Diterpenoids of terrestrial origin
James R.
Hanson
*
University of Sussex, Department of Chemistry, Brighton, Sussex, UK
First published on 2nd October 2014
Abstract
Covering: January to December 2013. Previous review, Nat. Prod. Rep., 2013, 30, 1346–1356
This review covers the isolation and chemistry of diterpenoids from terrestrial as opposed to marine sources and includes labdanes, clerodanes, abietanes, pimaranes, kauranes, cembranes and their cyclization products. There are 179 references.
1 Introduction
This report follows the pattern of its predecessors1 covering the identification and chemistry of diterpenoids of terrestrial as opposed to marine origin. The latter are thoroughly reviewed in the articles on marine natural products.2 In many cases diterpenoids from marine sources have carbon skeletons that are very different from those of terrestrial origin. The commercial production of stevia extracts as non-nutritive sweeteners has led to a number of studies on the chemistry of its diterpenoid aglycone, steviol, with the objective of exploring the biological activity of the related kauranoid diterpenes.116,117A number of reviews have appeared on the constituents of various diterpenoid bearing plant species including Salvia,3Chloranthus,4Xylopia,5 and Pinus species.6
2 Acyclic and related diterpenoids
An increasing number of dimeric diterpenoids have been identified and which are formed from two known compounds. The aphanamenes A and B are dimers which were isolated7 from a South Eastern Asian tree, Aphanamixis grandiflora (Meliaceae). They showed a significant inhibition of NO production and may be formed by a (4 + 2) cycloaddition of two known diterpene monomers, melidianolic acid A and nemoralisin C.3 Bicyclic diterpenoids
3.1 Labdanes
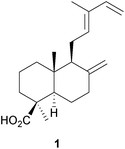
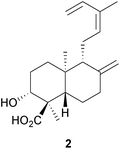
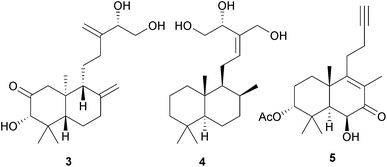
A diterpene synthase from the clary sage, Salvia sclarea, has been identified8 which catalyses the cyclization of geranylgeranyl diphosphate to (8R)-hydroxycopalyl diphosphate. The ready availability of a number of labdanes such as manool, larixol and sclareol has led to their use as chiral starting materials for synthesis. The application of manool in the partial synthesis of sesquiterpenes and diterpenes has been reviewed.9 The anti-malarial activity of 13-benzyl-15,16-bisnorlabdanes derived from manool has been examined.10 The synthesis of the drimane sesquiterpenoids (−)-albrassitriol and its 6-epimer from larixol has been reported,11 whilst larixol and sclareol provided the starting materials for the synthesis of some drimanes including 6-ketoeuryfuran and 6-ketowinterin.12 Modification of the side chain of communic acid 1, which is readily obtained from Juniper berries, has been used to confirm the structure of some labdanes.13 The total synthesis of the tetranorlabdane asperolide C by an iridium catalysed polyene cyclization has been described.14Variations between the labdane, pimarane and abietane components of the needles and twigs of different populations of the Siberian dwarf pine, Pinus pumila, have been reported.15 Sclareol was identified16 in an examination of the bio-active constituents of Salvia chrysophylla (Lamiaceae), whilst cis-sclareol with an unusual cis A/B ring junction was amongst the anti-mycobacterial labdanes obtained17 from Leucas stelligera (Lamiaceae). The labdorffianic acids A and B (e.g. A, 2) were isolated18 along with some atisane diterpenoids, xylodiol 7-acetate and xylopinone, from the tree Xylopia langsdorffiana (Annonaceae). The anti-bacterial activity of Alpinia nigra (Zingiberaceae) has been associated19 with the presence of some known labdane 15,16-dialdehydes. The pahangensins A and B are anti-bacterial dimeric and monomeric labdanes, respectively, which were obtained20 from another wild ginger, Alpinia pahangensis.
The 12(R)-12-hydroxylabda-8(17),13(16),14-trien-18- and 19-oic acids have been identified21 as constituents of the wood of the Taiwanese conifer, Cunninghamia konishii (Taxodiaceae). Examination of the stems of Mallotus japonicus (Euphorbiaceae), which has been used in Chinese traditional medicine, afforded22 the mallonicusins A–H (e.g. A, 3). Extraction of the fruits of Amomum kravanh (Zingiberaceae), a Chinese traditional medicine which is used to treat stomach disorders, yielded23 the labdane 4 together with some isospongiane diterpenoids known as the kravanhins A–C which possess a cis B/C ring junction. Further labdanes have been isolated24a from Leonotis leonurus, whilst a 15,16-dinorlabdane (5) with an unusual 13,14-acetylene has been identified24b in Leonurus japonicus (Lamiaceae). A series of ent-3,4-seco-labdan-3-oic acids have been obtained25,26 from the leaves of Callicarpa nudiflora (Verbenaceae). They were shown to have anti-inflammatory activity due to inhibition of NO production.
The cytotoxic biological activity of andrographolide and its derivatives has continued to attract attention and a number of aspects of its chemistry have been explored in this context.27–30 The tumour inhibitory effects of grindelic31 and imbricatolic32 acids have been examined. Andalusol has been shown to protect mitochondria from deleterious changes induced by hydrogen peroxide and other effects associated with age-related neurodegenerative diseases.33
Further labdanes have been isolated from Vitex species (Verbenaceae) which have been used in Chinese traditional medicine, including the vitextrifolins A–G (e.g. A, 6) from V. trifolia34 and viterotulin B 7 from V. rotundifolia.35 The metabolic diversity of Coleus forskohili (Lamiaceae) on the Indian sub-continent has been investigated36 in the context of forskolin production.
