Glycerol as feedstock in the synthesis of chemicals: a life cycle analysis for acrolein production†
D.
Cespi
a,
F.
Passarini
*ab,
G.
Mastragostino
a,
I.
Vassura
ab,
S.
Larocca
c,
A.
Iaconi
d,
A.
Chieregato
ab,
J.-L.
Dubois
e and
F.
Cavani
*ab
aDepartment of Industrial Chemistry “Toso Montanari”, Bologna University, Viale del Risorgimento 4, 40136 Bologna (BO), Italy. E-mail: fabrizio.passarini@unibo.it; fabrizio.cavani@unibo.it
bCentro Interdipardimentale di Ricerca Industriale - Energia e Ambiente, Via Angherà 22, 47900 Rimini (RN), Italy
cSO.G.I.S. SpA, Via Giuseppina 132, 26048 Sospiro (CR), Italy
dSpiga BD Srl, Via Pontevecchio 55, 16042 Carasco (GE), Italy
eARKEMA - Pierre Bénite Research Center (CRRA), Rue Henri Moissan - BP 63, 69493 Pierre Bénite Cedex, France
First published on 28th August 2014
Abstract
Glycerol is an important bio-platform molecule, potentially usable for the synthesis of various chemicals and fuel additives, the synthesis of acrolein by dehydration being one of the most studied reactions. Through the application of the life cycle assessment (LCA) methodology we investigated the production of acrolein from glycerol, by comparing two alternative scenarios in which glycerol is obtained as a co-product either in triglyceride trans-esterification to FAME or in hydrolysis to fatty acids. Our results show how the main impacts are not related to the energy involved in the two processes. In fact, the use of dedicated crops as a source of triglycerides in the biodiesel production entailed higher impacts in terms of land exploitation. On the other hand, beef tallow was assumed as a starting raw material in the production of fatty acids, and this involved some significant impacts associated with animal rearing. At the same time, however, avoiding the use of dedicated biomass ensured a lower global impact (in terms of single scores). Lastly, in order to validate the model created, a sensitivity analysis using the Monte Carlo method was performed. The two routes from glycerol were also compared with the classical chemical route where acrolein is produced by propylene oxidation.
1. Introduction
In the coming decades, bio-based feedstocks are going to play a crucial role in the chemical and fuel industries, where their use is expected to grow and surpass that of fossil raw materials.1 This sector, also known as bio-refineries, is expanding greatly and – in addition to the already well known environmental benefits – the coproduction of chemicals and biofuels may lead to a higher return on investment.2 An important emerging sector will be “glycerochemistry”,3 which consists of replacing oil with glycerol as a feedstock in several applications in the chemical industry, also as a solvent and fuel.4Indeed, glycerol is widely studied as a feedstock due to its chemical characteristics and availability on the market. Nowadays, it is mainly generated as a co-product in processes which involve reactions with triglycerides, such as the production of fatty acids by hydrolysis, and trans-esterification with methanol, which leads to the production of fatty acid methyl esters (FAME), also called biodiesel.5 Every year about 20 Mt of fats and oils are processed by the chemical industries; this leads to a great abundance of glycerol on the market; in 2012 its production was estimated at about 1.2 Mt,3 and it is expected to rise to 1.54 Mt in 20156 and around 2.5 Mt in 2020.7
As previously stated, glycerol's chemical and physical properties make it an extremely versatile compound, which can be used as a feedstock for the synthesis of a high number of molecules (e.g. ethers, esters, carboxylic acids, ethylene glycol, epichlorohydrin, syngas, oligomers, polymers and many others).6,8–11
The synthesis of acrolein by dehydration appears to be one of the most promising ways to valorize it,8,12 therefore companies’ efforts are focused substantially in that direction.13–15 In fact, as shown in the literature11 acrolein produced starting from glycerol (with a purity grade around 92 wt%) seems to have a good economic return and low raw material cost. Acrolein is an important drop-in chemical intermediate both in the industrial sector (e.g. acrylic acid)16–18 and in the agricultural field (methionine, annual world-wide production of around 0.5 Mt);19 nowadays it is commonly produced by partial propylene oxidation. Nevertheless, considering the issue of depletion of fossil fuels, glycerol use could become a competitive alternative.
Before being used as a feedstock, however, crude glycerol obtained as a co-product needs to be treated to remove impurities in organic synthesis, but due to the high price of these processes the availability of refined glycerol in Europe is now decreasing, with a corresponding increase in the non-upgraded glycerol, which is addressed to the renewable energy market or even to poorer markets (e.g. animal feed). This end of life results in a loss of a valuable product that could be exploited in different ways, to make the most of its great potential. As is well known the 7th principle of Green Chemistry20 encourages the use of renewable feedstock, in order to minimize fossil resource consumption, and to mitigate the greenhouse gas emissions associated with them. For this reason the aim of this study was to evaluate – from a life cycle perspective – the potential impacts on human health and the environment of the use of glycerol as an alternative and renewable feedstock in the production of acrolein. In fact, the glycerol production and its usage represent nowadays a crucial topic for chemical industry, in particular for companies with the aim of achieving a more sustainable production.
For this reason, two main synthesis routes entailing glycerol generation as a co-product were compared: the trans-esterification process to produce biodiesel, and the production of fatty acids by triglyceride hydrolysis. This approach is able to identify both the environmental issues and the potential benefits connected with each production step considered in the study, and may be considered as a support for the companies involved in the chemical sector in achieving the target of sustainability promoted by the principles of Green Chemistry.20
Furthermore, a comparison study was carried out with the traditional acrolein production process starting from propylene.
2. Methods and materials
2.1. Introduction to the methodology
The life cycle assessment (LCA) tool was used as a scientific approach to (i) create models which simulate – as realistically as possible – the production chains considered, and (ii) evaluate the potential environmental burdens associated with them. It is a standardized methodology,21,22 divided into four conceptual phases:- The goal and scope definition, in which researchers define the aim of the study by identifying system boundaries (as geographical, technological and temporal) and the functional unit, which is necessary to refer each data and compare different scenarios;
- The life cycle inventory (LCI), which represents the more time-consuming phase of the entire LCA, due to data search and utilization to create models as a snapshot of the system boundaries;
The life cycle impact assessment (LCIA), in which an analysis method is chosen to evaluate each scenario created from a life cycle perspective and obtain results in terms of ecosystem quality, human health and resources consumption;
- The results interpretation and improvement; here the results obtained by LCIA are discussed to identify the worst scenario and the processes with higher contribution. In this way researchers are able to detect the crucial hot-spots that should be modified to improve the process.
