Ruthenium-catalyzed hydrogen generation from glycerol and selective synthesis of lactic acid†
Yang
Li
a,
Martin
Nielsen
a,
Bin
Li
b,
Pierre H.
Dixneuf
b,
Henrik
Junge
a and
Matthias
Beller
*a
aLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert Einstein Str. 29a, 18059 Rostock, Germany. E-mail: Matthias.Beller@catalysis.de
bCatalyse et Organométalliques, Institut Sciences Chimiques de Rennes, UMR 6226-CNRS-Université de Rennes, France
First published on 18th September 2014
Abstract
An efficient hydrogen generation from glycerol and selective synthesis of lactic acid (67%) using pincer-type ruthenium complexes is described. Analysis of the products from glycerol dehydrogenation indicates that improving the efficiency of the decarboxylation step is a key point for further reforming processes.
The generation of sustainable energy is of general interest to our society because of depleting fossil fuel reserves and continuously increasing energy demand.1 Hydrogen is considered to play a key role in future clean energy technologies.2 Thus, its production from renewable resources, especially non-food related biomass or water is attracting research efforts. However, until now, efficient hydrogen production from water has remained difficult.3 Although important contributions have been made on hydrogen generation from biomass by enzyme fermentation,4 gasification,5 reforming in supercritical water and aqueous phase reforming by heterogeneous catalysts,6 the utilization of lignocellulosic biomass is still called “a chewy problem”.7 Thus, achieving hydrogen production from renewable sources under milder conditions is of actual interest. In this regard, “homogenous catalysis” might offer novel possibilities.
Hitherto most researches on acceptorless hydrogen generation from biomass derived alcohols by homogeneous catalysis only focused on simple alcohols, such as MeOH, EtOH and i-PrOH.8,9 Based on this situation and our experience in dehydrogenation of alcohols, we are interested in hydrogen generation from polyols, a “bridge” between simple alcohols and complex biomass. This study would give valuable insights into hydrogen production from complex biomass.10
Glycerol, an appropriate model system for polyols, is available on a bulk scale as a byproduct of the biodiesel generation. Indeed in 2010, 1.6 million tons of glycerol was produced from biodiesel.11 Notably, the selling price of the crude product was as low as 1–8 U.S. cents per pound in 2011. Currently, some of the glycerol is directly burned but the heat value is relatively low.12 Moreover, compared to other alcohols such as MeOH and EtOH, glycerol is advantageous because it is non-flammable, relatively non-toxic, and constitutes a high boiling point compound.13a
So far, hydrogen generation from glycerol has been mainly studied by bioconversion14 or using heterogeneous catalysts.13 Unfortunately, impurities in crude glycerol are inhibitory to microbial growth in the bioconversion procedure. On the other hand energy intensive harsh reaction conditions (above 220 °C with more than 2 MPa pressure or above 350 °C)13e are needed for successful heterogeneous catalysis. Compared to these processes, only few studies on hydrogen generation without an acceptor from glycerol by homogeneous catalysis are known.15 Seminal work was reported by Cole-Hamilton already in 1988, representing the state of the art activity of this reaction until today with 37.6 h−1 (2 h) [turnover frequencies (TOF)] under hυ at 150 °C applying RuH2N2(Ph3P)3 as the catalyst.15
As mentioned above, research on H2 generation from glycerol is important not only as a model system for H2 production from polyols or more complex biomass, but also for the direct utilization of the byproduct of the biodiesel. Herein, we report hydrogen generation from glycerol and selective synthesis of lactic acid (67%) under comparatively mild conditions by ruthenium pincer-type catalysts.16,17 Significantly improved catalyst performance for hydrogen generation was achieved (TOF: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 h−1, 2 h). Notably, even inexpensive industrial glycerol can be dehydrogenated with excellent catalyst activity (25
318 h−1, 2 h). Notably, even inexpensive industrial glycerol can be dehydrogenated with excellent catalyst activity (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 h−1, 2 h). The developed conditions were applied to other polyols, such as ethylene glycol and sorbitol.
088 h−1, 2 h). The developed conditions were applied to other polyols, such as ethylene glycol and sorbitol.
