Xylose yields and relationship to combined severity for dilute acid post-hydrolysis of xylooligomers from hydrothermal pretreatment of corn stover
Taiying
Zhang†
b,
Rajeev
Kumar†
b,
Yueh-Du
Tsai†
ab,
Richard T.
Elander
c and
Charles E.
Wyman
*ab
aDepartment of Chemical and Environmental Engineering, Bourns College of Engineering, University of California, Riverside, 446 Winston Chung Hall, 900 University Ave., Riverside, CA 92521, USA. E-mail: charles.wyman@ucr.edu; Fax: +1-951-781-5790; Tel: +1-951-781-5703
bCenter for Environmental Research and Technology (CE-CERT), Bourns College of Engineering, University of California, Riverside, 1084 Columbia Ave, Riverside, CA 92507, USA
cNational Bioenergy Center, National Renewable Energy Laboratory, 15013 Denver West Parkway, Golden, CO 80401, USA
First published on 12th September 2014
Abstract
To maximize yields of fermentable xylose monomer from hydrothermal pretreatment of corn stover at 200 °C, the xylooligomers-rich liquor produced was post hydrolyzed with dilute sulfuric acid over a range of times and acid concentrations. The results showed that application of 0.75% H2SO4 at 110 °C for 180 min or 0.50% H2SO4 at 110 °C for 240 min recovered almost 100% of the total xylose from the oligomers. Furthermore, adjusting one constant in the combined severity parameter (CSP) provided a rapid and accurate tool for trading off times, temperatures, and acid concentrations to reach the highest xylose yields from xylooligomers in hydrothermal pretreatment solutions. This adjusted CSP showed that an 8.4 °C temperature increase has the same impact as doubling the acid concentration or halving reaction time instead of the 10 °C change projected by the customary CSP and suggests that xylooligomers bonds are more easily broken than the hemicellulose from which they are derived.
1. Introduction
As petroleum reserves are depleted, it is important to develop economical and efficient alternative sources of liquid fuels for transportation,1,2 and biofuels derived from lignocellulosic biomass occupy a unique role in meeting this need. Cellulosic biomass including wood, grasses, and agriculture and forestry residues are inexpensive ($60 per dry ton biomass is competitive in unit energy cost with petroleum at about $20 per barrel) and abundant without competing with production of food or feed.3 In addition, fuels made from cellulosic biomass contribute much less to accumulation of carbon dioxide in that growing crops consume carbon dioxide emitted during production and use of biofuels provided minimal fossil fuels are employed in the overall production process.4Hemicellulose is typically the second most abundant portion of cellulosic biomass and is usually made up of xylose and other sugars. These sugars can in turn be fermented into ethanol that is now blended with gasoline for use in conventional vehicles or used as a pure fuel in appropriate engines. Alternatively, the sugars can be fermented into other products that are more fungible with gasoline or chemically reacted to furfural or other compounds that can in turn be converted into hydrocarbons that can be readily incorporated into the existing fuel infrastructure.5,6 Dilute acid pretreatments employ acid catalysis to hydrolyze a large portion of hemicellulose into mostly sugar monomers and some oligomers that are soluble in the aqueous phase. Because the hemicellulose in many forms of biomass is rich in xylose, a large portion of the dissolved sugars is xylose monomers and xylooligomers (XOs), some of which degrade into furfural and other products at conditions that favor high yields of sugars during pretreatment.7–9 These degradation products reduce sugar yields and also inhibit subsequent fermentations and enzymatic hydrolysis. Hydrothermal (just hot water or steam) pretreatment can use just heat for production of sugars, but a major fraction of the hemicellulose released at conditions that favor high yields of xylose monomers and oligomers and minimize sugar degradation is XOs.10,11 However, recent findings showed that XOs generated during pretreatment and/or enzymatic hydrolysis of residual xylan left in the pretreated solids strongly inhibit cellulase activity.12,13 In addition, because many organisms cannot ferment oligomers, enzymes or acids are needed following pretreatment to convert the oligomers into sugar monomers that are amenable to downstream processing.
Various processing options for dilute acid post-hydrolysis of XOs can be applied, each of which has implications for downstream operations and techno-economics. For instance, the whole slurry after hydrothermal pretreatment could be subjected to post-hydrolysis, which offers the opportunity to convert not only XOs contained in the liquid fraction but also potentially residual insoluble xylan left in the solid fraction. However, from an energy point of view, mixing the whole slurry at commercially viable loadings (∼>30 wt%) may prove to be energy intensive. In addition, as shown by Lloyd and Wyman and others earlier, ash in biomass solids can neutralize acid, thus, require more acid if the solids are left in than needed for post hydrolysis of the liquid alone.14,15 Alternatively, post-hydrolysis could be applied to just the liquid portion following hydrothermal pretreatment.16 Although this process configuration requires solid-liquid separation and washing steps to capture most of the available XOs in the liquid fraction, a simple stirred-tank or pipe reactor could be utilized in a commercial application, without the need for a complex high solids reactor configuration. This option is particularly important due to the usage of sulfuric acid in the post-hydrolysis reaction and resultant high cost metallurgy requirements for containment.