3.2 Halimanes and clerodanes
The scopariusins A–C (e.g. A, 8) together with the ent-halimane isoscoparins (e.g. M, 9) have been obtained37 from Isodon scoparius (Lamiaceae). The rearranged ent-halimanes may be formed by the migration of the C-9:C-10 bond to C-11. Ent-halimic acid has been used38 as the starting material for a synthesis of the anti-tumour indole:sesquiterpene alkaloid, 12-epi-ent-pentacyclindole.The structures and biological activity of the clerodanes have made them attractive synthetic targets. A Diels–Alder cycloaddition has been explored39 in the construction of the decalin core of the clerodanes. The structural features which contribute to the selective binding of the psychoactive salvinorin A to the κ-opioid receptor have continued to stimulate investigations into its chemistry and detection.40–42 The bio-transformation of clerodanes by Rhizopus stolonifer has been reported.43
Further investigations of Scutellaria barbata (Lamiaceae) have afforded44 the clerodanes scutebatas P–R (e.g. P, 10), whilst scopariusic acid from Isodon scoparius has been shown45 to be a meroditerpenoid containing a cyclobutane ring formed by the cycloaddition of the 13E double bond of isoscoparin P (11) to trans-4-hydroxycinnamate esters.
Amongst the cis-clerodanes that have been isolated from Chinese liverworts are the ciliatolides A–D (e.g. A, 12) from Scapania ciliata (scapaniaceae)46 and the cephaloziellins A–F (e.g. A, 13) from Cephaloziella kiaeri.47 Further investigations of the woody climber Tinospora cordifolia (Menispermaceae) afforded48 tincordin 14 which has insect anti-feedant activity, whilst the caseabalansins are a group of cytotoxic clerodanes which were obtained49 from Casearia balansae (Flacourtiaceae). Some of these clerodanes possess an unusual C-2:C-19 oxygen bridge.
Examination of Mexican Salvia species led50 to the isolation of the sepulturins A–F (e.g. A, 15) from S. shannoni and a revision of the structure of infuscatin to that of 16. The aerial parts of S. microphylla contained51 the unusual rearranged clerodane microphyllandiolide 17. The laevinoids A and B (e.g. A, 18) isolated from Croton laevigatus (Euphorbiaceae) were also assigned52 an unusual clerodane skeleton containing a cyclopropane ring.
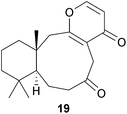
The amomaxins (e.g. A, 19) from Amomum maximum (Zingiberaceae) have an unusual skeleton, which may be derived53 from a labdane, in which the nine-membered ring is formed by oxidative cleavage of the C-8:C-9 bond and an aldol condensation between C-17 and C-12.
4 Tricyclic diterpenoids
4.1 Pimaranes
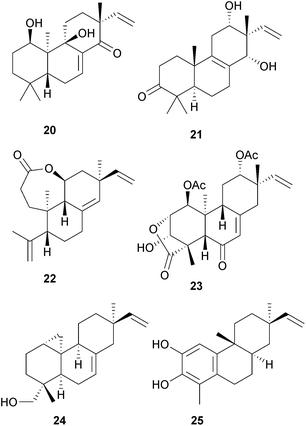
The norditerpenoid 8β-hydroxy-18-nor-4,15-isopimaradien-3-one has been isolated54 from Euonymus grandiflorus (Celastraceae). Investigation of the Chinese liverwort, Plagiochila pulcherrima yielded55 pimara-8(14),15-dien-11α-ol and the corresponding 1β,11α- and 7β,11α-diols, whilst the more highly oxygenated pedinophyllols A–J (e.g. A, 20) were obtained56 from Pedinophyllum interruptum. Further studies57 of the Chinese drug Gua-jin-ban, derived from Excoecaria acerifolia (Euphorbiaceae), afforded the excoecarins F–H (e.g. F, 21) whilst the 3,4-seco-ent-isopimarane agallochaexcoerin D 22 was obtained58 from the wood of the Indian mangrove plant E. agallocha. A study of the use of fungal pathogens as biocontrol agents of weeds such as ‘fat-hen’ (Chenopodium album) has led59 to the isolation of chenopodolin 23 from Phoma chenopodicola. This is a phytotoxic metabolite of this pathogen of the weed. The xylarianes are pimaranes which have been isolated60 from Xylaria species. The larvicidal activity of sphaeropsidin A against Aedes aegypti, a mosquito that is a vector of dengue fever, has been reported.61 An unusual 1:10-cyclopropyl-ent-7,15-pimaradiene 24 has been found62 in Calceolaria talcana (Scrophulariaceae). It has tyrosinase inhibitory activity. Since an abietatriene with the normal A/B stereochemistry was reported from the same plant, further evidence for the stereochemistry of 24 is required. The euphebracteolatins A and B (e.g. A, 25) are rosanes which were isolated63 from Euphorbia ebracteolata. However, there was little evidence for the absolute stereochemistry that was assigned to them.4.2 Abietanes and their relatives
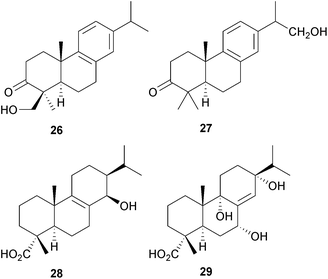
The oxidative enzyme system CYP76AH4, which catalyses the conversion of miltiradiene to abietatriene and ferruginol, has been characterized64 and a related system CYP76AH1 has been expressed65 in yeast in order to produce ferruginol from miltiradiene in the context of tanshinone biosynthesis. Resin acid conversions with CYP1O5A1 from Streptomyces griseus have also been explored.66The abietanes that are found in Pinus mugo have been considered67 as markers for these trees. Further abietanes including the triptobenzene R 26 have been isolated68 from the roots of the ‘Thunder God Vine’ (Tripterygium wilfordii, Celastraceae), a plant which is used in traditional Chinese medicine for the treatment of cancer. The evolutchuols A–E (e.g. A, 27) were obtained69 from the Okinawan shrub, Euonymus lutchuensis (Celastraceae). Amongst the abietanes isolated70 from the Thai medicinal herb Hyptis suaveolens (Lamiaceae) were isosuaveolic acid 28 and 8α,9α-epoxysuaveolic acid. Chinese eaglewood (Chenxiang, Aquilaria sinensis, Thymelaceae) has been used as a sedative and some of the aquilarabietic acids A–J (e.g. A, 29) that it contains have been shown71 to be active as anti-depressants.