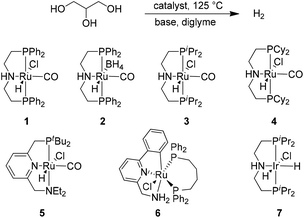
Based on our experience in hydrogen generation from MeOH,8 EtOH, and i-PrOH,9 the initial experiments were performed using the non-innocent pincer type catalyst Ru-MACHO (1, RuHCl(PNPPh)CO)18 in the presence of NaOH (0.07 equiv.) (Table 1, entry 1). Here, a TOF (2 h) of 483 h−1 for hydrogen production was observed at 125 °C. Due to the increased viscosity of glycerol compared to MeOH or EtOH, additional solvents were added to the reaction mixture. Distinctly improved activity was obtained in the presence of diglyme (Table 1, entry 2). Notably, no reaction activity was observed without glycerol. Subsequently, the amount of base was varied (Table 1, entries 2 to 4, Table S1,† entry 1). Experiments showed that 1.50 M NaOH gave improved results with a TOF (2 h) of 1010 h−1 (Table 1, entry 4). Increasing the amount of NaOH further led to lower activity (Table S1,† entry 1). Meanwhile, decreasing the reaction temperature from 125 °C to 95 °C resulted in lower reaction efficiency (Table S1,† entry 2).
| Entry | Catalyst | μmol, ppm | Base (M, equiv.) | V (1 h) | TOF (1 h) | V (2 h) | TOF (2 h) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: reactions were performed on glycerol (10.0 mL, 136.93 mmol) using diglyme (10.0 mL) as the solvent. Volumes (mL) were measured by gas burette with the removal of blank volumes. TOFs (h−1) were calculated with respect to volumes of H2. b Without solvent. c Reported on the average of 2 reactions and with an error margin of 10%. d The reaction was performed on glycerol (50.0 mL, 684.66 mmol) using diglyme (50.0 mL) as the solvent. | |||||||
| 1b | 1 | 4.20, 30.7 | NaOH (1.00, 0.07) | 65.0 | 629 | 100 | 483 |
| 2 | 1 | 4.17, 30.5 | NaOH (1.00, 0.15) | 129.8 | 1263 | 182.5 | 889 |
| 3 | 1 | 4.15, 30.3 | NaOH (0.50, 0.07) | 59.5 | 582 | 76.0 | 372 |
| 4c | 1 | 4.10, 30.0 | NaOH (1.50, 0.22) | 124.0 | 1231 | 202.0 | 1010 |
| 5 | 2 | 4.09, 29.9 | NaOH (1.50, 0.22) | 111.0 | 1103 | 173.5 | 861 |
| 6 | 3 | 3.97, 29.0 | NaOH (1.50, 0.22) | 93.5 | 957 | 138.5 | 709 |
| 7 | 4 | 4.07, 29.7 | NaOH (1.50, 0.22) | 89.0 | 889 | 137.5 | 686 |
| 8 | 5 | 4.06, 29.6 | NaOH (1.50, 0.22) | 66.0 | 661 | 116.0 | 581 |
| 9 | 6 | 4.14, 30.2 | NaOH (1.50, 0.22) | 39.5 | 387 | 47.5 | 233 |
| 10 | 7 | 4.11, 30.0 | NaOH (1.50, 0.22) | 50.0 | 494 | 114.5 | 566 |
| 11c | 1 | 4.02, 29.3 | KOH (1.50, 0.22) | 158.5 | 1606 | 260.8 | 1321 |
| 12 | 1 | 1.43, 10.5 | KOH (1.50, 0.22) | 130.5 | 3701 | 208.5 | 2957 |
| 13c,d | 1 | 0.35, 0.52 | KOH (1.50, 0.22) | 100.0 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477 477 |
179.8 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 318 |
To investigate the influence of the pincer ligand on the activity, several related organometallic complexes were studied (Table 1, entries 4 to 10). The hydrogen generation was slightly decreased (Table 1, entry 5) when changing the anion Cl− in catalyst 1 to BH4− (catalyst 2). Meanwhile, variation of phosphorous substituents in catalyst 1 from phenyl to i-propyl (catalyst 3, Table 1, entry 6) and cyclohexyl (catalyst 4, Table 1, entry 7), respectively, decreased the amount of hydrogen, too. In the presence of Milstein's catalyst (5)19 as well as the Baratta-type catalyst (6),20 lower activities were observed in both cases (Table 1, entries 8 and 9). Notably, iridium complex 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 (Table 1, entry 10) showed a slightly reduced efficiency compared with the ruthenium catalyst 3.
21 (Table 1, entry 10) showed a slightly reduced efficiency compared with the ruthenium catalyst 3.
Further, different kinds of bases were investigated (Table 1, entry 11, Table S1,† entries 3 and 4). To our delight, a higher TOF (2 h) of 1321 h−1 was obtained using KOH as a base (Table 1, entry 11). Although a similar reaction activity was observed using CsOH (Table S1,† entry 3), KOH was selected because of the lower cost.