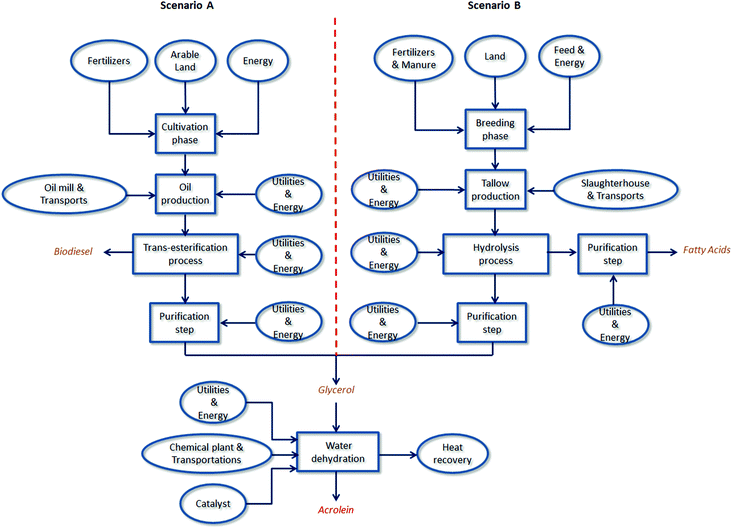
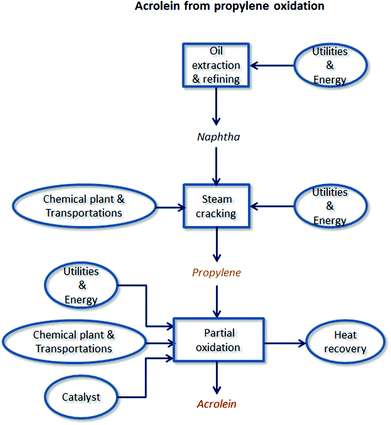
There are two main reasons for the use of LCA. First, it is able to investigate different domains of the industrial sector, but unlike other tools it is recognized by the international community due to its standardization. Secondly, the application of the LCA methodology to the chemical sector is rapidly expanding and involves different fields: industrial chemical production,23 the biofuel sector,24–27 the comparison between two processes with and without catalysts,28 and an expanding sector such as that of bio-refineries.29,30 As is well-known, the effectiveness of an LCA study is strongly influenced by the quality of the input data used in the various systems considered. For this reason, in this work LCI was carried out using mostly primary data directly provided by two Italian oleochemical companies and one French company: however, when not available, data were collected from the literature such as patents, encyclopaedia, and the Ecoinvent31 database (v. 2.2). LCA was conducted using the software developed by PRé Consultants, SimaPro32 (v.7.3.3). ReCiPe 200833 (I/A, v 1.07) and IPCC 200734 (20a) were used as LCIA analysis methods, both able to predict results with a twenty-year time horizon. The decision to choose these two methods stemmed from the need to express results in terms of midpoint categories, as well as in the form of the most consolidated way, using CO2-equivalents. Scenario modelling and their comparisons were carried out using the same amount of acrolein produced (1 kg) as a functional unit. A from-cradle-to-gate perspective was applied, considering the whole production chain for both scenarios: from raw material production (oil and fat) up to the synthesis of acrolein by glycerol dehydration, including the main intermediate stages such as triglyceride reaction processes and purification steps (if required). System boundaries are schematically depicted in Fig. 1 and 2. Below a detailed description of each inventory is reported.
2.2. Triglyceride trans-esterification process
As reported in the literature,35 the use of vegetable oils as a diesel source was investigated long before the oil crisis of the 1970s and 80s. Rudolf Diesel himself, in his book Liquid Fuel, mentioned the use of peanut oil (also known as arachis oil) in a small diesel engine seen during the Paris Exposition in 1900.35However, the patent developed by Chavanne36 in 1937 seems to be the first example of what we now call biodiesel. Nowadays, biodiesel is considered the best candidate to replace fuels in diesel engines, despite its higher cost37 and lower HHVs (higher heating values) than traditional fossil fuels.38 The biofuel importance is related to: (i) the possibility of applying it in a blend without involving any engine modification,39 and (ii) the benefits linked with its usage (e.g. greenhouse gas reduction).38 In contrast to Chavanne's work, which entailed the use of an acid catalyst, nowadays most industrial processes involve alkali-catalyst (NaOH or KOH) trans-esterification40 conducted at 60 °C under atmospheric pressure with a residence time of about 1 hour.35 The catalyst usage is also necessary in order to enhance the solubility of alcohols in oils.40 Triglycerides and an alcohol (methanol is the most used due to its low cost) are normally combined in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 and then allowed to react in continuous stirred tank reactors (CSTR).35 Below, eqn (1) represents the general reaction for the triglyceride trans-esterification process using methanol:
341 and then allowed to react in continuous stirred tank reactors (CSTR).35 Below, eqn (1) represents the general reaction for the triglyceride trans-esterification process using methanol:
| Triglycerides + 3CH3OH ⇄ 3RCOOCH3 + Glycerol | (1) |
The process yield of glycerol is about 10 wt%,7 producing empirically 1 ton of biodiesel and 100 kg of crude glycerol per ton of vegetable oil treated.41 As previously stated, the crude glycerol, generally with a purity grade of 80–88%,7 needs to be refined before its industrial usage. Generally speaking, the purification procedures require more processing steps and energy with respect to those used after hydrolysis, as salts and methanol have to be separated, and involve subsequent distillation steps until glycerol is obtained with the desired purity grade. Depending on the salt content, the equipment may also use ion exchange or thin-film distillation.42 As shown in Fig. 1, the system boundaries for acrolein obtained from glycerol by the trans-esterification process scenario (hereafter called Scenario A) cover the entire production chain: biomass cultivation and harvesting, the oil production process, and trans-esterification to obtain biodiesel and glycerol purification.
Obviously they also include the dehydration step to produce acrolein, which is common to both scenarios and is described separately in section 2.4. Rapeseed (Brassica napus) was chosen as the representative of energy crops since it is most commonly cultivated in Europe for obtaining biodiesel.28,43,44 Furthermore, Europe represents the World's biggest producer of biodiesel,24,45 with 178 thousand barrels per day in 201146 and thus rapeseed is now the dominant feedstock on the global scale.43Rape oil, at mill/RER U (Ecoinvent database), was chosen as the reference process to simulate both the rape cultivation and oil production phase. It includes all energy and mass flows used in rapeseed cultivation in Europe and all the utilities for treating seeds and extracting oil in an average European mill plant (including the average seed transportation to the mill).31 All this information was used in order to create two distinct models able to simulate both the Cultivation phase and the Oil production step. For more details see Table S1 reported in ESI.† As shown in Fig. 1, oil is then sent to the trans-esterification step. The default process considers that both plants are located in the same place (as in the case of hydrolysis). Spiga BD Srl, an Italian company which works in the field of biodiesel, glycerine, glycerine derivatives and renewable chemicals, provided the primary data needed to complete the life cycle inventory of trans-esterification and glycerol refining phases. The same operating conditions (temperature and pressure) described earlier were assumed. As suggested by the company, around 122 kg of glycerol are generated from 1005 kg of triglycerides treated to produce one ton of biodiesel. Moreover, the latter does not need to be refined; thus further purification steps were not included in the system boundaries. The use of glycerol as the starting raw material prevents its downgrading for energy use. For this reason a process avoiding glycerol burning was introduced in both scenarios. The energy produced by the combustion was modelled using the average values for glycerol LHV (18.74 MJ kg−1) reported in the literature.47 No reliable information on emissions was available, however in order not to neglect this environmental load, average air emissions from the combustion of natural gas31 were used as proxy data. In addition, due to the fact that glycerol combustion would provide energy in the form of heat, the process includes this avoided energy recovery. Lastly, in order to take into account the environmental benefits connected with the production of biofuel, the model created for Scenario A assumed that biodiesel is used to replace the traditional fossil fuel and included the avoided extraction of the same diesel amount. However, all input and output flows considered in the modeling of Scenario A are listed in ESI,† see Table S1.
