane-1,3-dione (2a) with phenylmethanamine (3a) using trimethylamine as a catalyst. We were delighted to find that the catalyst trimethylamine gave the desired 2-((benzylamino)(4-methoxyphenyl)methylene)cyclohexane-1,3-dione (4a) in 43% yield in the solvent of ethanol at room temperature (Table 1, entry 1). Subsequent experiments with various temperatures revealed that 65–75 °C was the optimal reaction temperature for the three multicomponent reaction, producing the desired product in 65% yield (Table 1, entries 2–5). Low conversion was observed at a lower reaction temperature, possibly due to the nucleophilic addition coordination of the amine to C3 of the furan ring with difficulties.
| Entry | Base/solvent/T(°C)/t(h) | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 3 mmol 1-methoxy-4-(2-nitrovinyl)benzene (1a), 3 mmol cyclohexane-1,3-dione (2a), 3 mmol phenylmethanamine (3a), 15 mL solvent, 6 h. b Yields were isolated. | ||
| 1 | Et3N (1.5 eq.)/EtOH/r.t./6 | 43 |
| 2 | Et3N (1.5 eq.)/EtOH/45/6 | 49 |
| 3 | Et3N (1.5 eq.)/EtOH/55/6 | 53 |
| 4 | Et3N (1.5 eq.)/EtOH/65/6 | 65 |
| 5 | Et3N (1.5 eq.)/EtOH/75/6 | 64 |
| 6 | Piperidine (1.5 eq.)/EtOH/65/6 | Trace |
| 7 | K2CO3 (1.5 eq.)/EtOH/65/6 | Trace |
| 8 | Li2CO3 (1.5 eq.)/EtOH/65/6 | 63 |
| 9 | NaOH(1.5 eq.)/EtOH/65/6 | None |
| 10 | Et3N (0.5 eq.)/EtOH/65/6 | 59 |
| 11 | Et3N (1.0 eq.)/EtOH/65/6 | 61 |
| 12 | Et3N (2.0 eq.)/EtOH/65/6 | 65 |
| 13 | Et3N (2.5 eq.)/EtOH/65/6 | 65 |
| 14 | Et3N (1.5 eq.)/DMF/65/6 | 58 |
| 15 | Et3N (1.5 eq.)/THF/65/6 | 45 |
| 16 | Et3N(1.5 eq.)/1,4-dioxane/65/6 | 60 |
When piperidine was used as the base, it gave no obvious reaction (Table 1, entry 6), indicating the important role of the tertiary amine. When an inorganic weak base K2CO3 was used, the desired product 4a was only a minor product (Table 1, entry 7). However, a decisive step-up in the yield of product 4a was achieved when replacing K2CO3 with Li2CO3 (Table 1, entry 8), which was less effective than triethylamine. As expected, no product 4a was observed using a strong base NaOH (Table 1, entry 9), possibly due to the difficulty in the formation of the hydroxyl group of the nitrogen. Reducing the amount of triethylamine to half or one equivalent led to decreased yields of product 4a (Table 1, entries 10 and 11). Further increase of the amount of triethylamine had no significant beneficial effect on the reaction (Table 1, entries 12 and 13). Further experiments revealed that alcohol was confirmed to be the most effective solvent (Table 1, entry 4). Other solvents, such as DMF, THF and 1,4-dioxane, gave lower product yields (Table 1, entries 14–16). These optimised reaction conditions employed the same equivalents of 1,3-cyclohexanediones, substituted β-nitrostyrenes and amines, and 1.5 equivalents of the base triethylamine (Table 1, entry 4), and the reaction time for this one-pot process was 6 hours.
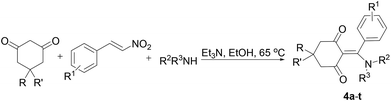
With these reaction conditions identified, our attention turned to examination of the scope of the multicomponent reaction. To determine the scope of the designed protocol, three-component reaction of a number of substituted β-nitrostyrenes 1a–n generated from aromatic aldehydes and nitromethane, commercially available substituted 1,3-cyclohexanediones 2a–c and amines 3a–j, was carried out under optimized reaction conditions, and the results are summarized in Table 2.