Decreasing the catalyst loading from 29.3 ppm to 0.5 ppm (Table 1, entries 11–13) under optimized conditions, very high TOFs (1 h, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477 h−1, 2 h, 10
477 h−1, 2 h, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 h−1) were achieved for hydrogen generation from glycerol on an about 700 mmol scale. Notably, this value is more than two orders of magnitude higher compared to the best reported TOF (2 h) of 37.6 h−1 using RuH2N2(Ph3P)3 as a catalyst (hυ at 150 °C).15
318 h−1) were achieved for hydrogen generation from glycerol on an about 700 mmol scale. Notably, this value is more than two orders of magnitude higher compared to the best reported TOF (2 h) of 37.6 h−1 using RuH2N2(Ph3P)3 as a catalyst (hυ at 150 °C).15
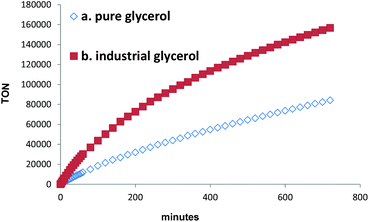
In order to test the stability of this catalytic system, hydrogen production was performed over 12 h. This resulted in 715.0 mL of H2 with a TON of 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027, without observation of CO and CH4. Notably, the catalyst was still active after 12 h (Fig. 1, a). Meanwhile, in the liquid reaction mixture, lactic acid (LA) (9), was identified as the major product (5%) accompanied with trace amounts of propane-1,2-diol (1,2-PDO) (10, <1%) (eqn (1)).
027, without observation of CO and CH4. Notably, the catalyst was still active after 12 h (Fig. 1, a). Meanwhile, in the liquid reaction mixture, lactic acid (LA) (9), was identified as the major product (5%) accompanied with trace amounts of propane-1,2-diol (1,2-PDO) (10, <1%) (eqn (1)).
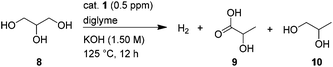 | (Eq 1) |
The increased availability of glycerol from biodiesel production and its very low price11,12 attracted significant research interest in recent years.13,14 Thus, industrial glycerol, containing only 86–88% glycerol,22 was investigated. Using 59.3 mL (684.66 mmol) of this substrate 427.0 mL of H2 was obtained with a TOF of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 h−1 after 2 h (Table 2, entry 1). To our surprise, the industrial glycerol displayed the highest reactivity (Table 2, entries 2 and 3).23 The higher reactivity of diluted glycerol (Table 2, entry 3) compared with pure glycerol (Table 2, entry 3) is explained by the improved solubility of base in the presence of water.
088 h−1 after 2 h (Table 2, entry 1). To our surprise, the industrial glycerol displayed the highest reactivity (Table 2, entries 2 and 3).23 The higher reactivity of diluted glycerol (Table 2, entry 3) compared with pure glycerol (Table 2, entry 3) is explained by the improved solubility of base in the presence of water.
| Entry | Substrate | V (mL, 1 h) | TOF (h−1, 1 h) | V (mL, 2 h) | TOF (h−1, 2 h) |
|---|---|---|---|---|---|
| a Reaction conditions: reactions were performed on 684.66 mmol of glycerol using the same volume of diglyme as the solvent at optimized reaction conditions (Table 1, entry 13). Volumes were measured by gas burette and blank volumes were removed. TOFs were reported on the average of 2 reactions and with an error margin of 10%. b The content of glycerol was determined as 86.5%. c The simulant mixture of glycerol and H2O is based on 86.5% glycerol, 13.5% H2O. | |||||
| 1 | Industrial glycerolb | 257.0 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 199 199 |
427.0 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 088 |
| 2 | Glycerol | 100.0 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477 477 |
179.8 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 318 |
| 3 | Glycerol + H2Oc | 165.0 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507 507 |
264.5 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834 834 |
In fact, using industrial glycerol 1334 mL of H2 was obtained with a TON of 156![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 after 12 h. Similar to the result of pure glycerol, no CO and CH4 were detected. Here, around 11% of lactic acid was generated as well as trace amounts of propane-1,2-diol. The presented reaction profiles in Fig. 1 demonstrate the potential application of industrial glycerol for H2 production.
691 after 12 h. Similar to the result of pure glycerol, no CO and CH4 were detected. Here, around 11% of lactic acid was generated as well as trace amounts of propane-1,2-diol. The presented reaction profiles in Fig. 1 demonstrate the potential application of industrial glycerol for H2 production.