2.3. Triglyceride hydrolysis process
Despite the fact that a higher quantity of glycerol is commonly obtained as a co-product from FAME manufacturing,7 biodiesel amounts produced in Europe in 2011 decreased by about 10% as compared to that in 2010.48 This trend affects the availability of glycerol on the market; therefore triglyceride hydrolysis was investigated as an alternative route. According to the literature7 fat splitting (followed by saponification) was the first source of glycerol in the past, until biodiesel production caught on. However, after a stagnant period, it is now considered to be the second major reserve of glycerol.7 Also, due to the synthesis of fatty acids, its industrial importance makes it a secure reserve process for glycerol production. In fact, today fat splitting using water is the most common way of obtaining synthetic fatty acids. This process occurs under homogeneous conditions in which water is dissolved in the lipid phase.49 The total equilibrium for the direct hydrolysis process is reported below (2):| Triglycerides + 3H2O ⇄ 3RCOOH + Glycerol | (2) |
After the pre-treatment procedures to remove impurities and settle triglyceride sources (such as filtration under heat, acidification, and degassing), hydrolysis takes place in the presence of demineralized water as a splitting agent (to increase the efficiency and prevent higher salt content).49 Ernst Twitchell was the first scientist to attempt to improve the process performance by introducing the Twitchell reagent as a catalyst in 1898. Later, either of the two different catalysts were used: lipases (cheaper due to lower process temperatures) or dibasic metal oxides (preferred over the acids, for corrosion prevention).49 However, the majority of modern units operate without a catalyst in continuous splitting columns, in the presence of high pressure steam, to achieve higher temperature and pressure (average values are 210–260 °C and 1.9–6.0 MPa).49 Fatty acid purity degrees obtained with the continuous process splitting are generally higher than 98%; crude glycerol obtained achieves a purity grade of about 20% and needs to be at first concentrated up to about 90% than purified (see the procedure previously described, distillation or ion exchange).49 As shown in Fig. 1, the system boundaries for the production of acrolein starting from glycerol as a by-product of hydrolysis (Scenario B) cover the entire manufacturing chain: from the triglyceride source (beef tallow) up to the dehydration step to produce acrolein, also including the hydrolysis and purification procedures. As for the previous scenario, in this case, also, primary data regarding the triglyceride splitting and purification procedures were provided by SO.G.I.S. SpA. This oleochemical company synthesizes fatty acids starting mainly from animal fats or palm oil as the triglyceride source. This raw material is obtained as a derivative from animal by-products in the meat production process and – as with other animal by-products – can be used in the industrial sector.50 In particular, beef tallow was considered in the model. Tallow, at plant/CH U was taken as the reference process in the Ecoinvent database to describe the average energy flows and other utilities (tap water, transportation, and infrastructure) involved in the production of tallow.31Beef (farm type 23)51 was chosen as the reference process to describe all the inputs and outputs connected with animal rearing, such as the occupation of arable land dedicated to the growth of cows, the animal feeds, the use of artificial fertilizers and in part of manure as the fertilizer, and all the energy usage during the breeding process. Given that tallow is considered a by-product, no mass flows and therefore no impacts related to animal rearing are included in the default process (Tallow, at plant/CH U).31 However, for the sake of prudence and considering that tallow has its own market price, an economical allocation was done by including the beef growth in the model. Therefore the amount of tallow obtained from the mass balance (able to produce 1 kg of acrolein) was multiplied by an economic allocation factor of 1.02 × 10−1 estimated from the ratio between the wholesale beef tallow price52 and the price of beef meat.53 In this way, the tallow impact is proportional to its market price. As previously stated, hydrolysis and purification were modelled using information directly supplied by SO.GI.S. SpA. According to this company, a production of around 90 kg of glycerol (and 880 kg of fatty acids) per ton of treated triglycerides was assumed. Also, inventory includes all the mass and energy flows involved in the fat splitting, as well as the utilities necessary for the purification step. In this case, the company suggests that refining procedures are necessary for both glycerol (from 20% to 99.5%) and fatty acids to reach the market purity grade. As in the case of Scenario A, avoided glycerol combustion was included in the model. Moreover, benefits derived from fatty acid production starting from tallow were estimated by considering an avoided production of vegetable oil (Rape oil, at oil mill/RER U)31 necessary for the synthesis of the same amount of acids. All the life cycle inventory details for Scenario B are listed in ESI,† see Table S2.
2.4. Glycerol dehydration process
Due to its importance as a chemical intermediate, mainly in the synthesis of acrylic acid and methionine, different acrolein production routes have been investigated in the past. In 1942, Degussa developed the first industrial synthesis process starting from acetaldehyde and formaldehyde through an aldol condensation reaction; this was subsequently replaced by partial propylene oxidation because of conversion and product separation problems.54 Given the affordability of propylene, today the latter process is still the most widespread manufacturing synthesis process despite the investigation into other routes – such as the partial oxidation of propane and the biological one.54 Nevertheless, the availability of a great abundance of glycerol as a co-product has led companies and research efforts to look into new synthesis procedures. Among these, the dehydration of glycerol is studied in depth in the literature,55–58 due to the fact that it represents one of the most promising and easiest ways to valorize it.8,11,12 As reported in Fig. 1, acrolein production represents the last step for both scenarios. The dehydration process generally occurs in a Packed Bed Reactor (PBR) at 280 °C and 101 kPa in the presence of an acid catalyst.54 In this study, a heteropoly acid catalyst with an empirical structure of H4SiW12O40/TiO2 was taken into account.55 Experimental data demonstrated55 that it leads to a 79% yield and selectivity of acrolein, with a glycerol conversion of 100%. As already stated, acrolein is the main intermediate in the production of acrylic acid; therefore, some data regarding utility consumption (e.g. electricity, natural gas, cooling and process water) as well as the amount of inert gas were assumed to be the same as the glycerol dehydration to acrylic acid and extrapolated from an internal report of a company.59 Instead, general information regarding the infrastructure (chemical plant), chemical auxiliary usage (e.g. hydroquinone as a stabilizing agent and ethyl acetate as a solvent for the extraction process) and average transportation was assumed to be the same as for the process Acrylic acid, at plant/RER U.31 Moreover the same average catalyst consumption for the synthesis of acrylic acid was assumed (0.3 g per functional unit)31 by modelling its structure on the basis of the already explained procedure reported in the literature.23 No information on regeneration and makeup, as well as on industrial energy consumption for the catalyst production, was available because such information usually constitutes corporate know-how. Therefore they were not included, assuming that their contribution to the overall impact is negligible, as reported in the literature.23 The amount of water in the form of steam, the inlet into the reactor (necessary to maintain the desired temperature), was estimated using the molar ratio reported in the literature.55 In order to limit the energy cost associated with the dehydration phase and to avoid side reactions, a concentrated solution of 50 wt% in glycerol is recommended as the inlet to the reactor.55,60 An energy recovery of half the heat exchanged in the reactor coils was assumed, calculating its amount through the enthalpy balance and the assumptions reported in the literature;23 half is recovered as heat and the rest as electricity (with a conversion efficiency of 31%). Lastly, the amount of steam for the purification step of acrylic acid (reported in a previous study)61 was assumed to be the same as in the case of acrolein. As can be seen, because of the lack of data due to corporate confidentiality, it was only possible to model the dehydration process using some proxy information, but it does not seem to affect the result of the study. Moreover, for the sake of prudence, it has been deliberately overestimated. A detailed description of the life cycle inventory for the dehydration process is shown in ESI,† see Table S3.2.5. Acrolein from propylene oxidation