| Entry | R/R′ | R1 | R2/R3 | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | H/H | p-MeO | Bn/H | 65 (4a) |
| 2 | H/H | p-MeO | PhCH(CH3)/H | 78 (4b) |
| 3 | H/H | m-MeO | Bn/H | 63 (4c) |
| 4 | H/H | o-MeO | Bn/H | 59 (4d) |
| 5 | Me/Me | p-Me | Bn/H | 81 (4e) |
| 6 | Me/Me | p-Me | HO2CCH2/H | 79 (4f) |
| 7 | Me/Me | p-MeO | n-C4H9/H | 87 (4g) |
| 8 | Me/Me | p-Me | n-C8H17/H | 84 (4h) |
| 9 | Me/Me | p-Me | n-C10H21/H | 86 (4i) |
| 10 | Me/Me | p-Me | n-C14H29/H | 81 (4j) |
| 11 | Me/Me | p-Me | n-C16H33/H | 80 (4k) |
| 12 | Me/Me | p-Cl | Bn/H | 80 (4l) |
| 13 | Me/Me | m-MeO | Bn/H | 87 (4m) |
| 14 | Me/Me | o-MeO | Bn/H | 78 (4n) |
| 15 | Me/Me | m-Br | Bn/H | 76 (4o) |
| 16 | Me/Me | p-MeO | Bn/H | 83 (4p) |
| 17 | Me/Me | p-MeO | HO2CCH(CH3)/H | 64 (4q) |
| 18 | Me/Me | p-MeO | n-C6H13/n-C6H13 | 55 (4r) |
| 19 | Me/Me | p-NO2 | Bn/H | 51 (4s) |
| 20 | iPr/H | p-Cl | Bn/H | 62 (4t) |
The substrate scope for this reaction was found to be broad for a variety of 1,3-cyclohexanediones, substituted β-nitrostyrenes and amines (Table 2). As shown in Table 2, 1,3-cyclohexanediones were found to afford the expected product, whereas 5,5-dimethyl-1,3-cyclohexanedione proved to be a good substrate. 1,3-Cyclopentanedione was also tested as a starting material in the reaction, however 1,3-cyclopentanedione was incompatible with this multicomponent reaction. This is because the intermediate cyclopentane[a]furan skeleton was formed difficultly. In general, reaction yields were good for substituted β-nitrostyrenes, regardless of the electronic nature and substitution pattern on the aryl moiety. In particular, the reaction was found to afford good to excellent yields for the desired β-enaminones for a variety of electron-rich β-nitrostyrenes.
In terms of the amine component, this assembly process enjoyed the wide aliphatic primary amines. A variety of functional groups, such as acid and aryl groups, were tolerated and installed. The reaction was also tolerant of higher fatty amines and secondary amines (Table 2, entries 8–11 and 18), furnishing the β-enaminone product in good yield. However, aromatic amines were incompatible with this multicomponent reaction due to their weak nucleophilicity. β-Enaminones 4a–t were fully characterized by spectroscopic methods and were confirmed by single-crystal X-ray diffraction studies performed for two representative compounds 4f and 4l (Fig. 2). As a similar reaction, Qi and co-workers have reported the L-proline-dependent chemoselective reactions of cyclohexane-1,3-dione and nitroolefins with aromatic amines in water, and for the generation of tetrahydro-4H-indol-4-one derivatives.11a In this domino reaction, the Michael addition of cyclohexanedione to 2-nitrovinylbenzene formed the 2-(1-aryl-2-nitroethyl)-cyclohexane-1,3-dione ion, which is followed by L-proline-promoting deprotonation to form a resonance-stabilized N-oxide, and a stable Schiff base also was yielded from the carbonyl of cyclohexane-1,3-dione and aromatic amine in the presence of L-proline. Finally, an intramolecular cyclization followed by nucleophilic addition of an intermediate enamine to N-oxide oxime afforded the desired tetrahydro-4H-indol-4-one. In addition, Saito et al. also reported one-pot synthesis of 2-amino-3-arylbenzofuran derivatives from cyclohexane-1,3-dione and nitroolefins.11b,c
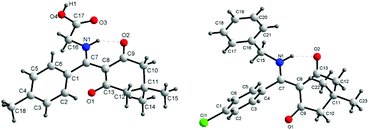 | ||
| Fig. 2 Molecular structure of β-enaminones 4f and 4l.12 | ||
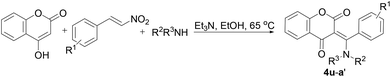
In particular, we were pleased to see that our conditions allowed for a highly efficient route to (E)-3-((alkylamino)(aryl)methylene) chroman-2,4-diones, and the results are summarized in Table 3. It was found that the three-component reaction showed broad tolerance for various R1, R2 and R3 groups of substrates. All selected substrates, β-nitrostyrenes bearing electron-rich (entries 1, 2, 6), electron deficient (entries 3–5) R1 groups, reacted smoothly with fatty amines and secondary amines, and 4-hydroxy-2H-chromen-2-one to give the corresponding polysubstituted (E)-3-((alkylamino)(aryl)methylene)chroman-2,4-diones 4u–4y in high yields at 65 °C for 6 h. In addition, in the case of β-nitrostyrene, the three-component reaction also worked well, yielding the desired product 4a′ in 70% yield (Table 3, entry 7).