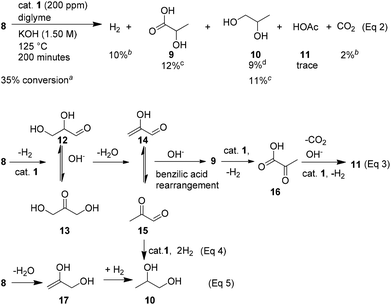
Improved conversion of glycerol (35%) was obtained in the presence of 200 ppm of catalyst 1 (Scheme 1). Here, 353.0 mL of H2 (10%) and 65.0 mL of CO2 (2%, 35 mL in the gas phase and 30.0 mL trapped in the reaction mixture) were obtained in 200 minutes. Detailed analysis of the reaction mixture revealed that LA (9, 12%), propane-1,2-diol (10, 11%) and trace amounts of acetic acid (11) were produced, too.
The possible reaction pathways for the formation of these products are proposed in Scheme 1. First, glycerol will be dehydrogenated in the presence of the catalyst to form hydrogen and intermediates 12 and/or 13. Dehydration and subsequent benzilic acid rearrangement should afford LA (9). This latter product can be dehydrogenated further on in the presence of ruthenium complex 1 to produce another molecule of H2 and 2-oxopropanoic acid (16). Intermediate 16 affords acetic acid (11) by decarboxylation and dehydrogenation in the presence of a base and a catalyst 1 (Scheme 1, eqn (3)). In addition, propane-1,2-diol (10) can be formed by hydrogenation of intermediates 14, 15 (Scheme 1, eqn (4)) and/or intermediate 17, which is generated by direct dehydration of glycerol (Scheme 1, eqn (5)).24 Compared to the reaction of 0.5 ppm of catalyst loading, it shows that depending on the active catalyst species and its concentration the selectivity for the different dehydrogenation and hydrogenation steps are influenced. Consequently, at higher catalyst loading the hydrogen productivity is decreased by the increased hydrogenation reactions. On the other hand, improving the efficiency of the decarboxylation is a key point for a reforming process.
Notably, LA, a biomass-derived platform chemical, is considered to be of importance in future bio-refineries. For example, catalytic transformations of LA lead to the selective production of green solvents, fine chemicals, commodity chemicals and even fuel precursors. Moreover, it is already used today as a precursor for biodegradable PLA (polylactic acid) polymers, which have a more positive impact on the environment compared to traditional polyolefins.17 Until today, LA is mainly produced by fermentation using biomass resources including carbohydrates and glycerol.25 However, the efficiency and productivity of the fermentation methods should be improved further on. In addition, this process suffers from complex separation steps. Hence, alternative methods for producing LA from glycerol were developed in recent years. However, they need high temperature (above 200 °C),17,24,26 a high ratio of base to glycerol (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and/or provide lower selectivity of LA compared to 1,2-PDO. To the best of our knowledge, there is no method known to give good yields of LA and hydrogen under comparably mild conditions.24a,d Thus, the preparation of LA attracted our attention.
1), and/or provide lower selectivity of LA compared to 1,2-PDO. To the best of our knowledge, there is no method known to give good yields of LA and hydrogen under comparably mild conditions.24a,d Thus, the preparation of LA attracted our attention.
The analysis of products from glycerol using different concentrations of the catalyst (eqn (1)–(5)), suggested the possibility to achieve high glycerol conversion as well as the high selectivity of lactic acid. Performing the reaction at high temperature and low concentration of the active catalyst should promote high selectivity of LA. Furthermore, according to the results obtained with industrial glycerol the presence of water will be beneficial for the reaction efficiency. With these considerations in mind, the conversion of glycerol to LA was optimized by variation of key reaction parameters.
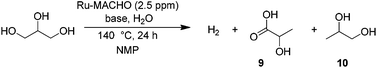
When the reaction temperature was increased to 140 °C, using NMP as the solvent in the presence of 1 equiv. of H2O and only 2.5 ppm of catalyst 1 LA was obtained in 21% yield (Table 3, entry 1). The amount of base displayed a key factor in the preparation of LA.17,24,26 Indeed, variation of the amount of base showed that using 1.08 equiv. of KOH gave the best result (57% yield of LA; Table 3, entry 4). Meanwhile, no 1,2-PDO was observed applying higher amounts of base (Table 3, entries 3 to 6). Using sodium hydroxide instead of potassium hydroxide, the yield of LA was slightly improved to 62%. Variation of the water concentration showed that 1.0 equiv. of water promoted the reaction most efficiently (Table 3, entries 7, 8 and 9).