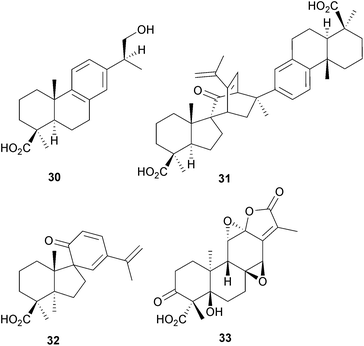
The majusanins A–C and majusanic acid E 30 have been obtained72 from the roots of Illicium majus (Schisandraceae) whilst the jiadifenoic acids isolated73 from the roots of I. jiadifengpi have anti-viral activity. These include an unusual spiranic dimer, jiadifenoic acid A (31), which is formed by a Diels–Alder reaction of a spirodienone (32) arising by rearrangement of an 8α,9α-epoxide. Acanthopanolide B 33 is a highly-oxygenated abietane obtained74 from the roots of Acanthopanax brachypus (Araliaceae).
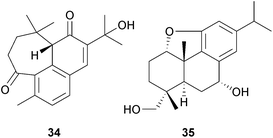
New quinones continue to be isolated from Salvia species such as S. corrugata,75a and xantoquinone, 5α,6α-dimethoxyabieta-8,12-diene-7,11,14-trione, has been isolated from S. xanthocheila75b whilst further degraded abietanes have been obtained from S. przewalskii76 and cell cultures of S. miltiorrhiza.77 Examination of Isodon lophanthoides var. gerardianus (Lamiaceae), which is used as ‘xihuangcao’ in Chinese herbal beverages, has afforded a number of abietanes including 11,12,15-trihydroxy-7-oxoabieta-8,11,13-trien-7-one78 and various relatives known as the graciflorins.79 A rearrangement involving the cleavage of ring A leads to the structure of graciflorin D 34. Rearranged abietanes in which a methyl group has migrated from C-4 to C-3, and in which the C-13 isopropyl group has been converted to an n-propyl group have been isolated from Clerodendrum trichotomum (Lamiaceae).80 Other abietanes, the ent-abierubesins, have been isolated from I. rubescens,81 and isoabietenin A 35 has been isolated from I. tenuifolius.82
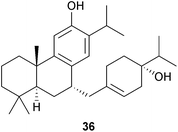
Salicassin is a chalcone:diterpene (sugiol) adduct which was obtained83 from the root bark of Maytenus salicifolia (Celastraceae). A giant invasive Brazilian water fern, the kariba weed, Salvinia molesta (Salviniaceae), was the source of salviniol 36 which possesses a ferruginol–menthane coupled skeleton and showed activity against several human cancer cell lines.84 Trichotomone is a dimeric abietane which was isolated85 from the roots of Clerodendrum trichotomum (Lamiaceae).
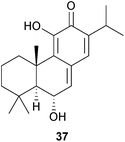
The oxidation of the methyl ether of 6,7-dehydroferruginol with dimethyldioxirane has been examined86 in the context of the synthesis of more highly oxidized abietanes. A series of dehydroabietic acid, dehydroabietylamine and maleopimaric acid derivatives have been synthesized and their activity against human cancer cell lines has been assessed.87–91 In another study of the abietanes derived from Salvia and Plectranthus species, taxodone (37) proved to be particularly active against human tumour cells.92 Some tanshinones from S. miltiorrhiza have been shown93 to inhibit the action of the carboxyesterases in the liver which compromise the activity of pro-drugs, whilst taxusabietane C from Taxus wallichiana is an inhibitor of lipoxygenase.94 Some arylamine fluorescent cell probes containing dehydroabietic acid units have been synthesized.95 An enantioselective synthesis has been reported96 of the pygmaeocins A and B which are irregular abietanes in which C-19 has migrated from C-10 to C-5.
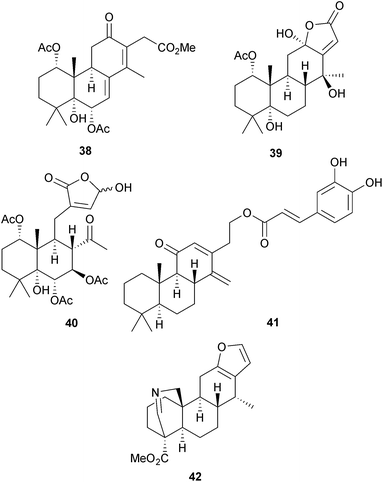
Cassanes are common constituents of Caesalpinia (Fabaceae) species and a number of new examples have been isolated including the caesalpins (e.g. A, 38)97 and some 16 → 12 lactones such as the caesalpinolides (e.g. F, 39) from C. minax98 and C. crista.99 Ring C has been cleaved in an unusual manner to afford the caesalminaxins (e.g. A, 40) which were also obtained100 from C. minax. The voulkensins (e.g. C, 41) are 11-oxocassanes that were obtained101 from C. volkensis, a plant which has anti-malarial activity. The caesanines (e.g. A, 42), which were isolated102 from the Chinese herb C. sappan, have a nitrogen bridge across ring A which is reminiscent of the tetracyclic diterpene alkaloids. Schaffnerine is a dimeric 7,8-seco-cassane diterpene which has been obtained103 from the Mexican tree, Acacia schaffneri (Leguminosae). The total synthesis of cassaine from (+)-carvone has been reported.104
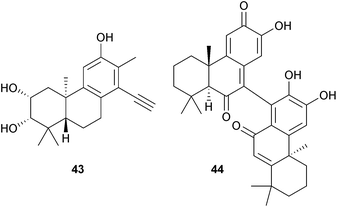
A number of degraded diterpenes have been reported which may have their origin in cassanes or cleistanthanes. These include the trigophyxins J–N from Trigonostemon xyphophylloides (Euphorbiaceae)105 and an unusual phenylacetylene, phyllanflexoid A (43) from Phyllanthus flexuosus (Euphorbiaceae) roots. This plant also contained a related cleistanthol.106 The bicelaphanols A (44), isolated as two atropisomers, and fimbricalyx A are norditerpene dimers which were obtained from the Oriental bittersweet vine Celastrus orbiculatus (Celastraceae)107 and Strophioblachia fimbricalyx (Euphorbiaceae).108
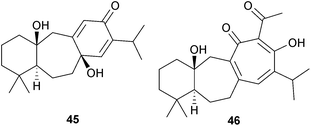
Some further icetexanes have been isolated, including fokihodgin J (45) from the Chinese medicinal plant Fokienia hodginsii (Cupressaceae),109 perovskatone A (46) from Perovskia atriplicifolia (Lamiaceae),110 and the dimeric obtusinones D and E from Premna obtusifolia (Lamiaceae) roots.111 Some synthetic studies in this area have been reported.112
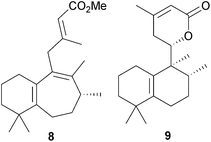