In 1959 Shell developed the first industrial synthesis of acrolein starting from propylene. The process was based on the vapour-phase oxidation of alkenes using cuprous oxide as a catalyst. However, due to lower conversion of propylene, Sohio investigated a new class of catalysts based on bismuth molybdate.62 These multicomponent metal oxide systems are still used nowadays, conducting the oxidation process in a tubular fixed-bed reactor operated at 300–320 °C and at an inlet pressure of 150–250 kPa.62 Although the aim of the study was to evaluate the positive and negative aspects in the use of glycerol as renewable feedstock (as suggested by the Green Chemistry principles),20 a system boundary expansion was carried out in order to perform a comparison with the traditional route starting from propylene. In order to match the requirements in the quality of data common to LCA studies, an internal report provided by a company63 was used to complete LCI for the Acrolein from the propylene oxidation scenario. As for the dehydration scenario, data regarding the chemical plant, average transportation, and the catalyst amount were collected from the Ecoinvent database (Acrylic acid, at plant/RER U).31 On the other hand, the catalyst composition was evaluated from the patent literature,64 by assuming a yield of 75%63 and a propylene conversion of 95%. The unreacted olefin was assumed to be released as CO2, with a combustion efficiency of 100%. As in the case of glycerol dehydration, enthalpy balances23 were applied to estimate the amount of energy dissipated and recovered for plant utilities. This process was taken into account as a reference scenario for a comparison with the two bio-based routes described above. System boundaries are depicted in Fig. 2, while a detailed LCI description is reported in ESI,† see Table S4.3. Results and discussion
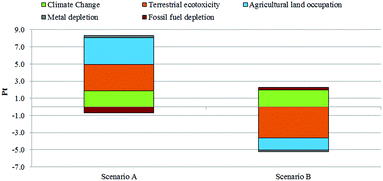
As previously stated, the LCIA phase was carried out using ReCiPe 200833 (v 1.07). This method makes it possible to estimate the impact category indicators,33 and is able to supply LCIA results in terms of midpoint impact categories; then the latter may be grouped into three endpoints based on (i) Damages to Human Health, measured in disability-adjusted life years – DALYs, which represents the sum of years of life lost (YLL) and years of life lived as disabled (YLD) due to the onset of a disease (e.g. cancer); (ii) Ecosystem Quality, measured in potentially vanished fractions of species – species year, which represents the disappearance of the species in all compartments (terrestrial, freshwater and marine water) due to anthropogenic factors; (iii) Resource Consumption, measured in terms of increased extraction costs – $, which takes into account that each resource extraction will cause a cost increase. An Average Individualist (I/A) cultural perspective was selected with the aim of analysing the scenarios considered in a twenty-year time horizon. In fact, the other two available choices for the Hierarchic (H) and Egalitarian (E) methods use a time horizon perspective of 100 and 500 years, respectively, which in our opinion are too broad a range for this case study (mainly because of economic implications). Results were expressed by selecting six midpoint impact categories, according to their significance with relation to the aim of the study: agricultural land occupation, terrestrial eco-toxicity, metal depletion, fossil fuel depletion, and climate changes with damage both on human health and ecosystem quality. The software elaborates data from the inventory phase and through a four-step procedure, which includes (i) fate analysis; (ii) effect analysis; (iii) damage analysis and (iv) normalization, it is able to estimate the environmental loads associated with each LCI choice, expressing them in terms of midpoint impact categories. This visualization of results, reported in Table 1, is also called “characterization analysis”. Each impact category is related to a particular damage on Human Health, Ecosystem Quality and Resources Depletion, which is expressed with the units described above. However, in order to compare the two acrolein production scenarios showing which route is the preferable solution in terms of cumulative global impacts, it is necessary to sum up all the above mentioned damage. The last step is (v) weighting, in which the software converts damages in terms of scores (Pt), also called “eco-indicator”. In this way, each impact category has the same unit (Pt) and a cumulative visualization called “ReCiPe 2008 single score” is possible. Results in terms of Pt are reported in Fig. 3. As shown, acrolein produced by glycerol obtained as a co-product of triglyceride trans-esterification is less sustainable compared to the route based on hydrolysis.| Impact category | Unit | Scenario A | Scenario B |
|---|---|---|---|
| Total impact | Pt | 8.6 × 100 | −3.8 × 100 |
| Agricultural land occupation | Species year | 1.5 × 10−6 | −6.4 × 10−7 |
| Terrestrial ecotoxicity | Species year | 1.4 × 10−6 | −1.7 × 10−6 |
| Climate change – Ecosystems | Species year | 3.7 × 10−7 | 3.9 × 10−7 |
| Climate change – Human health | DALY | 5.6 × 10−5 | 5.9 × 10−5 |
| Fossil fuel depletion | $ | −4.6 × 10−1 | 1.8 × 10−1 |
| Metal depletion | $ | 1.6 × 10−1 | −1.5 × 10−1 |
The lower sustainability of Scenario A is mainly due to significant impacts in terms of land occupation, terrestrial eco-toxicity and climate change (which includes process contributions to both human health and ecosystem damage categories), in spite of the benefits connected with an avoided fossil fuel consumption due to the avoided diesel extraction. This global negative trend is attributable to the high-intensity processes connected with the biomass growth phase as a source of triglycerides. On the other hand, Scenario B – based on hydrolysis – seems to have significant impacts on the climate change and the depletion of fossil fuels, which are related to the energy consumptions assumed in the scenario. In fact, both categories are strictly related to each other as well as to the energy need required by the system, for example during the very intensive phases of purification. In fact, as written previously in the description of LCI, the hydrolysis scenario implies a further purification stage than the trans-esterification scenario, in order to reach the market purity grade for fatty acids. This additional step leads to an increase of impacts related to energy use. Furthermore, although the just-mentioned negative effects are not negligible, the use of tallow as a substitute for vegetable biomass leads to potential environmental benefits in terms of avoided damage on land occupation and eco-toxicity. This cumulative representation shows a quick vision of the overall impacts of each scenario. However, considering only the figure above, it is not possible to clarify the environmental significance of each phase within both scenarios. Therefore, a contribution analysis was carried out in order to show the potential burdens on the environment which are associated with the system boundaries considered. To accomplish this, each scenario was split into its main phases and the analysis results are shown in terms of single score and characterization. The results from the analysis carried out for Scenario A (Fig. 4 and Table 2) show that the cultivation phase seems to contribute much more than the others to global impacts. As a confirmation of our previous suggestions, a detailed analysis conducted using the network tool provided by the software revealed that about 100% of global contribution for both categories of agricultural land occupation and eco-toxicity is associated with the cultivation phase.
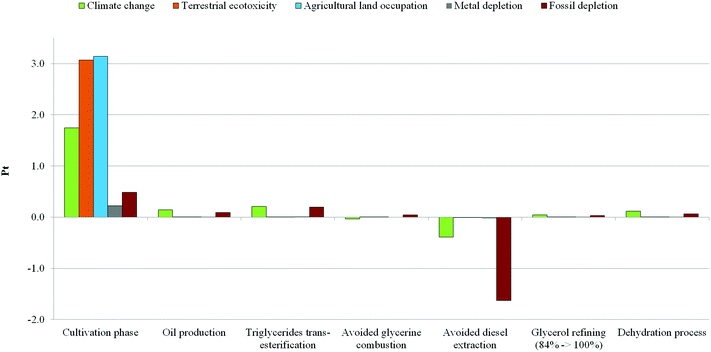 | ||
| Fig. 4 Contribution analysis Scenario A – acrolein produced from glycerol obtained as a co-product of triglyceride trans-esterification (ReCiPe 2008 I/A, Single score). | ||
| Impact category | Unit | Total | Cultivation phase | Oil production | Triglyceride trans-esterification | Avoided glycerine combustion | Avoided diesel extraction | Glycerol refining (84%→100%) | Dehydration process |
|---|---|---|---|---|---|---|---|---|---|
| Agricultural land occupation | Species year | 1.5 × 10−6 | 1.5 × 10−6 | 1.2 × 10−10 | 2.1 × 10−10 | 4.2 × 10−11 | −1.4 × 10−10 | 6.5 × 10−12 | 7.1 × 10−11 |
| Terrestrial ecotoxicity | Species year | 1.4 × 10−6 | 1.4 × 10−6 | 4.5 × 10−11 | 7.9 × 10−11 | 2.8 × 10−11 | −7.0 × 10−10 | 1.3 × 10−11 | 3.1 × 10−11 |
| Climate change Ecosystems | Species year | 3.7 × 10−7 | 3.5 × 10−7 | 2.9 × 10−8 | 4.2 × 10−8 | −6.3 × 10−9 | −7.7 × 10−8 | 9.8 × 10−9 | 2.4 × 10−8 |
| Climate change Human Health | DALY | 5.6 × 10−5 | 5.2 × 10−5 | 4.4 × 10−6 | 6.4 × 10−6 | −9.5 × 10−7 | −1.2 × 10−5 | 1.5 × 10−6 | 3.6 × 10−6 |
| Fossil fuel depletion | $ | −4.6 × 10−1 | 3.2 × 10−1 | 6.2 × 10−2 | 1.3 × 10−1 | 3.0 × 10−2 | −1.1 × 10 | 2.1 × 10−2 | 4.2 × 10−2 |
| Metal depletion | $ | 1.6 × 10−1 | 1.5 × 10−1 | 6.6 × 10−3 | 1.1 × 10−2 | −6.1 × 10−4 | −1.4 × 10−2 | 8.8 × 10−4 | 4.7 × 10−3 |
In particular, it was ascertained that the highest negative effect in terms of terrestrial eco-toxicity is related to the release of pesticides in soil, and that the exploitation of arable land contributes to its occupation. Also, as a confirmation of the scores reached in Fig. 4, Table 2 shows that the cultivation phase presents the highest negative load in terms of climate change. A contribution of approximately 77% was estimated for this impact category, associated with the energy consumptions during biomass growth. On the other hand the oil production phase has a contribution around 6% for the same category. Moreover, the trans-esterification, glycerol purification, and dehydration phases were estimated to make a non-negligible contribution to the same category (around 2–9% of the global impact) due to the energy flows involved. In particular, as previously described in the inventory analysis, mass and energy flows used to model the trans-esterification and purification processes were furnished directly by the company. For this reason the results may be considered a good simulation of the real case. Regarding the fossil fuel depletion category, the highest contribution is due to the cultivation phase (about 53%), followed by the trans-esterification process (22%), the oil production phase (10%), and the glycerol dehydration (7%) and refining (3%) phases. All these stages are energy-intensive, and industrial manufacturing involves higher consumption of fossil fuels for steam and electricity production. In particular, impacts regarding electricity generation are strictly related to the energy mix adopted by any country. In this case, according to the geographical system boundaries, an average energy mix for Italy was assumed (Electricity, production mix IT/IT U).31 Also regarding fossil fuel depletion, a contribution of about 5% was calculated due to the avoided glycerol combustion. This trend is related to the assumptions made during the inventory phase: it was considered that glycerol was commonly burned in the industrial sector in order to produce heat, and that in the case in which it is not incinerated (but recovered as a feedstock) the same energy amount should be produced by traditional fuels. On the other hand, the avoided emission of greenhouse gases from the combustion of glycerol helps to prevent, in part, the negative effects on climate change. However, the highest positive contribution to the environment and human health proves to be from the use of biodiesel in substitution for diesel. In fact, the avoided diesel extraction leads to significant benefits regarding both the climate change and the fossil fuel categories. The relative contribution of each life cycle phase for Scenario A, both in terms of Impacts and Avoided impacts, is reported in Table S5.