| Entry | Base (M. equiv.) | H2O (equiv.) | NMP (mL) | Conversionb (%) | 9 Yieldc (%) | 10 Yieldc (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: reactions were performed on glycerol (10.0 mL, 136.93 mmol). b The conversion was detected by GC. c 1H NMR yield. d Reported on the average of 2 reactions and with an error margin of 0.02%. e Industrial glycerol was used. f The reaction was performed at 125 °C for 48 h. | ||||||
| 1 | KOH (1.5, 0.27) | 1.0 | 12.5 | 35 | 21 | 1 |
| 2 | KOH (2.0, 0.36) | 1.0 | 12.5 | 40 | 27 | 1 |
| 3 | KOH (4.0, 0.72) | 1.0 | 12.5 | 71 | 43 | — |
| 4d | KOH (6.0, 1.08) | 1.0 | 12.5 | 93 | 57 | — |
| 5 | KOH (7.0, 1.28) | 1.0 | 12.5 | 90 | 52 | — |
| 6 | KOH (8.0, 1.46) | 1.0 | 12.5 | 89 | 43 | — |
| 7d | NaOH (6.0, 1.08) | 1.0 | 12.5 | 100 | 62 | — |
| 8 | NaOH (6.1, 1.08) | 0.8 | 12.5 | 100 | 48 | — |
| 9 | NaOH (5.9, 1.08) | 1.2 | 12.5 | 100 | 52 | — |
| 10 | NaOH (5.5, 1.08) | 1.0 | 15.0 | 100 | 63 | — |
| 11 | NaOH (7.3, 1.08) | 1.0 | 8.0 | 100 | 67 | — |
| 12d,e | NaOH (7.3, 1.08) | 1.0 | 8.0 | 100 | 67 | — |
| 13e,f | NaOH (7.3, 1.08) | 1.0 | 8.0 | 100 | 67 | — |
Decreasing the amount of the solvent NMP had no obvious effect on the reaction efficiency (Table 3, entries 7, 10 and 11). Thus, dehydrogenation of industrial glycerol using the minimum quantity of the solvent (8 mL) under optimized conditions (1 equiv. H2O, 1.08 equiv. NaOH) gave 67% yield of LA (Table 3, entry 12).‡ Notably, in this experiment the same yield of hydrogen is obtained (containing 36 ppm of CO). The TON of the catalyst remarkably reached 265![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 326. A similar yield and TON were observed at 125 °C (Table 3, entry 13).
326. A similar yield and TON were observed at 125 °C (Table 3, entry 13).
Next, ethylene glycol and sorbitol were tested in the presence of catalyst 1 using the conditions for glycerol dehydrogenation. To our delight, also for the dehydrogenation of ethylene glycol unprecedented catalyst activities were achieved (64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 459 h−1 after 1 h and 59
459 h−1 after 1 h and 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 253 h−1 after 2 h; Table 4, entry 1). Compared with the previously best reported TOF (1185.3−1 after 2 h) the reaction activity was improved by about 54 times.15 Finally, D-sorbitol was studied as an example for more complex polyols. Good TOFs were observed (1 h, 1025 h−1, 2 h, 765 h−1) as well (Table 4, entry 2).
253 h−1 after 2 h; Table 4, entry 1). Compared with the previously best reported TOF (1185.3−1 after 2 h) the reaction activity was improved by about 54 times.15 Finally, D-sorbitol was studied as an example for more complex polyols. Good TOFs were observed (1 h, 1025 h−1, 2 h, 765 h−1) as well (Table 4, entry 2).
| Entry | Substrate | Solvent | μmol, ppm | V (1 h) | TOF (1 h) | V (2 h) | TOF (2 h) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: reactions were performed at 125 °C with 1.50 M KOH, volumes (mL) were measured by gas burette and blank volumes were removed. TOFs (h−1) were reported on the average of 2 reactions with an error margin of 6%. | |||||||
| 1 |
|
Diglyme (50.0 mL) | 0.34, 0.38 | 535.5 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 459 459 |
984.5 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 253 253 |
| 2 |
|
Diglyme (8.0 mL) | 1.30, 15.9 | 32.8 | 1025 | 49.0 | 765 |
| H2O (2.0 mL) | |||||||
In conclusion, a novel protocol for hydrogen generation from glycerol and the selective synthesis of lactic acid (67%) is presented using pincer-type ruthenium complexes. The developed conditions were successfully applied to other polyols, such as ethylene glycol and sorbitol. Unprecedented TOFs were obtained for ethylene glycol and glycerol after 2 h at 125 °C, using less than 1 ppm of the Ru-MACHO catalyst. With respect to potential applications, it is important to note that inexpensive and available industrial glycerol can be used directly and showed even improved performance for hydrogen production compared to the pure substrate. Analysis of the products from glycerol dehydrogenation indicated that improving the efficiency of decarboxylation is a key point for further reforming processes. In this regard, this study gives insights into hydrogen generation from more complex carbohydrate-based biomass.