All the prepared compounds have been characterized by spectral and analytical data. The structure of 4x is shown in Fig. 3.12 X-ray crystallographic analysis determined that the product 4x possesses the E exocyclic double bond at C(2) of the β-dicarbonyl compound. On the basis of spectroscopic evidence the structure of compounds 4u–4a′ was identified as (E)-3-((alkylamino)(aryl)methylene) chroman-2,4-dione. The exocyclic double-bond E configuration seemed to be thermodynamically favorable because an intermolecular hydrogen bond was observed on the basis of their X-ray crystallographic data.
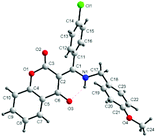 | ||
| Fig. 3 Molecular structure of (E)-3-((4-methoxybenzylamino)(4-chlorophenyl) methylene)chroman-2,4-dione (4x).12 | ||
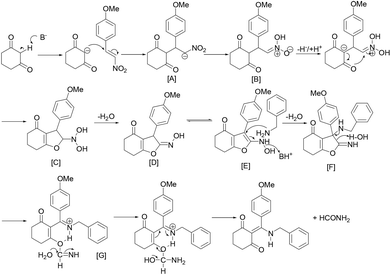
On the basis of the above results, a possible mechanism for the Et3N-promoted three component reaction from substituted β-nitrostyrenes, β-dicarbonyl compounds and amines is tentatively proposed as depicted in Scheme 1 (with the three component reaction of 1-methoxy-4-(2-nitrovinyl)benzene, β-cyclohexane-dione and benzylamine as an example).
Initially, the Michael addition of cyclohexanedione to 1-methoxy-4-(2-nitrovinyl)benzene formed the 2-(1-(4-methoxyphenyl)-2-nitroethyl)-cyclohexane-1,3-dione ion (Scheme 1, A), which is followed by base-promoting deprotonation to form a resonance-stabilized N-oxide oxime (Scheme 1, B) and a cyclic enolate. Next, intermediate enolate nucleophilic addition to N-oxide oxime (Scheme 1, B) gave an intermediate N-oxide hydroxylamine (Scheme 1, C), following dehydration yielded furan oxime (Scheme 1, D). Subsequently, the intermediate E, generated via C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond isomerization of intermediate D under basic conditions, undergoes nucleophilic addition from an amine, followed by dehydration to afford the furan imine intermediate F. Finally, the ring opening of the furan imine intermediate F produces the iminium G, which undergoes an intramolecular keto–enol tautomerism to give the β-enaminone product (Scheme 1).
N double bond isomerization of intermediate D under basic conditions, undergoes nucleophilic addition from an amine, followed by dehydration to afford the furan imine intermediate F. Finally, the ring opening of the furan imine intermediate F produces the iminium G, which undergoes an intramolecular keto–enol tautomerism to give the β-enaminone product (Scheme 1).
In summary, we have shown that a wide range of β-enaminones can be successfully synthesised using a one-pot three-component system that combines substituted β-nitrostyrenes and β-dicarbonyl compounds with amines. The reaction proceeds with easily accessible substituted β-nitrostyrenes bearing electron-rich and electron-poor groups, and triethylamine as a non-expensive promoter. Due to the described usefulness of β-enaminone derivatives, such simple reaction conditions and functional group tolerance are offering a new attractive method for access to such structures. Therefore, from these results, it can be envisioned that this procedure will find many applications in organic synthesis.