Dilute sulfuric acid can be attractive for this post-hydrolysis operation because it is effective and inexpensive, but little data is available to evaluate the viability of this approach. It would be highly desirable to have accurate kinetic models to predict a priori yields from dilute acid hydrolysis of liquors rich in XOs to monomers. However, detailed kinetic models fit key constants to data and have not proven to be particularly robust in predicting how yields change over reasonable ranges of time, temperature, and acid concentration.17 In addition, they are not particularly suited to use in the field. On the other hand, the severity parameter Ro, which is similar to H-factor or P-factor applied to the Kraft process decades ago, was developed for hydrothermal pretreatment employing steam explosion and later modified for dilute acid pretreatment of lignocellulosic biomass to indicate and predict xylan solubilization.18–21 In fact, its ability to rapidly tradeoff the effects of time and temperature for hydrothermal pretreatment have made it a valuable tool for selecting reaction conditions for lignocellulosic biomass pretreatment.18–20,22 The severity parameter was modified to make it possible to tradeoff the effects on pretreatment yields of acid/base concentrations against time and/or temperature in what is known as the combined severity parameter (CSP).23–27 It was recently further modified based on a pseudo-kinetic model of lignocellulosic saccharification with concentrated sulfuric acid.28,29
The CSP has limitations in that it does not accurately predict xylan solubilization at high severities.19,30 An inherent limitation in the activation energy constant in CSP for different substrates was recently discussed,31 and a combined hydrolysis factor (CHF) based on CSP was proposed for sulfite pretreatment (SPORL) of aspen wood.32 A recent paper shows that different acids followed slightly different CSP patterns even for pretreatment of the same substrate.33 The combined severity parameter has received little attention for relating yields for post-hydrolysis of XOs to reaction times, temperatures, and acid concentrations, and as a result, it is unclear if it would be a useful tool for identifying favorable conditions.
The general concept of converting XOs by dilute acid post-hydrolysis or applying enzymes has been considered by others at similar temperatures as needed for pretreatment.16,34–36 However, for post-hydrolysis to be commercially viable, it is important to realize high yields at lower temperatures than needed for pretreatment to keep containment costs low and have a potential economic advantage compared to direct use of dilute sulfuric acid in pretreatment. In addition, XO conversion by enzymes is not yet commercially viable due to their high cost, the need for a complex formulation to hydrolyze the range of oligomer bonds, and enzyme inhibition by background sugars and other compounds.36 Thus, in this study, yields from dilute acid hydrolysis of xylooligomers solubilized in hydrothermal pretreatment of corn stover at conditions that gave the highest total sugar yields were determined at relatively low temperatures (101 °C to 130 °C), and the effects of acid, time, temperature, and oligomers concentration on performance were determined. Furthermore, one of the CSP parameters was adjusted to facilitate relating xylose monomer yields from post-hydrolysis of hydrolyzate oligomers to a range of acid concentrations, times, and temperatures employed without the need for a detailed kinetics model.
2. Materials and methods
2.1. Substrate
The National Renewable Energy Laboratory in Golden, Colorado, provided corn stover that had been harvested in Iowa in the fall of 2010. The air-dried corn stover was milled (model 4, Arthur H. Thomas Company, Philadelphia, PA) to less than 2 mm and stored in sample bags at −18 °C in a laboratory freezer until needed.2.2. Compositional analysis
The moisture content of the prepared corn stover samples was determined with a laboratory moisture analyzer (Mettler Toledo, Model: HB43 Halogen Moisture Analyzer, Columbus, OH). Ash content was analyzed according to NREL Laboratory Analytical Procedures (Technical Report NREL/TP-510-42622)37 as was extractives content (Technical Report NREL/TP-510-42619).38 Klason lignin, glucan, and xylan contents were determined following the modified NREL Laboratory Analytical Procedure (Technical Report NREL/TP-510-42618)39 that employed two-step acid hydrolysis: (1) about 0.3 g substrate was placed into a vial and hydrolyzed in 72% (w/w) sulfuric acid at 30 °C for 1 h and (2) the slurry was further hydrolyzed in 4% (w/w) sulfuric acid at 121 °C for 1 h. The sugars in the liquid were determined by a Waters HPLC (2695, Waters corporation, Milford, MA), as described in the sugar analysis section.2.3. Batch hydrothermal pretreatment and XOs preparation
Tubular reactors (Hastelloy C-276, O.D. 0.0127 m (0.5′′) with wall thickness of 0.0008890 m (0.035′′), length of 0.1524 m (6′′), and volume of 0.0143 L (14.3 ml) were initially employed for batch hydrothermal pretreatment of corn stover to define conditions that gave the highest total xylose plus glucose yields from pretreatment and enzymatic hydrolysis combined. These reactors were heated in 4 kW fluidized sand baths (Model SBL-2D, Technical Co., Princeton, NJ), with the internal temperature monitored with a K type thermocouple probe (Omega KQSS-316G-12, Omega Engineering Co., Stamford, CT). The heat-up time to reach final reaction temperature was less than 200 seconds and included in the stated reaction time. The heat-up time was slightly longer for the higher temperature experiments than for lower temperatures. Cooling down in a water bath to room temperature took about 40 seconds, which was not included in the reaction time.Before pretreatment, milled corn stover was presoaked in deionized water (DI) overnight at a solids loading of 10 wt% for hydrothermal pretreatment at 160 °C, 180 °C, and 200 °C. Following pretreatments, the slurry was separated into a liquid hydrolyzate and pretreated solids by vacuum filtration using a 0.22 μm glass fiber filter (09-804-110A, Fisher Science, Pittsburgh, PA). The pretreated solids were washed thoroughly with a volume of room temperature DI water of about ∼30 times the wet weight of biomass before compositional analysis and subsequent enzymatic hydrolysis. Although we did not use the liquid wash from these experiments, it could be combined with the pretreatment liquor for dilute acid post hydrolysis and/or sent to water treatment. Sugar yields in the liquid from just hydrothermal pretreatment were designated as Stage 1 sugar yields, and those from subsequent enzymatic hydrolysis of the pretreated solids were labeled as Stage 2 sugar yields.