The taiwania quinones possess a 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ring system in which the five-membered ring B carries an aldehyde group. A number of syntheses of these diterpenes, including the ring contraction of sugiol methyl ether, have been reported.113–115
6 ring system in which the five-membered ring B carries an aldehyde group. A number of syntheses of these diterpenes, including the ring contraction of sugiol methyl ether, have been reported.113–115
5 Tetracyclic diterpenes
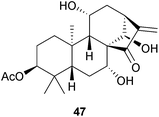
The approval of stevia as a non-nutritive sweetener for human consumption by the European Food Safety Authority in 2011 has meant that the aglycone steviol (ent-13-hydroxykaur-16-en-19-oic acid) and its beyerane Wagner–Meerwein rearrangement product, isosteviol, have become readily available for chemical transformations. The steviol glycosides found in Stevia rebaudiana [Asteraceae (Compositae)] and their metabolism, and the use of isosteviol as a starting material for a variety of transformations have been reviewed.116,117 Variations in the steviol content and major steviol glycosides isolated from S. rebaudiana grown in different regions and under different photoperiods have been documented.118 The transglycosylation of stevioside to improve its properties as a sweetener has been examined.119 A series of Beckmann rearrangements and fragmentation reactions of the bicyclo[3,2,1]octane ring system of steviol and isosteviol have been described.120 Some derivatives of isosteviol with vasorelaxant properties have been prepared.121 The cytotoxic and apoptosis-inducing activities of various steviol and isosteviol derivatives have been examined, including a number in which a heterocyclic ring has been added to ring D.122–124Seasonal variations in the formation of grandifloric acid derivatives by Mikania (Asteraceae) species have been noted.125 A number of kaurenes have been associated with the cytotoxic activity of medicinal plants. These include 18,19-dihydroxy-ent-kaur-16-en-3-one from Euryops arabicus (Asteraceae),126 17-hydroxy-ent-kaur-15-en-19-al from the ‘Custard-apple tree’ (Annona squamosa, Annonaceae)127 and 11α-hydroxyleukamenin E (47) from Salvia cavaleriei (Lamiaceae).128 Some epoxides obtained from the ent-kaurenoic acids of Wedelia paludosa have shown good activity against chloroquine resistant strains of Plasmodium falciparum,129 whilst some 19-nor ureas derived from 11β,15β-dihydroxy-ent-kaurenoic acid were selective inhibitors of 11β-hydroxysteroid dehydrogenase,130a and linearol and sidol have been shown130b to exert a cytoprotective effect against oxidative injury.
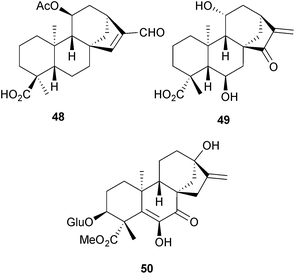
The Vietnamese plant Croton tonkinensis (Euphorbiaceae) is a rich source of diterpenoids, some of which have anti-inflammatory activity. New isolates include the crotonkinins C–J (e.g. C, 48).131 Further ent-kaurenes, including 49, have been obtained132 from Pteris semipinnata, whilst the graecumosides A and B (e.g. A, 50) are glycosides which were isolated133 from fenugreek seeds (Trigonella foenum-graecum, Fabaceae).
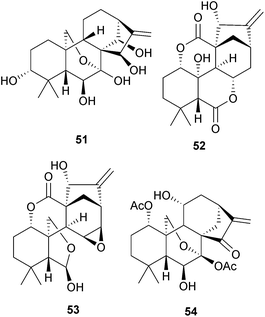
The investigations of Chinese Isodon species (Lamiaceae) have yielded over 600 diterpenoids, many of which possess cytotoxic and other medicinal properties. Further examples include 3α,l4β,16α-trihydroxy-ent-kaurane from I. japonicus,134 the rabdonervosins G–J (e.g. G, 51) from I. nervosus,135 the isorosthins A–P (e.g. the 20-norenmein isorosthin A, 52) from I. rosthornii,136 sculponin M (53) from I. sculponeatus,137 and effusanin F (54) from Rabdosia (Isodon) serra.138 The latter showed anti-bacterial activity. The chemistry of oridonin, which is one of the more readily available members of this series, has been explored in the context of anti-tumour structure–activity relationships.139,140
Further total syntheses of ent-kaurene and ent-beyerane diterpenoids have been reported.141,142 The transformation of ent-kaurenoic and trachylobanic acids from sunflower waste, using fluorosulfonic acid to form the carbocation that links the families of tetracyclic diterpenes, has led to the synthesis of some atisane diterpenes.143 The atisane diterpenoids, xylodiol acetate and xylopinone have been isolated18 from Xylopia langsdorffiana (Annonaceae).
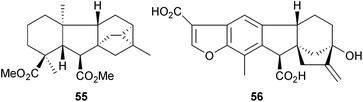
An interesting ring-contraction reaction of 7-oxoditerpenes with diacetoxyiodobenzene has afforded144 some gibberellin analogues (e.g.55) in the ent-kaurene, ent-trachylobane and ent-atisane series. The characterization of the gibberellin oxidases from cucumber, Cucumis sativus, has been reported.145 The microbiological transformation of 15α-hydroxy-ent-kaur-9(11),16-dienes by Fusarium (Gibberella) fujikuroi has shown146 that a 9(11) double bond impedes hydroxylation at C-7 and hence ring contraction, but not 6,7-dehydrogenation and the formation of the kaurenolides. Since the gibberellin plant hormones occur in very small amounts in plants, their separation and determination has continued to be an analytical challenge and further refinements to methods have been described.147 Pharbinilic acid (56) is a 9-epiallogibberic acid derivative which was obtained148 from Morning Glory (Pharbitis nil, Convolvulaceae).