This work was supported by generous grants from the National Natural Science Foundation of China (NNSFC 21173181). This project is funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Notes and references
- (a) M. Mladenovic, N. Vukovic, N. Niciforovic, S. Sukdolak and S. Solujic, Molecules, 2009, 14, 1495 CrossRef CAS PubMed; (b) N. Vukovic, S. Sukdolak, S. Solujic and N. Niciforovic, Food Chem., 2010, 120, 1011 CrossRef CAS PubMed; (c) D. L. Garmaise, D. T. W. Chu, E. Bernstein and M. Inaba, J. Med. Chem., 1979, 22, 559 CrossRef CAS; (d) B. B. Kuzmitskii, L. P. Malaeva, T. C. Khlebnikova and F. A. Lakhvich, Vestsi Akad. Navuk BSSR, Ser. Khim. Navuk, 1989, 5, 65 Search PubMed; (e) E. Badzisz, E. Brzezinska, U. Krajewska and M. Rozalski, Eur. J. Med. Chem., 2003, 38, 597 CrossRef; (f) E. V. Balebanova, V. V. Davydov, A. N. Zorin, B. Y. Syropyatov and Y. V. Shklyaev, Khim.-Farm. Zh., 1996, 30, 17 CAS; (g) D. S. Goldfarb, Method using lifespan-altering compounds for altering the lifespan of eukaryotic organisms, and screening for such compounds, US20090163545, 2009 Search PubMed.
- (a) S. R. Belan, A. F. Grapov and G. M. Melnikova, Novye pestitsidy, Spravochnik (New Perspective: Handbook), Khimiya, Moscow, 2001 Search PubMed; (b) F. A. Lakhvich, D. B. Rubinov and I. L. Rubinova, Zemled. Zashch. Rast., 2006, 47, 33 Search PubMed; (c) A. Serban, K. G. Watson, G. J. Bird, G. J. Farquharson and L. E. Cross, Preparation of 2-[1-(ethoxyimino)propyl]-5-indanylcyclohexene-1,3-diones and analogs as herbicides and plant growth regulators, US4952722A, 1990 Search PubMed.
- (a) E. Badzisz, B. K. Keppler, G. Giester, M. Wozniczka, A. Kufelnicki and B. Nawrot, Eur. J. Inorg. Chem., 2004, 4412 CrossRef; (b) E. Budzisz, M. Małecka, I.-P. Lorenz, P. Mayer, R. A. Kwiecien, P. Paneth, U. Krajewska and M. Rozalski, Inorg. Chem., 2006, 45, 9688 CrossRef CAS PubMed; (c) M. Sawaki, I. Iwataki, Y. Hirono and H. Ishikawa, Herbicidal 2-[1-(alkoxyamino)alkylidene]cyclohexane-1,3-diones, DE2439104, 1975 Search PubMed.
- (a) P. Barraja, V. Spanò, P. Diana, A. Carbone and G. Cirrincione, Tetrahedron Lett., 2009, 50, 5389 CrossRef CAS PubMed; (b) A. N. Pyrko, Russ. J. Org. Chem., 2010, 46, 1843 CrossRef CAS; (c) Y. Miyamoto, Y. Banno, T. Yamashita, T. Fujimoto, S. Oi, Y. Moritoh, T. Asakawa, O. Kataoka, H. Yashiro, K. Takeuchi, N. Suzuki, K. Ikedo, T. Kosaka, S. Tsubotani, A. Tani, M. Sasaki, M. Funami, M. Amano, Y. Yamamoto, K. Aertgeerts, J. Yano and H. Maezaki, J. Med. Chem., 2011, 54, 831 CrossRef CAS PubMed; (d) J. Kang, F. Liang, S. G. Sun, Q. Liu and X. Bi, Org. Lett., 2006, 8, 2547 CrossRef CAS PubMed; (e) B. Stanovnik and J. Svete, Chem. Rev., 2004, 104, 2433 CrossRef CAS PubMed; (f) A.-Z. A. Elassara and A. A. El-Khair, Tetrahedron, 2003, 59, 8463 CrossRef; (g) N. D. Eddingtona, D. S. Coxa, R. R. Robertsb, J. P. Stablesc, C. B. Powelld and K. R. Scotte, Curr. Med. Chem., 2000, 7, 417 CrossRef; (h) R. Bernini, G. Fabrizi, A. Sferrazza and S. Cacchi, Angew. Chem., Int. Ed., 2009, 48, 8078 CrossRef CAS PubMed; (i) J.-P. Wan and Y.-J. Pan, Chem. Commun., 2009, 2768 RSC; (j) A. Ilangovan and R. G. Kumar, Chem. – Eur. J., 2010, 16, 2938 CrossRef CAS PubMed; (k) A. Saito, T. Konishi and Y. Hanzawa, Org. Lett., 2010, 12, 372 CrossRef CAS PubMed; (l) Y. Chen, T. Ju, J. Wang, W. Yu, Y. Du and K. Zhao, Synlett, 2010, 231 Search PubMed; (m) J. Lu, X. Cai and S. M. Hecht, Org. Lett., 2010, 12, 5189 CrossRef CAS PubMed.