† Results from the contribution analysis for Scenario B are shown in Fig. 5 and Table 3. The avoided use of rapeseed as a source of triglycerides entails several environmental benefits. First, the avoided burdens in terms of climate change (including damage to both human health and the ecosystem) and fossil fuel depletion are related to the lower energy consumption in the production of chemicals used as fertilizers. In particular, nitrogenous fertilizers produced by a synthesis starting from ammonia, whose manufacturing is highly energy-intensive, as well as the production of nitric acid, seem to be the major causes. Also, unlike in Scenario A, several advantages are linked to the avoided use of pesticides, and to the avoided occupation of arable areas which may be devoted to other agricultural activities. Moreover, the partial replacement of fertilizers with animal manure reduces the contribution of the breeding phase to the terrestrial ecotoxicity (around 4%), the latter category being highly influenced by both the release of substances and waste during the energy-consuming steps which characterize the tallow production (29%) and the other steps such as the fatty acid purification that contributes for the 24%. However, even in this scenario the triglyceride supply is not without impacts. In fact, the phases of animal breeding and tallow production are the two steps with the highest environmental impacts along the entire manufacturing chain considered. As is well known, animal rearing implies an intense use of resources and energy. In fact, despite the economic allocation of tallow, the breeding phase achieves the highest contribution in terms of agricultural land occupation (100%) and climate change (around 72%) categories. Also the contribution to the fossil fuel depletion is not negligible, around 17%. On the other hand, the intense energy consumption for the production of tallow is responsible for the highest contribution in terms of fossil fuel depletion (around 46%) and contributes for a 16% in terms of the climate change category. Also, the energy consumption involved in the fatty acid purification stage is not so negligible as it contributes for 14% to the fossil fuel depletion and for 5% to the climate change category. Conversely, the energy used during hydrolysis and dehydration processes and for the glycerol refining procedure is not so significant as far as the contributions to climate change (2–3%) and fossil fuel depletion (5–7%) are concerned. However, as for Scenario A, the impacts related to the production and purification of fatty acids and for glycerol refining should be considered a reliable approximation, since data were furnished directly by the enterprises. Also, it should be considered that the further purification stage of fatty acids increases the total impacts reached by Scenario B regarding both fossil fuel depletion and climate change categories of around 17% and 5% respectively. Moreover, this additional step entails approximately 5% and 47% increases in impacts respectively on the same categories (fossil fuel depletion and climate change, including damage to both human health and the ecosystem), compared to results achieved by Scenario A for the same categories. As in the previous case, the contribution of each life cycle phase for Scenario B, both in terms of Impacts and Avoided impacts, is reported in Table S6.† From results, all energy consumptions involved in the glycerol purification stage produce a contribution around 2% in terms of climate change and about 3–6% with regard to fossil fuel depletion (depending on the steps involved in refining). However, as reported in the literature,65 it seems not to be possible to avoid the glycerol purification stage, especially if glycerol is obtained as a co-product in the FAME process, due to the higher amount of impurities present in raw glycerol: water, salts derived from basic medium neutralization, traces of methanol and NGOM (non-glycerin organic matter). A typical composition of various glycerol, produced at different industrial sites by trans-esterification, was found in the literature65 and reported in Table S7.† Basic compounds such as sodium and potassium salts might deactivate the acid catalyst used to dehydrate glycerol to acrolein, so prejudicing the entire process yield. Company efforts are focused on the development of new technologies in order to solve this issue.65,66 Moreover, NGOM includes several different substances extracted during the seed crush (e.g. lignocellulosic materials, such as phenolic compounds) that end up in the oil and finally in glycerine. This material contributes to coke formation and accelerates catalyst deactivation. Therefore, in order to reduce the content of these compounds, a glycerol distillation process is necessary. These issues could be solved by technology improvements that lead to new processes able to use crude glycerol as the feedstock for dehydration. Therefore, in order to verify how the global impact of the entire process could change avoiding the purification stage for glycerol, the LCIA phase was repeated for both scenarios, excluding the steps of glycerol refining. As expected, due to the low contribution of the purification stage, results reported in Table S8† show that differences compared with the scores obtained previously (Table 1) were negligible. Moreover, the climate change category was also investigated using IPCC 2007.34 This analysis method was developed by the Intergovernmental Panel on Climate Change (IPCC) which is able to assess the Global Warming Potential (GWP) while expressing results in terms of CO2 equivalents. In agreement with the ReCiPe 2008 method, a perspective of a 20-year time horizon was chosen. Results from this study are shown in Table 4. In this table, IPCC confirms the scores achieved using ReCiPe 2008 as regards the climate change category (Table 1). Furthermore, this method is able to quantify the exact amount of CO2 emitted or saved during each stage of the manufacturing process. Although the scores are quite similar, Scenario B achieves higher values (49.7 kg of CO2 eq.) than the trans-esterification-based scenario (45.2 kg of CO2 eq.). In fact, despite the high CO2 savings due to the avoided use of vegetable biomass as a source of triglycerides (−54.7 kg of CO2 eq.), the emissions associated with the animal rearing (70.0 kg of CO2 eq.) and energy consumption involved in tallow production (16.6 kg of CO2 eq.) contribute to increasing the global amount. On the other hand, the cultivation (42.5 kg of CO2 eq.) and oil production phases (3.6 kg of CO2 eq.) achieve higher values than the total CO2 amount emitted by Scenario A (46.1 kg of CO2 eq.); however, the use of biodiesel as a substitute for traditional fuels seems to produce several environmental benefits (−9.4 kg of CO2 eq.) by reducing their global amount. Lastly, the metal depletion category was also investigated. It was introduced at first to verify the overall environmental impact associated with metal extraction for the production of catalyst systems which are used for the dehydration process. However, the contribution analysis conducted for both scenarios shows that the catalyst assembly is not so significant for that category, which is mainly influenced by the consumption of metals in the form of salts used mainly as fertilizers (e.g. ammonium nitrate and ammonium nitrate phosphate). In fact, the scores (Pt) achieved by both scenarios with regard to metal consumption are similar (Table 1) and, in both cases, the higher contribution (positive and negative) is due to phases which involve biomass cultivation (Tables 2 and 3). The rest may be attributable to the great quantity of infrastructure involved (e.g. chemical plant, oil mill, transportation). Therefore, catalyst contribution to the global impact seems to be negligible, even though, as previously described, the catalytic system was modelled using only proxy data regarding the amount of acrolein per kg (see section 2.4). As stated above, in order to evaluate the environmental trends of both bio-based scenarios, a comparison with the traditional process for producing acrolein from the partial oxidation of propylene was also carried out using ReCiPe2008 as the test method. The results in terms of single scores are given in Fig. 6. The radar chart is a quick representation to show scores achieved by each scenario for each impact category. The closer the line to the triangle vertex, the higher the score meaning a negative effect on the environment. This picture shows a considerable difference between bio-based scenarios and that starting from olefin. The latter seems to have lower impacts than the scores achieved by Scenario A. The avoided use of dedicated crops entails no impacts related to land exploitation, occupation, or the use of pesticides. In contrast to the common opinion that attributes higher impacts on fossil fuel depletion and climate change to propylene partial oxidation (due to the use of oil as a raw material), the high energy consumption mainly related to glycerol purification in bio-based scenarios greatly affects the results. In both categories (in particular the fossil fuel depletion) the impact of acrolein from partial oxidation is lower if compared with that of the two bio-based scenarios.