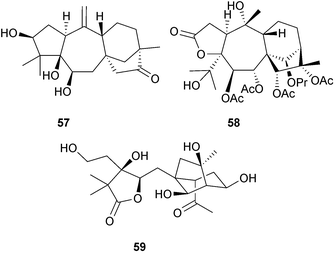
Grayane diterpenoids are characteristic diterpenoids of Rhododendron and Pieris (Ericaceae) species. Micranthanone A (57) is an ent-beyerane analogue which was isolated149 from Rhododendron micranthum. A number of 3,4-seco-grayanes including the pierisformotoxins A–D (e.g. A, 58) from Pieris formosa150 and their chlorine-containing relatives, the neopierisoids A and B from P. japonica, have insect anti-feedant activity.151 Mollolide A (59), with a 1,10:2,3-diseco-grayane carbon skeleton, is a more highly degraded example that was isolated152 from R. molle.
6 Macrocyclic diterpenoids and their cyclization products
The identification of bacterial diterpene cyclases that synthesize the cembrane skeleton153 and precursors to the fusicoccanes154 have been reported. Nephthenol 15-O-β-D-quinovoside is a cembrane glycoside which was obtained155 from Asterothamnus central-asiaticus (Asteraceae). Strategies for the synthesis of cembranolides have been examined.156 Some casbane diterpenoids have been isolated157 from the roots of Euphorbia pekinensis.The Canadian yew, Taxus canadensis, is a prolific source of taxanes. A review listing their isolation and discussing their chemistry has appeared.158 A short enantioselective synthesis of a taxol ring A fragment has been reported.159
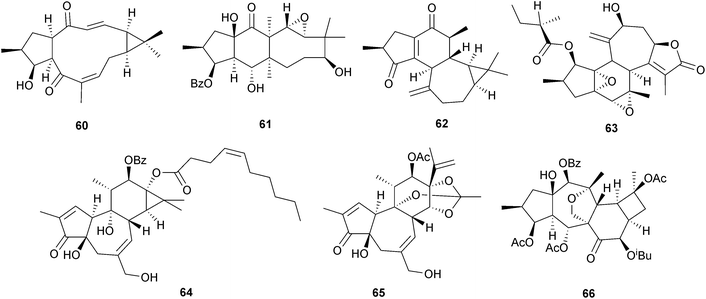
Diterpenoids of the lathyrane, tigliane, ingenane, daphnane and related carbon skeletons possess a range of biological activities, including interaction with protein kinase C which plays a role in regulating cellular growth and differentiation. Diterpenoids with these skeletons are characteristic constituents of plants of the euphorbiaceae and thymelaceae, a number of which are used in Chinese traditional medicine. The medicinal properties and phytochemistry of several Jatropha species have been reviewed.160 The ekanpenoids (e.g. A, 60) are norlathyranes which were isolated161a from the roots of the Chinese herbal medicine Euphorbia kansuensis, whilst further investigations of E. micractina afforded161b the euphactins (e.g. E, 61). Some jatrophanes have been obtained162a from E. dendroides, whilst the sikkimenoids (e.g. A, 62) were isolated162b from E. sikkimensis. The crotocascarins (e.g. A, 63) were isolated163 from Croton cascarilloides (Euphorbiaceae) whilst further cytotoxic phorbol esters have been obtained164 from C. tiglium. The tigliane stelleracin A (64) from the Nepalese medicinal plant Stellera chamaejasme (Thymelaceae) was reported165 to show anti-HIV activity. A number of tigliane and daphnane esters showing inhibitory activity against human cancer cell lines have been isolated166–168 from the flower buds of Daphne genkwa (Thymelaceae), which are used in Chinese traditional medicine. These include the daphneresinferins (e.g. A, 65).168 Acerifolin A is a tigliane which was obtained169 from Excoecaria acerifolia (Euphorbiaceae), whilst the cyclomyrsinol structure 66 has been assigned170 to an immunosuppressive diterpenoid from Euphorbia kopetdaghi.
The 13C NMR spectra of the daphnane diterpenoids have been reviewed.171 The pharmaceutical potential of phorbol esters obtained from Jatropha curcas oil has been examined172 in the context of their relationship with prostratin. Some structure–activity relationships amongst ingenol 3-angelate analogues in the treatment of actinic keratosis and non-melanoma skin cancer have been explored.173 Approaches to the synthesis of the tigliane–daphnane ring system have been reported.174
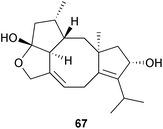
The roussoeliols A and B (e.g. A, 67) are fusiccane metabolites of the fungus, Roussella hysteroides.175
7 Miscellaneous diterpenoids
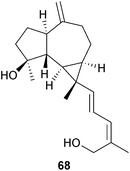
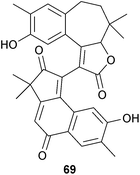
The total synthesis of the cyanthiwigins A, C, G and H has been described.176 Further prenylated sesquiterpenes have been reported including the boscartols (e.g. A, 68) which are prenyl aromadendranes that were isolated177 from frankincense resin, Boswellia carterii (Burseraceae). The serrulatane leubethanol has been synthesized178 from isopulegol. This anti-bacterial compound from Leucophyllum frutescens has potentially useful activity against resistant strains of bacteria.The trigoxyphins O–T from Trigonostemon xyphylloides (Euphorbiaceae) have been assigned179 structures (e.g. O, 69) which may be derived from two degraded diterpenes.
8 References
- J. R. Hanson, Nat. Prod. Rep., 2013, 30, 1346–1356 RSC.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2014, 31, 160–258 RSC.
- Y. B. Wu, Z. Y. Ni and Q. W. Shi, Chem. Rev., 2012, 112, 5967–6026 CrossRef CAS PubMed.
- Y. J. Xu, Chem. Biodiversity, 2013, 10, 1754–1773 CAS.
- I. C. Moreira, N. F. Roque, W. Vilegas, C. A. Zalewski, J. H. G. Lago and M. Funasaki, Chem. Biodiversity, 2013, 10, 1921–1943 CAS.
- B. Li, Y.-H. Shen, Y.-R. He and W.-D. Zhang, Chem. Biodiversity, 2013, 10, 2133–2160 CAS.
- H.-J. Zhang, J. Luo, S.-M. Shan, X.-B. Wang, J.-G. Luo, M.-H. Yang and L.-Y. Kong, Org. Lett., 2013, 15, 5512–5515 CrossRef CAS PubMed.