- A. N. Pyrko, Russ. J. Org. Chem., 2010, 12, 46 Search PubMed.
- (a) T. S. Khlebnikova, V. G. Isakova and F. A. Lakhvich, Russ. J. Gen. Chem., 2011, 81, 2 CrossRef; (b) S. H. Üngören, Molecules, 2009, 14, 1429 CrossRef PubMed.
- D. Kumar, D. N. Kommi, P. Chopra, M. I. Ansari and A. K. Chakraborti, Eur. J. Org. Chem., 2012, 6407 CrossRef CAS.
- (a) L. F. Tietze, S. Dietz, N. Boehnke, M. A. Duefert, I. Objartel and D. Stalke, Eur. J. Org. Chem., 2011, 6574 CrossRef CAS; (b) G. Xiong, M. Wei, Y. Zhou, Y. Li, F. Zhang and Y. Gong, Synthesis, 2011, 3439 CAS; (c) W. Song, W. Lu, J. Wang, P. Lu and Y. Wang, J. Org. Chem., 2010, 75, 3481 CrossRef CAS PubMed.
- (a) N. Ono, The Nitro Group in Organic Synthesis, Wiley-VCH, New York, 2001 CrossRef; (b) S. B. Tsogoeva, Eur. J. Org. Chem., 2007, 1701 CrossRef CAS; (c) D. Almasi, D. A. Alonso and C. N. Jera, Tetrahedron: Asymmetry, 2007, 18, 299 CrossRef CAS PubMed; (d) Z. H. Zhang, X. Q. Dong, D. Chen and C. J. Wang, Chem. – Eur. J., 2008, 14, 8780 CrossRef CAS PubMed; (e) Z. P. Yu, X. H. Liu, L. Zhou, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2009, 48, 5195 CrossRef CAS PubMed; (f) O. M. Berner, L. Tedeschi and D. Enders, Eur. J. Org. Chem., 2002, 1877 CrossRef CAS; (g) D. Almasi, D. A. Alonso, E. Gomez-Bengoa and C. Najera, J. Org. Chem., 2009, 74, 6163 CrossRef CAS PubMed.
- (a) Z. Zhou, H. Liu, Q. Sun, Y. Li, J. Yang, J. Liu, P. Yan and C. D. Wang, Synlett, 2012, 2255 CAS; (b) H. Liu, Z. Zhou, Q. Sun, Y. Li, Y. Li, J. Liu, P. Yan, D. Wang and C. D. Wang, ACS Comb. Sci., 2012, 14, 366 CrossRef CAS PubMed; (c) Z. Zhou, H. Liu, Y. Li, J. Liu, Y. Li, J. Liu, J. Yao and C. D. Wang, ACS Comb. Sci., 2013, 15, 363 CrossRef CAS PubMed; (d) Y. Li, H. Liu, L. Sun, J. Liu, Z. Xue, J. Yao and C. D. Wang, Synlett, 2013, 1851 Search PubMed; (e) Y. Li, Z.-H. Xue, W.-J. Ye, J.-J. Liu, J. Yao and C. D. Wang, ACS Comb. Sci., 2014, 16, 113 CrossRef CAS PubMed.
- (a) F. Zhang, C. Li and C. Qi, Synthesis, 2013, 3007 CAS; (b) M. Arai, Y. Miyauchi, T. Miyahara, T. Ishikawa and S. Saito, Synlett, 2009, 122 CAS; (c) T. Ishikawa, T. Miyahara, M. Asakura, S. Higuchi, Y. Miyauchi and S. Saito, Org. Lett., 2005, 7, 1211 CrossRef CAS PubMed.
- Crystallographic data for β-enaminones 4f, 4l and 4x were deposited with the Cambridge Crystallographic Data Centre with the deposition number CCDC 965964, 959044 and 990835.
Footnote |
| † Electronic supplementary information (ESI) available: Reaction conditions and spectra. CCDC 965964, 959044 and 990835. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4gc01234h |
| This journal is © The Royal Society of Chemistry 2015 |