Once conditions had been identified to give high yields in tubular reactors, large quantities of xylooligomers enriched solution were prepared in a 1 L Parr reactor (Parr Instruments, Moline, IL). In this case, a 10% (w/w) solid loading of corn stover based on dry weight was impregnated with DI water at room temperature for at least 4 h. Then the pre-soaked slurry was transferred into 1 L Hastelloy C Parr reactor fitted with a 3.5 in. diameter helical impeller on a two-piece shaft. The impeller was driven by a variable speed DC motor (A1750HC, Parr Instruments, Moline, IL) set to about 200 RPM. The reactor was sealed and lowered into a 4 kW sand bath (model SBL-2D TechNet, Princeton, NJ) set at 360 °C for fast heat up. Once the Parr reactor temperature reached 200 °C (monitored by K-type thermo-couple), it was raised out of the sand bath to keep the bottom about 1 to 2 cm above the sand surface. The temperature was controlled within ±2 °C of target temperature of 200 °C by raising or dropping the reactor slightly as needed.9,40 At the end of reaction period, the reactor was transferred into a bucket filled with room temperature water for cooling. After the content's temperature dropped below 60 °C, the reactor was opened, and the pretreated slurry was filtered through 125 mm diameter Whatman filter paper. A portion of the filtrate was treated according to the post-hydrolysis procedure to determine concentrations of xylooligomers and xylose. The rest was stored in 1 L glass bottles at room temperature to avoid substantial precipitation of xylooligomers at lower temperatures.41
2.4. Xylooligomers acid hydrolysis employing high throughput system (HTP)
A high throughput system (HTP) was used for acid hydrolysis of XOs contained in the liquid fraction after hydrothermal pretreatment to initially determine the effects of temperature, acid concentration, and reaction time on xylose yields. The custom made well-plate consisted of 96 Hastelloy round cups (i.d. 6.9 mm × 16.0 mm inside length) with reaction volumes of 450 μL resting on an aluminum bottom plate with their tops covered with a silicone gasket that was held in place by a stainless steel plate, with the resulting sandwich clamped tightly to contain the contents at pretreatment pressures and temperatures.42 A reaction volume of 450 μL with half xylooligomer liquor and half acid solution (8 channel pipetter, 30–300 μL, Eppendorf) was added to each well of the HTP system to provide the target acid concentration prior to clamping the assembly together. For each acid concentration, samples were run in quadruplicate. The sealed HTP system was placed horizontally and lengthwise inside a custom steam chamber made of readily available steam rated (to 1 MPa steam pressure) 316 stainless steel 0.102 m (4′′) diameter fittings (McMaster, Santa Fe Springs, CA). Steam generated by a high pressure steam boiler (FB-075-L, Fulton Companies, Pulaski, NY) rapidly heated the plate to 101 °C, 110 °C, 120 °C, or 130 °C for 3 min to 500 min. After the plate was cooled down to room temperature by flooding with tap water, half of the samples were centrifuged, and the liquid portion was then analyzed by HPLC to measure the xylose monomer concentration. Additional sulfuric acid was added into the other half of the samples to bring the acid concentration to 4% for post-hydrolysis directly to determine the oligomers concentration.432.5. Sugar analysis
Sugar monomers in the liquids from pretreatment and enzymatic hydrolysis were analyzed quantitatively with a Waters HPLC system (model 2695) equipped with a 2414 refractive index (RI) detector and a Waters 2695 auto sampler using Waters Empower™ 2 software (Waters Co., Milford, MA). Bio-Rad Aminex HPX-87H and Bio-Rad Aminex HPX-87P columns (Bio-Rad Laboratories, Hercules, CA) were employed for separation of sugars for quantification. The Aminex HPX-87H column was heated to 65 °C, with 5 mM sulfuric acid at a flow rate of 0.6 ml min−1 as the carrier solvent. Whereas for Aminex HPX-87P, the column was heated to 80 °C, with double deionized water at a flow rate of 0.6 ml min−1 as the carrier solvent. The concentrations of total xylan and glucan in the liquid hydrolyzate were determined by post-hydrolysis with 4% w/w sulfuric acid at 121 °C for 1 h according to NREL Laboratory Analytical Procedure (Technical Report NREL/TP-510-42623).43 Both glucan and xylan yields in Stage 1 were reported as the sum of monomer and oligomer yields.2.6. Enzymatic hydrolysis
Washed solids from hydrothermal pretreatment of corn stover in the batch tubes were enzymatically hydrolyzed in 50 mM sodium citrate buffer (pH 4.8) at 2% solids loadings at 50 °C in duplicates by following modified NREL Laboratory Analytical Procedure (Technical Report NREL/TP-510-42629)44 using Cellic®CTec2 (175 mg of protein g−1 of formulated enzyme product, Novozymes North America, Franklinton, NC) and Cellic®HTec2 (168 mg of protein g−1 of formulated enzyme product, Novozymes North America, Franklinton, NC). Protein numbers were provided by Novozymes. A total enzyme protein loading of 40 mg cellulase + 20 mg xylanase g−1 of total glucan plus xylan in raw corn stover was used for enzymatic hydrolysis. Hydrolysis samples were collected at 72 h, and sugar concentrations were determined for calculation of Stage 2 glucan and xylan yields as a percent of the original glucan and xylan content in the raw corn stover. Although there were very small amounts of xylan remaining in solids from pretreatments at high severities, optimization for total sugar yields resulted in considerable amounts of xylan remaining in solids from lower severity pretreatments. Therefore, the cellulase (CTec2) was supplemented with xylanase (HTec2) for enzymatic hydrolysis of all pretreated solids.2.7. Calculations
The log of the severity parameter (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro) for hydrothermal pretreatment was defined as a function of pretreatment temperature T (°C) and pretreatment time t (min), while the combined severity parameter (CSP) included the effect of pH from added acid on severity:18,21
Ro) for hydrothermal pretreatment was defined as a function of pretreatment temperature T (°C) and pretreatment time t (min), while the combined severity parameter (CSP) included the effect of pH from added acid on severity:18,21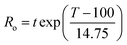 | (1) |
CSP = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro − pH Ro − pH | (2) |
Because the hydronium ions activity of sulfuric acid solutions is influenced by temperature and it was very difficult to measure pH above 100 °C, the pH values at each acid concentration were calculated by the following equation:14
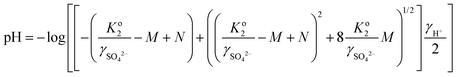 | (3) |
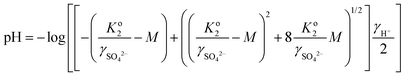 | (4) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ko2 = 56.889 − 19.8858 log Ko2 = 56.889 − 19.8858 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) T − 2307.9/T − 0.006473T T − 2307.9/T − 0.006473T | (5) |
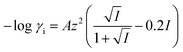 | (6) |
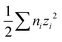 , γi = ionic activity coefficient, A = Debye–Huckel constant = 1.825 × 106 (εT)−1.5, ε = dielectric constant of water = 132.88 −0.208T, z = ionic charge, n = ion molarity, T = temperature, K.