- N. Gunnewich, Y. Higashi, X. Feng, K.-B. Choi, J. Schmidt and T. M. Kutchan, Phytochemistry, 2013, 91, 93–99 CrossRef CAS PubMed.
- (a) F. J. Salazar and J. E. Villamizar, J. Chem. Res., 2013, 37, 1–5 CrossRef CAS; (b) F. J. Salazar and J. E. Villamizar, J. Chem. Res., 2013, 37, 63–70 CrossRef CAS.
- F. J. Salazar, A. Quintero, N. G. Dominguez, J. L. Concepcion, M. E. Acosta, E. Tropper and J. E. Villamizar, J. Chem. Res., 2013, 37, 657–661 CrossRef CAS.
- P. F. Vlad, A. Ciocarlan, M. Coltsa, C. Edu, A. Biriiac, A. Barba, C. Deleanu, A. Nicolescu, M. D'Ambrosio and A. de Groot, Nat. Prod. Res., 2013, 27, 809–817 CrossRef CAS PubMed.
- P. F. Vlad, A. Ciocarlan, C. Edu, A. Aricu, A. Biriiac, M. Coltsa, M. D'Ambrosio, C. Deleanu, A. Nicolescu, S. Shava, N. Vornicu and A. de Groot, Tetrahedron, 2013, 69, 918–926 CrossRef CAS PubMed.
- D. J. Mack and J. T. Njardson, Angew. Chem., Int. Ed., 2013, 52, 1543–1547 CrossRef CAS PubMed.
- O. F. Jeker, A. G. Kravina and E. M. Carreira, Angew. Chem., Int. Ed., 2013, 52, 12166–12169 CrossRef CAS PubMed.
- A. V. Shpatov, S. A. Popov, O. I. Salnikova, E. N. Schmidt, S. W. Kang, S. M. Kim and B. H. Um, Chem. Biodiversity, 2013, 10, 198–208 CAS.
- B. Çulhaoğlu, G. Yapar, T. Dirmenci and G. Topçu, Nat. Prod. Res., 2013, 27, 438–447 CrossRef PubMed.
- R. R. Kulkarni, K. Shurpali, V. Puranik, D. Sarkar and S. P. Joshi, J. Nat. Prod., 2013, 76, 1836–1841 CrossRef CAS PubMed.
- P. F. dos Santos, M. C. Duarte, D. A. C. Bezerra, M. d. F. Agra, J. M. Barbosa Filho, M. S. da Silva and J. F. Tavares, Helv. Chim. Acta, 2013, 96, 1085–1092 CrossRef CAS.
- S. Ghosh, K. Indukuri, S. Bondalapati, A. K. Saikia and L. Rangan, European J. Medic. Chem., 2013, 66, 101–105 CrossRef CAS PubMed.
- Y. Sivasothy, H. Ibrahim, A. S. Paliany, S. A. Alias and K. Awang, Bioorg. Med. Chem. Lett., 2013, 23, 6280–6285 CrossRef CAS PubMed.
- Y. C. Chen, Y.-C. Li, H. L. Chiu, W.-Y. Cheng, Y.-H. Hong, P. J. Sung, C. C. Kuo, B. T. Wu and Y. H. Kuo, Helv. Chim. Acta, 2013, 96, 2282–2287 CrossRef CAS.
- D.-Z. Li, C. Tang, R. J. Quinn, Y. Feng, C.-Q. Ke, S. Yao and Y. Ye, J. Nat. Prod., 2013, 76, 1580–1585 CrossRef CAS PubMed.
- H. Yin, J.-G. Luo and L.-Y. Kong, J. Nat. Prod., 2013, 76, 237–242 CrossRef CAS PubMed.
- (a) H. Wu, J. Li, F. R. Fronczek, D. Ferreira, C. L. Burandt, V. Setola, B. L. Roth and J. K. Zjawiony, Phytochemistry, 2013, 91, 229–235 CrossRef CAS PubMed; (b) F. Peng, L. Xiong and X. M. Zhao, Molecules, 2013, 18, 13904–13909 CrossRef CAS PubMed.
- L. Zhang, J. Huang, M.-S. Liu, C. Zhang, G. Li, K. Zhang, L. Dong and J. Wang, Heterocycles, 2013, 87, 1561–1569 CrossRef CAS.
- L. Dong, X.-P. Zhang, M.-S. Liu, Y.-M. Li, J. H. Wang and Y. Wang, J. Asian Nat. Prod. Res., 2013, 15, 30–34 CrossRef CAS PubMed.
- D. Chen, Y. Song, Y. Lu and X. Xue, Bioorg. Med. Chem. Lett., 2013, 23, 3166–3169 CrossRef CAS PubMed.
- S. Wei, Y.-B. Tang, H. Hua, E. Ohkoshi, M. Goto, L.-T. Wong, K. H. Lee and Z. Xiao, Bioorg. Med. Chem. Lett., 2013, 23, 4056–4060 CrossRef CAS PubMed.
- D. Singh and P. K. Chaudhuri, Nat. Prod. Res., 2013, 27, 680–683 CrossRef CAS PubMed.
- X.-W. Xiao, H.-Z. Fu, Y.-H. Luo and X.-Y. Wei, J. Asian Nat. Prod. Res., 2013, 15, 809–818 CrossRef CAS PubMed.
- G. F. Reta, A. J. Chiaramello, C. Garcia, L. G. Leon, V. S. Martin, J. M. Padron, C. E. Tonn and O. J. Donadel, European J. Medic. Chem., 2013, 67, 23–28 Search PubMed.
- M. W. Pertino, C. Theoduloz, M. Bastias and G. Schmeda-Hirschmann, Molecules, 2013, 18, 5936–5953 CrossRef PubMed.
- E. Gonzalez-Burgos, A. I. Duarte, M. E. Carretero, P. I. Moreira and M. P. Gomez-Serranillos, J. Nat. Prod., 2013, 76, 933–938 CrossRef CAS PubMed.
- C.-J. Zheng, J.-Y. Zhu, W. Yu, X.-Q. Ma, K. Rahman and L.-P. Qin, J. Nat. Prod., 2013, 76, 287–291 CrossRef CAS PubMed.