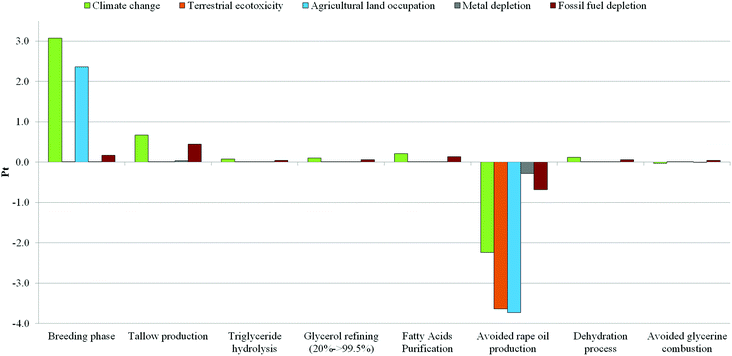 | ||
| Fig. 5 Contribution analysis Scenario b – acrolein produced from glycerol obtained as a co-product of triglyceride hydrolysis (ReCiPe 2008 I/A, Single score). | ||
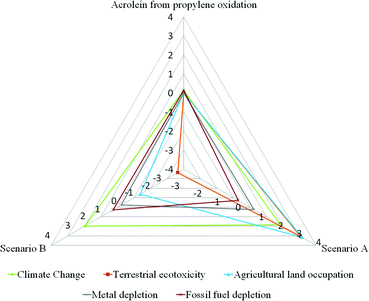 | ||
| Fig. 6 Comparison between the three acrolein production scenarios, in terms of ReCiPe I/A – Single score (radar chart). | ||
| Impact category | Unit | Total | Breeding phase | Tallow production | Triglyceride hydrolysis | Glycerol refining (20%→99.5%) | Fatty acid purification | Avoided rape oil production | Dehydration process | Avoided glycerine combustion |
|---|---|---|---|---|---|---|---|---|---|---|
| Agricultural land occupation | Species year | −6.4 × 10−7 | 1.1 × 10−6 | 5.4 × 10−10 | 1.2 × 10−11 | 5.8 × 10−11 | 2.4 × 10−11 | −1.7 × 10−6 | 7.1 × 10−11 | 4.2 × 10−11 |
| Terrestrial ecotoxicity | Species year | −1.7 × 10−6 | 1.2 × 10−11 | 8.0 × 10−11 | 2.2 × 10−11 | 3.3 × 10−11 | 6.5 × 10−11 | −1.7 × 10−6 | 3.1 × 10−11 | 2.8 × 10−11 |
| Climate change Ecosystems | Species year | 3.9 × 10−7 | 6.1 × 10−7 | 1.3 × 10−7 | 1.4 × 10−8 | 2.0 × 10−8 | 4.2 × 10−8 | −4.5 × 10−7 | 2.4 × 10−8 | −6.3 × 10−9 |
| Climate change Human Health | DALY | 5.9 × 10−5 | 9.2 × 10−5 | 2.0 × 10−5 | 2.2 × 10−6 | 3.0 × 10−6 | 6.3 × 10−6 | −6.7 × 10−5 | 3.6 × 10−6 | −9.5 × 10−7 |
| Fossil fuel depletion | $ | 1.8 × 10−1 | 1.1 × 10−1 | 2.9 × 10−1 | 3.1 × 10−2 | 4.1 × 10−2 | 9.0 × 10−2 | −4.5 × 10−1 | 4.2 × 10−2 | 3.0 × 10−2 |
| Metal depletion | $ | −1.5 × 10−1 | 9.7 × 10−4 | 2.4 × 10−2 | 5.8 × 10−4 | 3.6 × 10−3 | 1.6 × 10−3 | −1.8 × 10−1 | 4.7 × 10−3 | −6.1 × 10−4 |
| Scenario A | kg CO2 eq. | Scenario B | |
|---|---|---|---|
| Tot. | 45.2 | 49.7 | Tot. |
| Cultivation phase | 42.5 | 70.0 | Breeding phase |
| Oil production | 3.6 | 16.6 | Tallow production |
| Triglyceride trans-esterification | 5.2 | 1.8 | Triglyceride hydrolysis |
| Glycerol refining (84%→100%) | 1.2 | 2.5 | Glycerol refining (20%→99.5%) |
| Avoided glycerine combustion | −0.8 | −0.8 | Avoided glycerine combustion |
| Avoided diesel extraction | −9.4 | −54.7 | Avoided rape oil production |
| — | — | 5.2 | Fatty acid purification |
| Dehydration process | 3.0 | 3.0 | Dehydration process |
This trend is ascribable to both the massive consumption of fossil fuels that characterizes all the purification steps (e.g. glycerol, fatty acids) and to the triglyceride transformation (e.g. trans-esterification process) and the upstream stages: on one hand, the rapeseed cultivation and oil production, and on the other hand the breeding and the tallow production. In fact, as previously reported, they represent the most intensive steps in terms of energy and resource requirement. The results in terms of characterization analysis are shown in Table 5. However, if the comparison is made considering the global impacts achieved by each process alone, Scenario B seems to be the most environmentally sustainable one due to the benefits associated with the avoided dedicated crop usage.