, γi = ionic activity coefficient, A = Debye–Huckel constant = 1.825 × 106 (εT)−1.5, ε = dielectric constant of water = 132.88 −0.208T, z = ionic charge, n = ion molarity, T = temperature, K.
Glucan and xylan yields from pretreatment and overall glucan and xylan yields from both pretreatment and enzymatic hydrolysis were both calculated as:
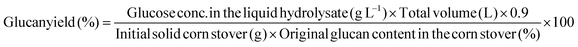 | (7) |
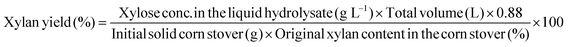 | (8) |
 | (9) |
These equations were applied to determine yields from Stage 1 (pretreatment) and Stage 2 (enzymatic hydrolysis). For Stage 1, sugar yields included both monomer and oligomers determined through post hydrolysis of the liquid hydrolyzate. Sugar yields were calculated as a percent of the theoretical maximum on the basis of original glucan and/or xylan content in corn stover unless otherwise specified. Monomeric sugars in the liquid hydrolyzate were measured after pretreatment and enzymatic hydrolysis on the Waters HPLC described in section 2.5, and sugar yields were calculated by eqn (7)–(9).
Xylose yields from dilute acid post-hydrolysis of the liquid hydrolyzate of corn stover by hydrothermal pretreatment were calculated as:
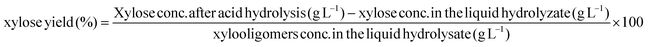 | (10) |
In eqn (10), the XOs concentration in the liquid hydrolyzate was expressed as equivalent xylose determined by the difference between total xylose and xylose monomer concentrations.
3. Results and discussion
3.1. Definition of conditions for hydrothermal pretreatment followed by enzymatic hydrolysis
Hydrolyzate for post-hydrolysis of oligomers was prepared by hydrothermal pretreatment of corn stover that contained 30.8 ± 1.23% glucan, 21.70 ± 1.12% xylan, 17.20 ± 0.49% lignin, and 8.08 ± 0.14% ash including 5.72 ± 1.50% acid insoluble ash, all on a dry weight basis. To identify conditions that gave the highest total xylose plus glucose yields from the combined operations of pretreatment and enzymatic hydrolysis, hydrothermal pretreatment was performed for reaction times of 2 to 271 min at temperatures of 160, 180, and 200 °C in small tube reactors at a 10% w/w solids loading followed by enzymatic hydrolysis.9Fig. 1 presents glucan and xylan sugar yields from batch hydrothermal pretreatment (Stage 1) of corn stover and from subsequent enzymatic hydrolysis of the washed solids (Stage 2) at the conditions noted. The total xylan plus glucan yields for Stage 1 and 2 combined increased initially with pretreatment time at 160, 180, and 200 °C to peak values at 171, 44, and 14.3 min, respectively. From this, we see that the highest total sugar yield of ∼92% from pretreatment followed by enzymatic hydrolysis at an enzyme loading of 40 mg cellulase + 20 mg xylanase g−1 of total glucan plus xylan in the raw corn stover was achieved for a 14.3 min pretreatment at 200 °C.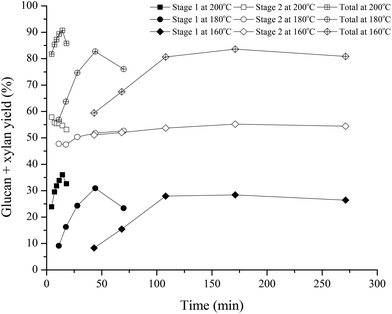 | ||
| Fig. 1 Glucan and xylan sugar yields from batch hydrothermal pretreatment of corn stover and subsequent enzymatic hydrolysis of the washed solids at 160, 180, and 200 °C versus pretreatment time. | ||
The xylose monomer, oligomers, and total xylose yields from pretreatment versus severity in Fig. 2 show that although yields of xylose monomers plus oligomers from pretreatment at different temperatures peaked at similar severities, the value of the highest total xylose yield changed with temperature. In particular, the highest total xylose yields from hydrothermal pretreatment were 58.5% and 66.0% at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 4.0 for 160 °C and 180 °C, respectively, and 77.3% at log
Ro = 4.0 for 160 °C and 180 °C, respectively, and 77.3% at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro = 4.1 for 200 °C. Furthermore, xylose losses were lower at the lowest temperature run of 160 °C than at 180 °C at log
Ro = 4.1 for 200 °C. Furthermore, xylose losses were lower at the lowest temperature run of 160 °C than at 180 °C at log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ro of 4.2. The highest yield of xylose monomers and oligomers of ∼77.3% occurred when corn stover was pretreated at 200 °C for 14.3 min. The solution contained monomeric sugars (glucose 0.86 g L−1, xylose 1.84 g L−1, arabinose 1.12 g L−1), oligomers (cellooligomers 1.61 g L−1, xylooligomers 14.57 g L−1), total sugars (glucose 2.47 g L−1, xylose 16.41 g L−1), organic acids (lactic acid 1.05 g L−1, glycerol 0.44 g L−1, acetic acid 2.14 g L−1), and degradation products (5-HMF 0.14 g L−1 and furfural 0.34 g L−1). Fig. 2 also shows that liquid prepared at these conditions was rich in oligomers, with only 3.6% of the total being xylose monomers. The solutions for all post-hydrolysis experiments were prepared by hydrothermal pretreatment of corn stover at these conditions in a 1 L Parr reactor as described in materials and methods.