- C. Lee, J. W. Lee, Q. Jin, H. J. Lee, S.-J. Lee, D. Lee, M. K. Lee, C.-K. Lee, J. T. Hong, M. K. Lee and B. Y. Hwang, Bioorg. Med. Chem. Lett., 2013, 23, 6010–6014 CrossRef CAS PubMed.
- E. J. Tamboli, M. Singh, Y. T. Kamal, M. Garg, R. Parveen, M. Mujeeb and S. Ahmad, Nat. Prod. Res., 2013, 27, 1737–1742 CrossRef CAS PubMed.
- M. Zhou, H.-C. Geng, H.-B. Zhang, K. Dong, W.-G. Wang, X. Du, X.-N. Li, F. He, H.-B. Qin, Y. Li, J.-X. Pu and H. D. Sun, Org. Lett., 2013, 15, 314–317 CrossRef CAS PubMed.
- I. S. Marcos, R. F. Moro, I. Costales, P. Basabe, D. Diez, F. Mollinedo and J. G. Urones, Tetrahedron, 2013, 69, 7285–7289 CrossRef CAS PubMed.
- S. C. Butler and C. J. Forsyth, J. Org. Chem., 2013, 78, 3895–3907 CrossRef CAS PubMed.
- B. Ivanova and M. Spiteller, Med. Chem. Res., 2013, 1–14 Search PubMed.
- P. R. Polepally, K. White, E. Vardy, B. L. Roth, D. Ferreira and J. K. Zjawiony, Bioorg. Med. Chem. Lett., 2013, 23, 2860–2862 CrossRef CAS PubMed.
- (a) A. P. Riley, V. W. Day, H. A. Navarro and T. E. Prisinzano, Org. Lett., 2013, 15, 5936–5939 CrossRef CAS PubMed; (b) M. K. Paude1, O. Shirota, K. Sasaki-Tabata, H. Tanaka, S. Sekita and S. Morimoto, J. Nat. Prod., 2013, 76, 1654–1660 CrossRef PubMed.
- M. I. Choudhary, M. Y. Mohamad, S. G. Musharraf, I. Onajobi, A. Mohammad, I. Anis, M. R. Shah and Atta-ur-Rahman, Phytochemistry, 2013, 90, 56–61 CrossRef PubMed.
- Y.-Y. Li, X.-L. Tang, T. Jiang, P.-F. Li, P.-L. Li and G.-Q. Li, J. Asian Nat. Prod. Res., 2013, 15, 941–949 CrossRef CAS PubMed.
- M. Zhou, H.-B. Zhang, W.-G. Wang, N.-B. Gong, R. Zhan, X.-N. Li, X. Du, L.-M. Li, Y. Li, J. Lu, J.-X. Pu and H. D. Sun, Org. Lett., 2013, 15, 4446–4449 CrossRef CAS PubMed.
- N. Liu, R.-X. Zhu, S. Wang, J.-Z. Zhang, Z.-M. Lin, R.-J. Li and H. X. Lou, Chem. Biodiversity, 2013, 10, 1606–1612 CAS.
- R.-J. Li, R.-X. Zhu, Y.-Y. Li, J.-C. Zhou, J.-Z. Zhang, S. Wang, J.-P. Ye, Y.-H. Wang, S. L. Morris-Natschke, K.-H. Lee and H.-X. Lou, J. Nat. Prod., 2013, 76, 1700–1708 CrossRef CAS PubMed.
- A. Sivasubramanian, K. K. G. Narasimha, R. Rathnasamy and A. M. F. Oliveira Campos, Nat. Prod. Res., 2013, 27, 1431–1436 CrossRef CAS PubMed.
- B. Wang, X.-L. Wang, S.-Q. Wang, T. Shen, Y.-Q. Liu, H. Yuan, H.-X. Lou and X.-N. Wang, J. Nat. Prod., 2013, 76, 1573–1579 CrossRef CAS PubMed.
- E. Bautista, R. A. Toscano, F. Calzada, E. Diaz, L. Yepez-Mulia and A. Ortega, J. Nat. Prod., 2013, 76, 1970–1975 CrossRef CAS PubMed.
- E. Bautista, R. A. Toscano and A. Ortega, Org. Lett., 2013, 15, 3210–3213 CrossRef CAS PubMed.
- G.-C. Wang, H. Zhang, H.-B. Liu and J.-M. Yue, Org. Lett., 2013, 15, 4880–4883 CrossRef CAS PubMed.
- H. Yin, J. G. Luo, S. M. Shan, X. B. Wang, J. Luo, M.-H. Yang and L.-Y. Kong, Org. Lett., 2013, 15, 1572–1575 CrossRef CAS PubMed.
- C. Li, B. Li, J. Ye, W. Zhang, Y. Shen and J. Yin, Nat. Prod. Res., 2013, 27, 1716–1721 CrossRef CAS PubMed.
- S. Wang, S. S. Liu, Z. M. Lin, R. J. Li, X. N. Wang, J. C. Zhou and H.-X. Lou, J. Asian Nat. Prod. Res., 2013, 15, 473–481 CrossRef CAS PubMed.
- N. Liu, R. J. Li, X.-N. Wang, R.-X. Zhu, L. Wang, Z.-M. Lin, Y. Zhao and H.-X. Lou, J. Nat. Prod., 2013, 76, 1647–1653 CrossRef CAS PubMed.
- S. Z. Huang, Q. Y. Ma, W. W. Fang, F. Q. Xu, H. Peng, H. F. Dai, J. Zhou and Y. X. Zhao, J. Asian Nat. Prod. Res., 2013, 15, 750–755 CrossRef CAS PubMed.
- M. G. Ponnapalli, M. Anikireddy, S. C. V. A. Rao Annam, S. Ravirala, S. Sukki and V. R. Tuniki, Tetrahedron Lett., 2013, 54, 2942–2945 CrossRef PubMed.
- A. Cimmino, A. Andolfi, M. C. Zonno, F. Avolio, A. Santini, A. Tuzi, A. Berestetskyi, M. Vurro and A. Evidente, J. Nat. Prod., 2013, 76, 1291–1297 CrossRef CAS PubMed.
- P. Xu, H. You, Z. Hu, Y. Li and Y. Shen, Chinese J. Org. Chem., 2013, 33, 2618–2621 CrossRef CAS.