Acknowledgements
We thank Dr Elisabetta Alberico for providing catalysts 3 and 4, A.+E. Fischer Chemie for providing industrial glycerol. Y. L. and M. N. thank the Alexander von Humboldt Foundation for financial support.Notes and references
- K. Christopher and R. Dimitrios, Energy Environ. Sci., 2012, 5, 6640 CAS.
- N. Armaroli and V. Balzani, ChemSusChem, 2011, 4, 21 CrossRef CAS PubMed.
- For selected recent reviews, see: (a) T. Maschmeyer and M. Che, Angew. Chem., Int. Ed., 2010, 49, 1536 CrossRef CAS PubMed; (b) X. Chen, S. Shen, L. Guo, S. S. Mao, Y. Wang, Y. Wang and R. Xu, Chem. Rev., 2010, 110, 6503 CrossRef CAS PubMed; (c) V. Artero, M. Chavarot-Kerlidou and M. Fontecave, Angew. Chem., Int. Ed., 2011, 50, 7238 CrossRef CAS PubMed; (d) E. S. Andreiadis, M. Chavarot-Kerlidou, M. Fontecave and V. Artero, Photochem. Photobiol., 2011, 87, 946 CrossRef CAS PubMed; (e) H. Ozawaa and K. Sakai, Chem. Commun., 2011, 47, 2227 RSC; (f) S. C. Warren and E. Thimsen, Energy Environ. Sci., 2012, 5, 5133 RSC.
- (a) C.-L. Cheng, Y.-C. Lo, K.-S. Lee, D.-J. Lee, C.-Y. Lin and J.-S. Chang, Bioresour. Technol., 2011, 102, 8514 CrossRef CAS PubMed; (b) R. R. O. Barros, R. S. Paredes, T. Endo, E. P. S. Bon and S.-H. Lee, Bioresour. Technol., 2013, 136, 288 CrossRef PubMed.
- P. Azadi and R. Farnood, Int. J. Hydrogen Energy, 2011, 36, 9529 CrossRef CAS.
- (a) R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature, 2002, 418, 964 CrossRef CAS PubMed; (b) D. C. Elliott, Biofuels, Bioprod. Biorefin., 2008, 2, 254 CrossRef CAS; (c) P. Azadi and R. Farnood, Int. J. Hydrogen Energy, 2011, 36, 9529 CrossRef CAS.
- K. Sanderson, Nature, 2011, 474, S12 CrossRef CAS PubMed.
- (a) M. Nielsen, E. Alberico, W. Baumann, H.-J. Drexler, H. Junge, S. Gladiali and M. Beller, Nature, 2013, 495, 85 CrossRef CAS PubMed; (b) R. E. Rodríguez-Lugo, M. Trincado, M. Vogt, F. Tewes, G. Santiso-Quinones and H. Grützmacher, Nat. Chem., 2013, 5, 342 CrossRef PubMed; (c) E. Alberico, P. Sponholz, C. Cordes, M. Nielsen, H.-J. Drexler, W. Baumann, H. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 14162 CrossRef CAS PubMed; (d) A. Monney, E. Barsch, P. Sponholz, H. Junge, R. Ludwig and M. Beller, Chem. Commun., 2014, 50, 707 RSC.
- (a) H. Junge and M. Beller, Tetrahedron Lett., 2005, 46, 1031 CrossRef CAS; (b) H. Junge, B. Loges and M. Beller, Chem. Commun., 2007, 522 RSC; (c) M. Nielsen, A. Kammer, D. Cozzula, H. Junge, S. Gladiali and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 9593 CrossRef CAS PubMed; (d) M. Nielsen, H. Junge, A. Kammer and M. Beller, Angew. Chem., Int. Ed., 2012, 51, 5711 CrossRef CAS PubMed.