Ro of 4.2. The highest yield of xylose monomers and oligomers of ∼77.3% occurred when corn stover was pretreated at 200 °C for 14.3 min. The solution contained monomeric sugars (glucose 0.86 g L−1, xylose 1.84 g L−1, arabinose 1.12 g L−1), oligomers (cellooligomers 1.61 g L−1, xylooligomers 14.57 g L−1), total sugars (glucose 2.47 g L−1, xylose 16.41 g L−1), organic acids (lactic acid 1.05 g L−1, glycerol 0.44 g L−1, acetic acid 2.14 g L−1), and degradation products (5-HMF 0.14 g L−1 and furfural 0.34 g L−1). Fig. 2 also shows that liquid prepared at these conditions was rich in oligomers, with only 3.6% of the total being xylose monomers. The solutions for all post-hydrolysis experiments were prepared by hydrothermal pretreatment of corn stover at these conditions in a 1 L Parr reactor as described in materials and methods.
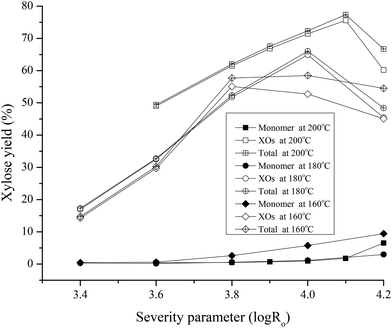 | ||
| Fig. 2 Xylose monomer, oligomers, and total xylose yields from hydrothermal pretreatment versus the severity parameter for pretreatment at 160, 180, and 200 °C. | ||
3.2. Acid hydrolysis of xylooligomers with high throughput system (HTP)
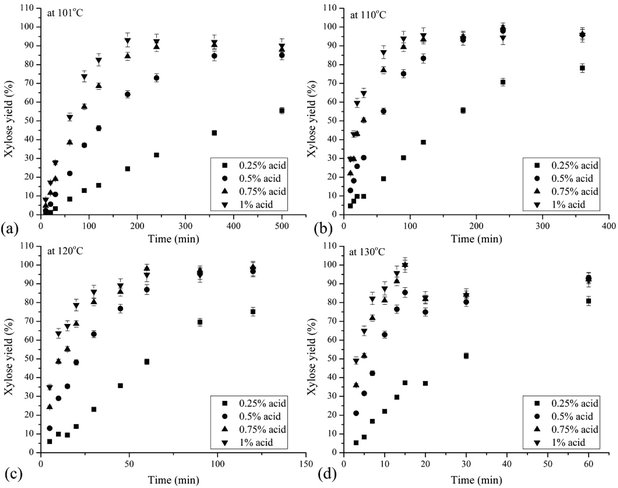
The liquid fraction from hydrothermal pretreatment of corn stover at 200 °C for 14.3 min was treated with 0.25%, 0.50%, 0.75%, and 1.00% w/w sulfuric acid concentrations at temperatures of 101, 110, 120, and 130 °C in a custom built 96-well plate HTP system.42,45Fig. 3 shows the resulting average xylose yields of quadruplicate as a percent of the theoretical maximum that could be obtained from the xylooligomers in the liquid fraction at each temperature versus reaction time. As acid loading increased, xylose yields increased rapidly at shorter reaction times and became close and even overlapped at higher acid concentrations at longer times.Table 1 summarizes conditions that realized the highest overall xylose yields for each combination of acid concentration and temperature applied to the liquors resulting from hydrothermal pretreatment of corn stover. Xylose yields reached over 90% before the end of the allowed reaction time except at the 0.25% acid loading. For the latter, the maximum xylose yields were 78% at 110 °C, 75% at 120 °C, and 81% at 130 °C. However, the xylose yield at 101 °C only reached 55.5% after 500 min and still climbing at this longest time applied. Overall, xylose yields continually increased because xylooligomers primarily formed monomeric xylose and xylose degradation was very low at the low temperatures applied. Furfural, the major product from xylose degradation, was negligible in the liquid sample after acid hydrolysis as analyzed by HPLC.
| Temperature (°C) | Acid concentration (%) | Reaction time (min) | Xylose + xylooligomer yield (%) |
|---|---|---|---|
| Considering the error bar, the closest to the highest xylose yield with shorter reaction time were listed instead of the highest xylose yield with longer reaction time (a500 min for 85%; b120 min for 96.61%). | |||
| 101 | 0.25 | 500 | 55.5% |
| 0.5 | 360 | 84.6%a | |
| 0.75 | 240 | 89.29% | |
| 1 | 180 | 93.1% | |
| 110 | 0.25 | 360 | 78.2% |
| 0.5 | 240 | 98.0% | |
| 0.75 | 240 | 99.7% | |
| 1 | 120 | 95.7% | |
| 120 | 0.25 | 120 | 75.1% |
| 0.5 | 90 | 95.2%b | |
| 0.75 | 60 | 98.0% | |
| 1 | 60 | 94.9% | |
| 130 | 0.25 | 60 | 80.8% |
| 0.5 | 60 | 93.2% | |
| 0.75 | 15 | 100.0% | |
| 1 | 15 | 100.0% | |
These results showed that very high yields of xylose can be realized from xylooligomers at low temperatures with dilute sulfuric acid. However, the reaction times were too long to be useful at the lowest acid concentration of 0.25%. Furthermore, temperatures of 110 or 120 °C would be needed to achieve xylose yields of nearly 100% while 101 °C was apparently too low to overcome the activation energy of all the bonds that must be broken to release xylose monomers. Overall, the condition that gave the highest xylose yield of 99% from post hydrolysis was reaction with 0.75% sulfuric acid for 240 min at 110 °C.