| Impact category | Unit | Amount |
|---|---|---|
| Total impact | Pt | 3.1 × 10−1 |
| Agricultural land occupation | Species year | 9.3 × 10−11 |
| Terrestrial ecotoxicity | Species year | 5.8 × 10−12 |
| Climate change – Ecosystems | Species year | 2.9 × 10−8 |
| Climate change – Human health | DALY | 4.3 × 10−6 |
| Metal depletion | $ | 9.9 × 10−3 |
| Fossil fuel depletion | $ | 9.0 × 10−2 |
3.1. Sensitivity analysis
Lastly, in order to evaluate the robustness of the models created, a sensitivity analysis was carried out using the Monte Carlo statistical method. Data uncertainties were evaluated by combining the pedigree matrix developed by Weidema and Wesnaes67 with the method previously reported in the literature.68 A lognormal statistical distribution with a 95% confidence interval was assumed; by performing an iterative calculation of 1000 simulations, both of the bio-based acrolein production scenarios were compared using ReCiPe2008 as the analysis method. The results of the sensitivity analysis are shown below, in Fig. 7 and Table 6. On the y-axis the six midpoint impact categories are reported, while the x-axis shows the frequency in terms of percentage. The frequency indicates how many times a scenario shows impacts higher than the other one for a particular impact category. A frequency equal to 100% (or −100%) means that for all the 1000 iterations the results are confirmed; on the other hand a frequency of 0% means that this situation does not occur. Green bars stand for the case in which Scenario A attains higher environmental impacts than B; red bars, on the other hand, indicate the opposite. As can be seen, the Monte Carlo method confirms the results obtained previously in the LCIA. 100% frequency was achieved for fossil fuels and metal depletion as well as for the impact categories related to land exploitation (agricultural land occupation and terrestrial ecotoxicity). Furthermore, higher frequencies (62–63%) were achieved by both climate change categories, thus confirming results’ robustness.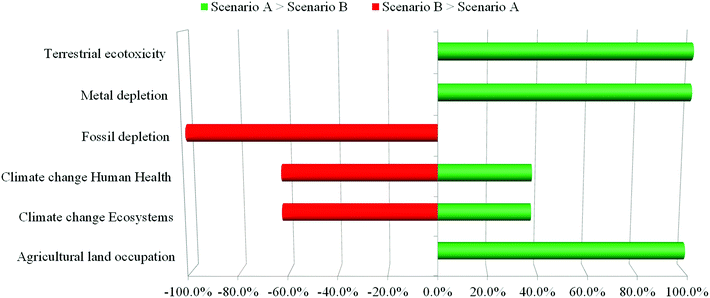 | ||
| Fig. 7 Monte Carlo analysis – comparison between two bio-based acrolein scenarios, in terms of ReCiPe impact categories. | ||
| Impact category | A > B | B > A |
|---|---|---|
| Agricultural land occupation | 100% | 0% |
| Climate change – Ecosystems | 38% | 63% |
| Climate change – Human health | 38% | 62% |
| Fossil fuel depletion | 0% | 100% |
| Metal depletion | 100% | 0% |
| Terrestrial ecotoxicity | 100% | 0% |
4. Conclusions
This work presents a life cycle assessment “from cradle to gate” for the production of 1 kg of acrolein starting from glycerol generated as an industrial co-product. Two main industrial alternatives for the production of feedstock were compared: triglyceride trans-esterification and hydrolysis, which led respectively to the production of biodiesel and fatty acids as the main products. The aim of the study was to verify, from a life-cycle perspective, the environmental sustainability associated with the application of principles of Green Chemistry, i.e. the use of renewable feedstocks,20 by evaluating the possibility of using glycerol as a valuable chemical raw material instead of downgrading it for energy use. Inventory analysis, which includes the main stages of the manufacturing chain (from the source of triglycerides up to dehydration to obtain acrolein, also including the intermediate stages which lead to glycerol production and purification), was carried out using the data supplied by two companies (regarding the trans-esterification and hydrolysis processes and purification procedures) and extracted from the literature (also including patents). The results show that the acrolein produced from glycerol and obtained as a co-product in biodiesel production seems to be less sustainable in terms of global impacts if compared with the hydrolysis-based scenario. However, contribution analysis indicates that the higher significance is not associated with the industrial consumptions, but with the triglyceride supply.Due to the land occupation and exploitation, the vegetable source of triglycerides seems to have higher environmental loads. Nevertheless, if rapeseed is substituted (even in part) by a marginal cultivation which does not require the use of pesticides and does not subtract space from agricultural cultivation, lower impacts could be achieved. For example, as suggested by the literature,69 marginal lands could be exploited for the cultivation of jatropha curcas and castor beans, which however need a higher quantity of freshwater.69 In addition, it should be considered that although the cultivation phase represents the more stressful step for the environment,25 it could be also influenced by many variables connected to different agricultural practices (e.g. higher or lower use of fertilizers) or different soil characteristics, typical of each geographical area.25 On the other hand it is not possible to feed the entire glycerol industry using only tallow as the source of triglycerides (despite the fact that meat consumption is increasing due to the world's population growth). Moreover, this source is not without impacts, due to the significant environmental loads associated with both animal rearing and tallow production. For this reason, alternative routes should be pursued. The literature2 points out the increasing attention paid to the use of biomass waste as a possible source for bio-based industry. This solution could be an interesting opportunity, considering that in 2012 the European production of organic waste was estimated to range between 118 and 138 million tonnes, with an estimated increase of 10% expected by 2020.70 Also, this alternative could be even more advantageous for the Italian case study, thanks to its contribution to the reduction of CO2 emissions: in Italy, in fact, the average percentage of organic waste not recovered (composted) amounts to around 60–70% of the total production.70 Anyway, it should be considered that the use of alternative raw materials for the production of biofuels and chemicals in Europe will also be affected by EU and national fiscal incentive policies. In conclusion, this study highlights the importance of the application of the LCA methodology as an assessment tool for evaluating the potential impacts associated with the industrial chemical sector. However, as commonly remembered in LCA studies, results should be considered valid only within the system boundaries concerned in the study. Further considerations regarding possible changes due to future trends could be estimated by an extension of the system boundaries to include economic and social variables also, but this would clearly go beyond the aim of this study.
Notes and references
- F. W. Lichtenthaler, Carbohydrates as Organic Raw Materials, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2010 DOI:10.1002/14356007.n05_n07.
- R. Sheldon, Green Chem., 2014, 16, 950–963 RSC.
- C. F. M Pestana, A. C. O. Guerra, G. B. Ferreira, C. C. Turci and C. J. A. Mota, J. Braz. Chem. Soc., 2013, 24, 100–105 CrossRef PubMed.
- M. Pagliaro, R. Ciriminna, H. Kimura, M. Rossi and C. Della Pina, Eur. J. Lipid Sci. Technol., 2009, 111, 788–799 CrossRef CAS.
- A. Behr, J. Eilting, K. Irawadi, J. Lechinski and F. Lindner, Green Chem., 2008, 10, 13–30 RSC.
- L. Tao, B. Yan, Y. Liang and B. Xu, Green Chem., 2013, 15, 696–705 RSC.
- M. Ayoub and A. Z. Abdullah, Renewable Sustainable Energy Rev., 2012, 16, 2671–2686 CrossRef CAS PubMed.
- B. Katryniok, H. Kimura, E. Skrzyńska, J. S. Girardon, P. Fogarland, M. Capron, R. Ducoulombier, N. Mimura, F. Dumeignil and S. Paul, Green Chem., 2011, 13, 1960–1979 RSC.
- C. Zhou, J. N. Beltramini, Y. Fan and G. Q. Lu, Chem. Soc. Rev., 2008, 37, 527–549 RSC.
- J. A. Kenar, Lipid Technol., 2007, 19, 249–253 CrossRef CAS.
- J. A. Posada, L. E. Rincón and C. A. Cardona, Bioresour. Technol., 2012, 111, 282–293 CrossRef CAS PubMed.
- B. Katryniok, S. Paul, V. Bellière-Baca, P. Rey and F. Dumeignil, Green Chem., 2010, 12, 2079–2098 RSC.
- J.-L. Dubois, US Pat., 8 686 195 B2, 2014 Search PubMed.
- J.-L. Dubois, US Pat., 8 378 136 B2, 2013 Search PubMed.
- J.-L. Dubois, Y. Magatani and K. Okumura, US Pat., 8 252 960 B2, 2012 Search PubMed.
- A. Chieregato, M. D. Soriano, F. Basile, G. Liosi, S. Zamora, P. Conceptión, F. Cavani and J. M. López Nieto, Appl. Catal., B, 2014, 150–151, 37–46 CrossRef CAS PubMed.
- A. Chieregato, F. Basile, P. Conceptión, S. Guidetti, G. Liosi, M. D. Soriano, C. Trevisanut, F. Cavani and J. M. López Nieto, Catal. Today, 2012, 197, 58–65 CrossRef CAS PubMed.
- M. D. Soriano, P. Conceptión, J. M. López Nieto, F. Cavani, S. Guidetti and C. Trevisanut, Green Chem., 2011, 13, 2954 RSC.
- B. Katryniok, S. Paul, M. Capron and F. Dumeignil, ChemSusChem, 2009, 2, 719–730 CrossRef CAS PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed.
- DIN EN ISO 14040 Environmental Management, Life Cycle Assessment, Principles and Framework, DIN (Deutsches Institut für Normung e.V.), Beuth Verlag, Berlin, Germany, 2006 Search PubMed.
- DIN EN ISO 14044 Environmental Management, Life Cycle Assessment, Requirements and Guidelines, DIN (Deutsches Institut für Normung e. V.), Beuth Verlag, Berlin, Germany, 2006 Search PubMed.
- D. Cespi, F. Passarini, E. Neri, I. Vassura, L. Ciacci and F. Cavani, J. Cleaner Prod., 2014, 69, 17–25 CrossRef CAS PubMed.