- N. Taccardi, D. Assenbaum, M. E. M. Berger, A. Bösmann, F. Enzenberger, R. Wölfel, S. Neuendorf, V. Goeke, N. Schödel, H.-J. Maass, H. Kistenmacherc and P. Wasserscheid, Green Chem., 2010, 12, 1150 RSC.
- V. F. Wendisch, S. N. Lindner and T. M. Meiswinkel, ed. G. Montero, InTech, 2011, p. 305.
- B. Sims, “Clearing the Way for Byproduct Quality: Why quality for glycerin is just as important for biodiesel”, Biodiesel Magazine. October 25, 2011.
- For selected samples, see: (a) P. D. Vaidya and A. E. Rodrigues, Chem. Eng. Technol., 2009, 32, 1463 CrossRef CAS; (b) A. C. C. Souzaa and J. L. Silveira, Renewable Sustainable Energy Rev., 2011, 15, 1835 CrossRef; for selected samples, see: (c) G. W. Huber, J. W. Shabaker and J. A. Dumesic, Science, 2003, 300, 2075 CrossRef CAS PubMed; (d) D. L. King, L. Zhang, G. Xia, A. M. Karim, D. J. Heldebrant, X. Wang, T. Petersona and Y. Wang, Appl. Catal., B, 2010, 99, 206 CrossRef CAS; (e) S. H. Cho and D. J. Moon, J. Nanosci. Nanotechnol., 2011, 11, 7311 CrossRef CAS PubMed; (f) F. D. Alvarado and F. Gracia, Int. J. Hydrogen Energy, 2012, 37, 14820 CrossRef; (g) J.-J. Wang, Z.-J. X.-B. Li, X.-B. Fan, Q.-Y. Meng, S. Yu, C.-B. Li, J.-X. Li, C.-H. Tung and L.-Z. Wu, ChemSusChem, 2014, 7, 1468 CrossRef CAS PubMed.
- J. Sarma, S. K. Brar, E. B. Sydney, Y. L. Bihan, G. Buelna and C. R. Soccol, Int. J. Hydrogen Energy, 2012, 37, 6473 CrossRef.
- D. Morton and D. Cole-Hamilton, J. Chem. Soc., Chem. Commun., 1988, 1154 RSC.
- For selected reviews on pincer and non-innocent ligands, see: (a) M. Albrecht and G. Koten, Angew. Chem., Int. Ed., 2001, 40, 3750 CrossRef CAS; (b) M. E. Boom and D. Milstein, Chem. Rev., 2003, 103, 1759 CrossRef PubMed; (c) H. Grützmacher, Angew. Chem., Int. Ed., 2008, 47, 1814 CrossRef PubMed; (d) J. I. Vlugt and J. N. H. Reek, Angew. Chem., Int. Ed., 2009, 48, 8832 CrossRef PubMed; (e) W.-H. Zhang, S. W. Chien and T. S. A. Hor, Coord. Chem. Rev., 2011, 255, 1991 CrossRef CAS.
- M. Dusselier, P. Van Wouwe, A. Dewaele, E. Makshina and B. F. Sels, Energy Environ. Sci., 2013, 6, 1415 CAS.
- (a) W. Kuriyama, T. Matsumoto, Y. Ino and O. Ogata, PCT Int. Appl, WO/2011/048727 A1, Takasago International Corporation, 2011 Search PubMed; (b) M. Bertoli, A. Choualeb, A. J. Lough, B. Moore, D. Spasyuk and D. G. Gusev, Organometallics, 2011, 30, 3479 CrossRef CAS.
- For reviews, see: (a) D. Milstein, Top. Catal., 2010, 53, 915 CrossRef CAS; (b) C. Gunanathan and D. Milstein, Acc. Chem. Res., 2011, 44, 588 CrossRef CAS PubMed; (c) C. Gunanathan and D. Milstein, Science, 2013, 341, 1229712 CrossRef PubMed.
- (a) W. Baratta, E. Herdtweck, K. Siega, M. Toniutti and P. Rigo, Organometallics, 2005, 24, 1660 CrossRef CAS; (b) W. Baratta, G. Chelucci, S. Gladiali, K. Siega, M. Toniutti, M. Zanette, E. Zangrando and P. Rigo, Angew. Chem., Int. Ed., 2005, 44, 6214 CrossRef CAS PubMed; (c) W. Baratta, K. Siega and P. Rigo, Adv. Synth. Catal., 2007, 349, 1633 CrossRef CAS; (d) W. Baratta, K. Siega and P. Rigo, Chem. – Eur. J., 2007, 13, 7479 CrossRef CAS PubMed; (e) W. Baratta, M. Ballico, G. Esposito and P. Rigo, Chem. – Eur. J., 2008, 14, 5588 CrossRef CAS PubMed; (f) W. Baratta, G. Chelucci, S. Magnolia, K. Siega and P. Rigo, Chem. – Eur. J., 2009, 15, 726 CrossRef CAS PubMed; (g) W. Baratta, G. Bossi, E. Putignano and P. Rigo, Chem. – Eur. J., 2011, 17, 3474 CrossRef CAS PubMed.