Because laboratory reactors large enough to produce sufficient quantities of oligomers could not handle higher solids concentrations, hydrothermal pretreatment was performed at lower solids loading (∼10 wt%) that resulted in lower XO concentrations (∼10 g L−1) than would be desirable commercially (50–70 g L−1) at high solids loading (>35 wt%).16,35 In addition, operation at lower solids provided enough water to efficiently recover the oligomers. However, we anticipate the results should not change much with XOs concentration, at least for the XOs concentrations (50–70 g L−1) realized at commercially relevant solids loadings as hydrolysis depends on the hydronium ion concentration. In addition, although further investigation is warranted, we anticipate background sugars and other soluble compounds (such as soluble lignin and furfural) produced at high solids loadings (25–35 wt%) would not appreciably affect XOs hydrolysis rates and/or yields at the low reaction temperature determined to be optimum in this study. Thus, lower XO concentrations should not appreciably affect the main goals of this study to define conditions to hydrolyze xylooligomers at low severity and determine whether the conventional severity parameter can correlate yields well.
3.3. Combined severity parameter for acid hydrolysis of xylooligomers
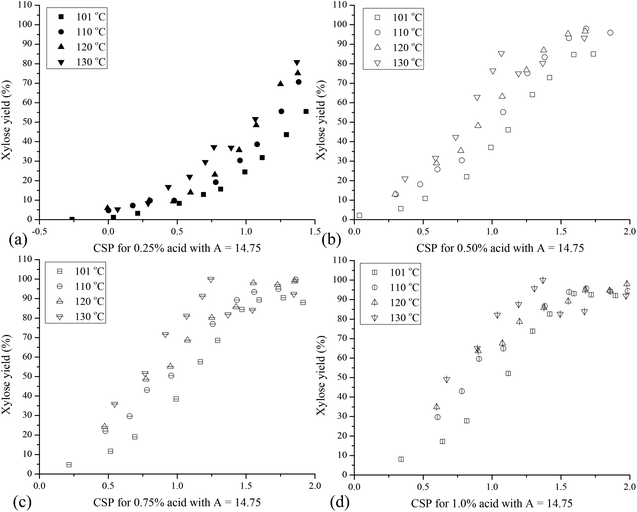
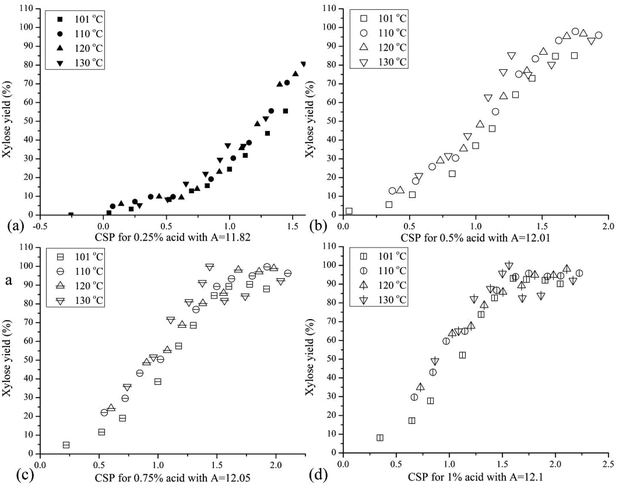
Because the combined severity parameter serves as a valuable tool with which to rapidly estimate tradeoffs among time, temperature, and acid concentration to realize similar sugar yields from biomass pretreatment, it was applied here to relate xylose yields from hydrolysis of XOs over the range of conditions applied. Accordingly, Fig. 4 presents xylose yields for all four temperatures and four acid concentrations applied to hydrolyze XOs in hydrolyzates from hydrothermal pretreatment of corn stover versus the combined severity parameter (eqn (2)) based on the adjusted pH. It can be seen that xylose yields were typically higher at higher acid concentrations at the same value of the combined severity parameter, and xylose yields at the same value of the combined severity parameter were greater at higher temperatures. Maximum xylose yields of 90%–95% at 101 °C and 95%–99% at 110 °C, 120 °C and 130 °C were reached at all acid concentrations applied except 0.25 wt% that would have required excessive run times. It can be seen that xylose yields were greater for higher temperatures at the same CSP for each acid concentration run. These results suggest that the activation energy for some of the oligomer bonds was higher than for others, as would be expected for hemicellulose that involves a number of different bond types among a variety of compounds (e.g., xylose, arabinose, acetic acid). Significant differences were also observed in the values of the combined severity parameter to give a 90% xylose yield at these four temperatures: Ro ∼1.3 at 101 °C, ∼1.0 at 110 °C and 120 °C, and ∼0.7 at 130 °C. This outcome is not surprising in that the values of the empirical constants in the combined severity parameter were fit to data from hemicellulose hydrolysis of hardwood18 and would not necessarily be expected to apply to lower temperature hydrolysis of XOs with different chemical bonds and activation energies.The constants in the combined severity parameter correlation were adjusted to better relate yields over the lower temperature range employed for xylooligomer hydrolysis. The combined severity parameter has only two constants that can be adjusted: Tc that is usually set at 100 °C and A with a customary value of 14.75. The parameter Tc only affects the absolute value of CSP for a particular set of conditions but does not adjust for the effects of temperature on CSP. Thus, changing Tc would not improve the grouping of xylose yields versus severities at different temperatures. On the other hand, changing the parameter A would adjust the grouping of yields versus CSP with changing temperature. To isolate how A would change with acid concentration, values of A were determined by least squares fitting over the range of temperatures applied at pH values of 1.293 for 0.25% sulfuric acid, 0.992 for 0.50%, 0.815 for 0.75%, and 0.690 for 1%. The resulting values of A increased with acid concentration as follows: 11.82 for 0.25%, 12.01 for 0.50%, 12.05 for 0.75%, and 12.10 for 1% acid. As shown in Fig. 5, xylose yields were lower at lower temperatures at equal severity, but changing the values of the parameter A significantly reduced the spread among xylose yields at each acid concentration.