- D. Kralisch, C. Staffel, D. Ott, S. Bensaid, G. Saracco, P. Bellantoni and P. Loeb, Green Chem., 2013, 15, 463–477 RSC.
- J. Malça, A. Coelho and F. Freire, Appl. Energy, 2014, 114, 837–844 CrossRef PubMed.
- A. Vlysidis, M. Binns, C. Webb and C. Theodoropoulos, Energy, 2011, 36, 4671–4683 CrossRef CAS PubMed.
- C. M. Gasol, J. Salvia, J. Serra, A. Antón, E. Sevigne, J. Rieradevall and X. Gabarrell, Biomass Bioenergy, 2012, 40, 71–81 CrossRef CAS PubMed.
- H. A. van Kalkeren, A. L. Blom, F. P. J. T. Rutjes and M. A. J. Huijbregts, Green Chem., 2013, 15, 1255–1263 RSC.
- L. Soh, M. Montazeri, B. Haznedaroglu, C. Kelly, J. Peccia, M. Eckelman and J. Zimmerman, Bioresour. Technol., 2014, 151, 19–27 CrossRef CAS PubMed.
- S. Pereira and J. E. A. Seabra, Appl. Energy, 2013, 102, 5–12 CrossRef PubMed.
- Ecoinvent Centre (formerly Swiss Centre for Life Cycle Inventories), Ecoinvent 2.2 Database, 2009.
- PRé Consultants, SimaPro7, PhD version 7.3.3, Amersfoort, The Netherlands, 2013 Search PubMed.
- M. Goedkoop, R. Heijungs, M. Huijbregts, A. De Schryver, J. Struijs and R. van Zelm, ReCiPe 2008 – A life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level, First edition (version 1.07), Ministry of Housing, Spatial Planning and the Environment (VROM), Netherlands, 2012 Search PubMed.
- IPCC - Intergovernmental Panel on Climate Change, Climate Change 2007. Fourth Assessment Report. The Physical Science Basis, Cambridge University Press, United Kingdom, 2007 Search PubMed.
- G. Knothe, J. Van Gerpen and J. Krahl, The Biodiesel Handbook, AOCS Press, United States of America, 2005 Search PubMed.
- G. Chavanne, Belgian Pat., 422 877, 1937 Search PubMed.
- I. B. Bankovíc-Ilíc, O. S. Stamenkovíc and V. B. Veljkovíc, Renewable Sustainable Energy Rev., 2012, 16, 3621–3647 CrossRef PubMed.
- A. Demirbas, Energy Convers. Manage., 2009, 50, 14–34 CrossRef CAS PubMed.
- M. Balat, Energy Convers. Manage., 2011, 52, 1479–1492 CrossRef CAS PubMed.
- A. Karmakar, S. Karmakar and S. Mukherjee, Bioresour. Technol., 2010, 101, 7201–7210 CrossRef CAS PubMed.
- M. Pagliaro and M. Rossi, The Future of Glycerol – New Usages for a Versatile Raw Material, RCS Publishing, Cambridge, UK, 2008 Search PubMed.
- R. Christoph, B. Schmidt, U. Steinberner, W. Dilla and R. Karinen, Glycerol, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006 DOI:10.1002/14356007.a12_477.pub2.
- Food and Agricultural Organization of the United Nations, FAO, Biofuels and the sustainability challenge: A global assessment of sustainability issues, trends and policies for biofuels and related feedstocks, 2013, http://www.fao.org/docrep/017/i3126e/i3126e.pdf (accessed August 2014).
- M. T. Firisia, I. Van Duren and A. Voinov, Energy Effic., 2014, 7, 79–95 CrossRef PubMed.
- BP, Statistical Review of World Energy, 2013, http://www.bp.com/content/dam/bp/pdf/statistical-review/statistical_review_of_world_energy_2013.pdf (accessed August 2014).
- EIA – International Energy Statistics, Independent Statistics & Analysis - U.S. Energy Information Administration, http://www.eia.gov/cfapps/ipdbproject/iedindex3.cfm?tid=79&pid=81&aid=1&cid=regions,&syid=2007&eyid=2011&unit=TBPD (accessed August 2014).
- M. D. Bohon, B. A. Metzger, W. P. Linak, C. J. King and W. L. Roberts, Proc. Combust. Inst., 2011, 33, 2717–2724 CrossRef CAS PubMed.
- EBB - European Biodiesel Board, Statistics - The EU biodiesel industry 2011, http://www.ebb-eu.org/stats.php (accessed August 2014).
- D. J. Anneken, S. Both, R. Christoph, G. Fieg, U. Steinberner and A. Westfechtel, Fatty Acids, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006 DOI:10.1002/14356007.a10_245.pub2.
- Council Regulation (EC) 1069/2009 of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by-products Regulation) OJ L 300/1.
- P. H. Nielsen, A. M. Nielsen, B. P. Weidema, R. Dalgaard and N. Halberg, LCA food data base, 2003 Search PubMed.
- Gov.uk, Ecofys, Tallow review: a report on the current animal market, 2012, http://www.gov.uk/government/uploads/system/.../tallowreview.pdf (accessed August 2014).
- W. Hahn, USDA Economic Research Service calculations based on Bureau of Labor Statistics and USDA Agricultural Marketing Service Data, 2013, http://www.ers.usda.gov/data-products/meat-price-spreads.aspx (accessed August 2014).
- L. Liu, X. P. Ye and J. J. Bozell, ChemSusChem, 2012, 5, 1162–1180 CrossRef CAS PubMed.
- Y. Magatani, K. Okumura, J.-L. Dubois and J.-F. Devaux, US Pat., 0 053 595 A1, 2013 Search PubMed.
- J.-L. Dubois, US Pat., 8 143 454 B2, 2012 Search PubMed.
- L. E. Bogan and M. A. Silvano, US Pat., 8 198 477 B2, 2012 Search PubMed.
- L. Shen, H. Yin, A. Wang, X. Lu, C. Zang, F. Chen, Y. Wang and H. Chen, J. Ind. Eng. Chem., 2014, 20, 759–766 CrossRef CAS PubMed.
- Chem Systems – Process Evaluation/Research Planning (PERP program), Acrylic Acid 08/09-3, 2010.
- J.-L. Dubois and G. Patience, US Pat., 8 212 070 B2, 2012 Search PubMed.
- P. A. Holman, D. R. Shonnard and J. H. Holles, Ind. Eng. Chem. Res., 2009, 48, 6668–6674 CrossRef CAS.
- D. Arntz, A. Fischer, M. Höpp, S. Jacobi, J. Sauer, T. Ohara, T. Sato, N. Shimizu and H. Schwind, Acrolein and Methacrolein, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007 DOI:10.1002/14356007.a01_149.pub2.
- Chem Systems – Process Evaluation/Research Planning (PERP program), Epichlorohydrine 99/00S11, 2000.
- A. Heiko, K. Harth, H.-P. Neumann, U. Hammon and R. Felder, EU Pat., 1 005 908 A2, 2000 Search PubMed.
- J.-L. Dubois and G. Patience, US Pat., 8 530 697 B2, 2013 Search PubMed.
- J.-L. Dubois and G. Patience, US Pat., 0 171 685 A1, 2014 Search PubMed.
- B. P. Weidema and M. Wesnaes, J. Cleaner Prod., 1996, 4, 167–174 CrossRef.
- D. Cespi, F. Passarini, L. Ciacci, V. Castellani, E. Collina, A. Piazzalunga and L. Morselli, Int. J. Life Cycle Assess., 2014, 19, 89–99 CrossRef CAS.
- S. Liang, M. Xu and T. Zhang, Bioresour. Technol., 2013, 129, 72–77 CrossRef CAS PubMed.
- ISPRA – Istituto Superiore per la Protezione e la Ricerca Ambientale, Urban waste report - edition 2013, http://www.isprambiente.gov.it/ (accessed August 2014).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc01497a |
| This journal is © The Royal Society of Chemistry 2015 |