- (a) Z. E. Clarke, P. T. Maragh, T. P. Dasgupta, D. G. Gusev, A. J. Lough and K. Abdur-Rashid, Organometallics, 2006, 25, 4113 CrossRef CAS; (b) X. Chen, W. Jia, R. Guo, T. W. Graham, M. A. Gullons and K. Abdur-Rashid, Dalton Trans., 2009, 1407 RSC.
- Contents of the industrial glycerol are determined as 86–88% glycerol, 12–14% H2O, and less than 0.01% sulphate ash by A.+E. Fischer Chemie.
- Both pure glycerol and industrial glycerol were analyzed by inductively coupled plasma mass spectroscopy (ICP-MS). There was no detection of Ru, Rh, Ir, Co, Cu, Fe, Mo, Pd (less than 1 ppm).
- (a) H. Kishida, F. Jin, Z. Zhou, T. Moriya and H. Enomoto, Chem. Lett., 2005, 34, 1560 CrossRef CAS; (b) Y. Shen, S. Zhang, H. Li, Y. Ren and H. Liu, Chem. – Eur. J., 2010, 16, 7368 CrossRef CAS PubMed; (c) F. Auneau, S. Noël, G. Aubert, M. Besson, L. Djakovitch and C. Pinel, Catal. Commun., 2011, 16, 144 CrossRef CAS; (d) Y. Zhang, Z. Shen, X. Zhou, M. Zhang and F. Jin, Green Chem., 2012, 14, 3285 RSC.
- P. Mäki-Arvela, I. L. Simakova, T. Salmi and D. Y. Murzin, Chem. Rev., 2013, 114, 1909 CrossRef PubMed.
- (a) Z. Shen, F. Jin, Y. Zhang, B. Wu, A. Kishita, K. Tohji and H. Kishida, Ind. Eng. Chem. Res., 2009, 48, 8920 CrossRef CAS; (b) C. A. Ramírez-López, J. R. Ochoa-Gómez, M. Fernández-Santos, O. Gómez-Jiménez-Aberasturi, A. Alonso-Vicario and J. Torrecilla-Soria, Ind. Eng. Chem. Res., 2010, 49, 6270 CrossRef; (c) A. Yuksel, H. Koga, M. Sasaki and M. Goto, Ind. Eng. Chem. Res., 2010, 49, 1520 CrossRef CAS; (d) D. Roy, B. Subramaniam and R. V. Chaudhari, ACS Catal., 2011, 1, 548 CrossRef CAS; (e) F. Auneau, L. S. Arani, M. Besson, L. Djakovitch, C. Michel, F. Delbecq, P. Sautet and C. Pinel, Top. Catal., 2012, 55, 474 CrossRef CAS; (f) A. Costine, J. S. C. Loh, F. Busetti, C. A. Joll and A. Heitz, Ind. Eng. Chem. Res., 2013, 52, 5572 CrossRef CAS; (g) X. Jin, D. Roy, P. S. Thapa, B. Subramaniam and R. V. Chaudhari, ACS Sustainable Chem. Eng., 2013, 1, 1453 CrossRef CAS; (h) Y. Li, S. Chen, J. Xu, H. Zhang, Y. Zhao, Y. Wang and Z. Liu, Clean: Soil, Air, Water, 2013, 41, 1 Search PubMed; (i) J. Xu, H. Zhang, Y. Zhao, B. Yu, S. Chen, Y. Li, L. Hao and Z. Liu, Green Chem., 2013, 15, 1520 RSC; (j) R. K. P. Purushothaman, J. van Haveren, D. S. van Es, I. Melian-Cabrera, J. D. Meeldijk and H. J. Heeres, Appl. Catal., B, 2014, 147, 92 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: General information, detail reaction procedure, GC and 1H NMR spectrum. See DOI: 10.1039/c4gc01707b |
| ‡ The reaction mixture was analyzed by 1H NMR and GC-MS; however no clear information was obtained for unidentified side products. |
| This journal is © The Royal Society of Chemistry 2015 |