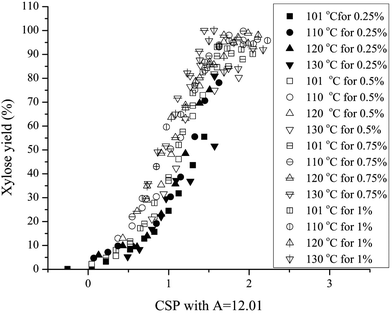
Because a single combined severity parameter equation based on a single value of A is more valuable for relating the effects of acid, time, and temperature, the parameter A was fit to xylose yield data for the four temperatures and acid concentrations based on the pH values noted above. The result was a value of A = 12.01, with the data fit to the adjusted correlation plotted in Fig. 6. Now, peak xylose yields occurred at or very near the modified log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CSP of 2. However, the maximum yields were lower at lower temperatures even though the CSP values corresponding to the yield peaks were tightly grouped.
CSP of 2. However, the maximum yields were lower at lower temperatures even though the CSP values corresponding to the yield peaks were tightly grouped.
It is noteworthy that with the value of A adjusted to 12.01, an 8.35 °C increase in temperature would cut the time required to reach a particular yield in half in contrast to the 10 °C change required to cut the time in half according to the traditional CSP formula. Thus, this result suggests that dissolved oligomers are more labile to breakdown to form monomers than is the solid hemicellulose in biomass.
The adjusted combined severity parameter improved the grouping of post-hydrolysis yields to provide a useful tool for estimating how changes in time, temperature, and acid concentration can be traded off against one another to give similar results. Only the one empirical parameter A must be known to use the adjusted combined severity parameter, and even that can be viewed as a doubling of the reaction rate for every 8.4 °C. Consequently, this simple adjustment in combined severity parameter increases its utility for rapidly translating from one set of conditions that give the highest yields from post-hydrolysis of XOs to a different set of conditions. For example, one could rapidly predict that doubling the acid concentration could be compensated for by halving the time or dropping the temperature by 8.4 °C to give the same yield.
Although kinetic models may also predict similar trends, they are also empirical curve fits with many more parameters employed to describe yield data. Furthermore, these models are not very robust in their ability to predict results with different acid concentrations, temperatures, and times for different biomass materials.17 And they cannot be readily employed in the field to estimate tradeoff among conditions. Thus, while more detailed kinetic models can provide some useful insights into such features as changes in concentrations of monomers and various oligomers that the simple combined severity parameter model cannot, they serve different roles in guiding definition of xylooligomer hydrolysis conditions and understanding the reactions involved. In line with this reasoning, a more detailed reaction model is being developed to predict how acid concentrations and temperatures affect yields of monomers from oligomers, better understand factors limiting yields at lower temperatures, and suggest strategies for further performance optimization.
4. Conclusions
Post-hydrolysis of XOs with dilute acid at low temperatures gave nearly theoretical monomeric xylose yields, and modification of one CSP constant to 12.01 instead of the customary value of 14.75 resulted in tight grouping of CSP values at maximum yields. The adjusted CSP also showed that the time to reach a particular xylose yield for post-hydrolysis of XOs would be cut in half for every 8.4 °C increase instead of the 10 °C change projected by the customary CSP, indicating differences in bond energy and providing a valuable tool for estimating tradeoffs among time, temperature, and acid concentrations to achieve similar yields.Acknowledgements
We are grateful to National Renewable Energy Laboratory for funding this research through Subcontract no. XGV-2-11467-01. We also acknowledge the Center for Environmental Research and Technology (CE-CERT) of the Bourns College of Engineering for providing facilities used in this research and the Ford Motor Company for funding the Chair in Environmental Engineering that facilitates projects such as this one.References
- R. A. Kerr, Science, 2011, 331, 1510–1511 CrossRef CAS PubMed.
- C. de Castro, L. J. Miguel and M. Mediavilla, Energy Policy, 2009, 37, 1825–1833 CrossRef PubMed.
- R. D. Perlack and B. J. Stokes, U.S. Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry, U.S. Department of Energy, Oak Ridge National Laboratory, Oak Ridge, TN, 2011 Search PubMed.
- A. Farrell, Science, 2006, 312, 1748–1748 Search PubMed.
- W. De Jong and G. Marcotullio, Int. J. Chem. React. Eng., 2010, 8, A69 Search PubMed.
- G. W. Huber and J. A. Dumesic, Catal. Today, 2006, 111, 119–132 CrossRef CAS PubMed.
- J. Shi, M. A. Ebrik and C. E. Wyman, Bioresour. Technol., 2011, 102, 8930–8938 CrossRef CAS PubMed.
- R. Kumar, F. Hu, P. Sannigrahi, S. Jung, A. J. Ragauskas and C. E. Wyman, Biotechnol. Bioeng., 2013, 110, 737–753 CrossRef CAS PubMed.
- T. A. Lloyd and C. E. Wyman, Bioresour. Technol., 2005, 96, 1967–1977 CrossRef CAS PubMed.
- C. E. Wyman, V. Balan, B. E. Dale, R. T. Elander, M. Falls, B. Hames, M. T. Holtzapple, M. R. Ladisch, Y. Y. Lee, N. Mosier, V. R. Pallapolu, J. Shi, S. R. Thomas and R. E. Warner, Bioresour. Technol., 2011, 102, 11052–11062 CrossRef CAS PubMed.
- Y. Kim, N. S. Mosier, M. R. Ladisch, V. R. Pallapolu, Y. Y. Lee, R. Garlock, V. Balan, B. E. Dale, B. S. Donohoe, T. B. Vinzant, R. T. Elander, M. Falls, R. Sierra, M. T. Holtzapple, J. Shi, M. A. Ebrik, T. Redmond, B. Yang, C. E. Wyman and R. E. Warner, Bioresour. Technol., 2011, 102, 11089–11096 CrossRef CAS PubMed.
- R. Kumar and C. E. Wyman, Biotechnol. Bioeng., 2009, 102, 457–467 CrossRef CAS PubMed.
- R. Kumar and C. E. Wyman, Biotechnol. Bioeng., 2014, 111, 1341–1353 CrossRef CAS PubMed.
- T. Lloyd and C. Wyman, Appl. Biochem. Biotechnol., 2004, 115, 1013–1022 CrossRef.
- M. Neureiter, H. Danner, C. Thomasser, B. Saidi and R. Braun, Appl. Biochem. Biotechnol., 2002, 98–100, 49–58 CrossRef CAS.
- G. Yang, M. S. Jahan, H. Liu and Y. Ni, Ind. Eng. Chem. Res., 2012, 51, 13902–13907 CrossRef CAS.
- S. Jacobsen and C. Wyman, Appl. Biochem. Biotechnol., 2000, 84–86, 81–96 CrossRef CAS.
- R. P. Overend and E. Chornet, Philos. Trans. R. Soc. London, Ser. A, 1987, 321, 523–536 CrossRef CAS.
- N. Abatzoglou, E. Chornet, I. Belkacemi and R. Overend, Chem. Eng. Sci., 1992, 47, 1109–1122 CrossRef CAS.
- N. Abatzoglou, P. Koeberle, E. Chornet, R. Overend and E. Koukios, Can. J. Chem. Eng., 1990, 68, 627–638 CrossRef CAS.
- H. Chum, D. Johnson, S. Black and R. Overend, Appl. Biochem. Biotechnol., 1990, 24–25, 1–14 CrossRef CAS.
- M. Heitz, E. Capek-Ménard, P. G. Koeberle, J. Gagné, E. Chornet, R. P. Overend, J. D. Taylor and E. Yu, Bioresour. Technol., 1991, 35, 23–32 CrossRef CAS.
- M. A. Kabel, G. Bos, J. Zeevalking, A. G. J. Voragen and H. A. Schols, Bioresour. Technol., 2007, 98, 2034–2042 CrossRef CAS PubMed.
- G. Kalman, E. Varga and K. Reczey, Chem. Biochem. Eng. Q., 2002, 16, 151–157 CAS.
- F. Schutt, J. Puls and B. Saake, Holzforschung, 2011, 65, 453–459 CrossRef.
- D. Montane, J. Salvado, X. Farriol and E. Chornet, Biomass Bioenergy, 1993, 4, 427–437 CrossRef CAS.
- J.-W. Lee and T. W. Jeffries, Bioresour. Technol., 2011, 102, 5884–5890 CrossRef CAS PubMed.
- S. T. Moe, K. K. Janga, T. Hertzberg, M.-B. Hägg, K. Øyaas and N. Dyrset, Energy Procedia, 2012, 20, 50–58 CrossRef CAS PubMed.
- K. K. Janga, K. Oyaas, T. Hertzberg and S. T. Moe, Bioresources, 2012, 7, 2728–2741 CAS.
- Y. Kim, T. Kreke, N. S. Mosier and M. R. Ladisch, Biotechnol. Bioeng., 2014, 111, 254–263 CrossRef CAS PubMed.
- M. Pedersen and A. S. Meyer, New Biotechnol., 2010, 27, 739–750 CrossRef CAS PubMed.
- W. Zhu, C. J. Houtman, J. Y. Zhu, R. Gleisner and K. F. Chen, Process Biochem., 2012, 47, 785–791 CrossRef CAS PubMed.
- T. Zhang, R. Kumar and C. E. Wyman, Carbohydr. Polym., 2013, 92, 334–344 CrossRef CAS PubMed.
- M. Saska and E. Ozer, Biotechnol. Bioeng., 1995, 45, 517–523 CrossRef CAS PubMed.
- Y. Kim, T. Kreke and M. R. Ladisch, AIChE J., 2013, 59, 188–199 CrossRef CAS.
- J. Shekiro, E. Kuhn, M. Selig, N. Nagle, S. Decker and R. Elander, Appl. Biochem. Biotechnol., 2012, 168, 421–433 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of Ash in Biomass, Laboratory Analytical Procedures (LAPs), National Renewable Energy Laboratory, Golden, CO, 2008 Search PubMed.
- A. Sluiter, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, Determination of Extractives in Biomass, National Renewable Energy Laboratory, Golden, CO, 2005 Search PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedures (LAPs), National Renewable Energy Laboratory, Golden, CO, 2008 Search PubMed.
- B. Yang and C. E. Wyman, Methods Mol. Biol., 2009, 581, 103–114 CAS.
- M. C. Gray, A. O. Converse and C. E. Wyman, Ind. Eng. Chem. Res., 2007, 46, 2383–2391 CrossRef CAS.
- M. H. Studer, J. D. DeMartini, S. Brethauer, H. L. McKenzie and C. E. Wyman, Biotechnol. Bioeng., 2010, 105, 231–238 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples, Laboratory Analytical Procedures (LAPs), National Renewable Energy Laboratory, 2006 Search PubMed.
- M. Selig, N. Weiss and Y. Ji, Enzymatic Saccharification of Lignocellulosic Biomass, Laboratory Analytical Procedures (LAPs), National Renewable Energy Laboratory, Golden, CO, 2008 Search PubMed.
- M. H. Studer, S. Brethauer, J. D. DeMartini, H. L. McKenzie and C. E. Wyman, Biotechnol. Biofuels, 2011, 4 Search PubMed.
Footnote |
| † These authors made equal contribution to this work. |
| This journal is © The Royal Society of Chemistry 2015 |