- A. Cimmino, A. Andolfi, F. Avolio, A. Ali, N. Tabanca, I. A. Khan and A. Evidente, Chem. Biodiversity, 2013, 10, 1239–1251 CAS.
- E. Muňoz, J. G. Avila, J. Alarćon, I. Kubo, E. Werner and C. L. Céspedes, J. Agric. Food Chem., 2013, 61, 4336–4343 CrossRef PubMed.
- S. Z. Mu, C. R. Jiang, T. Huang and X. J. Hao, Helv. Chim. Acta, 2013, 96, 2299–2303 CrossRef CAS.
- J. Zi and R. J. Peters, Org. Biomol. Chem., 2013, 11, 7650–7652 CAS.
- J. Guo, Y. J. Zhou, M. L. Hillwig, Y. Shen, L. Yang, Y. Wang, X. Zhang, W. Liu, R. J. Petes, X. Chen, Z. K. Zhao and L. Huang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12108–12113 CrossRef CAS PubMed.
- S. Janocha, J. Zapp, M. Hutter, M. Kleser, J. Bohlmann and R. Bernhardt, ChemBioChem, 2013, 14, 467–473 CrossRef CAS PubMed.
- A. Venditti, A. M. Serrilli, S. Vittori, F. Papa, M. D. Cecca, G. Ciaschetti, M. Bruno, S. Roselli and A. Bianco, Chem. Biodiversity, 2013, 10, 2091–2100 CAS.
- J.-Y. Li, Y. Png, L.-Z. Li, P.-Y. Gao, C. Gao, S.-X. Xia and S.-J. Song, Helv. Chim. Acta, 2013, 96, 313–319 CrossRef CAS.
- Y. Inaba, T. Hasuda, Y. Hitotsuyanagi, Y. Aoyagi, N. Fujikawa, A. Onozaki, A. Watanabe, T. Kinoshita and K. Takeya, J. Nat. Prod., 2013, 76, 1085–1090 CrossRef CAS PubMed.
- S. Prawatsri, A. Suksamrarn, A. Chindaduang and T. Rukachaisirikul, Chem. Biodivsersity., 2013, 10, 1494–1500 CrossRef CAS PubMed.
- L. Yang, L. Qiao, C. Ji, D. Xie, N.-B. Gong, Y. Lu, J. Zhang, J. Dai and S. Guo, J. Nat. Prod., 2013, 76, 216–222 CrossRef CAS PubMed.
- Y.-D. Wang, G.-J. Zhang, J. Qu, Y.-H. Li, J.-D. Jiang, Y.-B. Liu, S.-G. Ma, Y. Li, H.-N. Lv and S.-S. Yu, J. Nat. Prod., 2013, 76, 1976–1983 CrossRef CAS PubMed.
- G.-J. Zhang, Y.-H. Li, J.-D. Jiang, S.-S. Yu, J. Qu, S.-G. Ma, Y.-B. Liu and D.-Q. Yu, Tetrahedron, 2013, 69, 1017–1023 CrossRef CAS PubMed.
- H.-B. Hu, X.-D. Zhang, H.-S. Hu and Y. Wu, Chem. Biodiversity, 2013, 10, 1623–1629 CAS.
- (a) E. Giacomelli, S. Bertrand, A. Nievergelt, V. Zwick, C. Simoes-Pires, L. Marcourt, E. Rivara-Minten, M. Caudet, A. Bisio and J.-L. Wolfender, Phytochemistry, 2013, 96, 257–264 CrossRef CAS PubMed; (b) Z. Habibi, S. Gandomkar, M. Yousefi and S. Ghasemi, Nat. Prod. Res., 2013, 27, 266–269 CrossRef CAS PubMed.
- H.-L. Jiang, X.-Z. Wang, J. Xiao, X.-H. Luo, X.-J. Yao, Y.-Y. Zhao, Y.-J. Chen, P. Crews and Q.-X. Wu, Tetrahedron, 2013, 69, 6687–6692 CrossRef CAS PubMed.
- D.-W. Zhang, X. Liu, D. Xie, R. Chen, S.-J. Tao, J.-H. Zou and J. Dai, Chem. Pharm. Bull., 2013, 61, 576–580 CrossRef CAS PubMed.
- L. Yang, S. Liu, H. Wang and Y. Suo, J. Chem. Res., 2013, 37, 28–29 CrossRef CAS.
- (a) W. Zhou, H. Xie, P. Wu and X. Wei, Food Chem., 2013, 136, 1110–1116 CrossRef CAS PubMed; (b) Y. Liang, H. Xie, P. Wu, Y. Jiang and X. Wei, Food Chem., 2013, 136, 1177–1182 CrossRef CAS PubMed.
- W.-X. Wang, J. Xiong, Y. Tang, J.-J. Zhu, M. Li, Y. Zhao, G.-X. Yang, G. Xia and J.-F. Hu, Phytochemistry, 2013, 89, 89–95 CrossRef CAS PubMed.
- X. Liu, R. Zhan, W.-G. Wang, X. Du, X.-N. Li, J.-H. Yang, P. Zhang, Y. Li, J.-X. Pu, J. Z. Wu and H. D. Sun, Chem. Pharm. Bull., 2013, 61, 90–95 CrossRef CAS PubMed.
- J.-H. Yang, X. Du, F. He, H.-B. Zhang, X.-N. Li, J. Su, Y. Li, J.-X. Pu and H.-D. Sun, J. Nat. Prod., 2013, 76, 256–264 CrossRef CAS PubMed.
- C. G. Magalhães, G. D. Fátima Silva, L. P. Duarte, I. L. Bazzocchi, A. J. Diaz, L. Moujir, M. P. Lopez, R. C. Figueiredo and S. A. Vieira Filho, Helv. Chim. Acta, 2013, 96, 1046–1054 CrossRef.
- S. Li, P. Wang, G. Deng, W. Yuan and Z. Su, Bioorg. Med. Chem. Lett., 2013, 23, 6682–6687 CrossRef CAS PubMed.
- W.-X. Wang, J.-J. Zhu, Y. Zou, Z.-L. Hong, S.-T. Liu, M. Li, Y. Huang, J. Xiong, Y. Zhao, G.-X. Yang, G. Xia and J.-F. Hu, Tetrahedron Lett., 2013, 54, 2549–2552 CrossRef CAS PubMed.
- I. Córdova-Guerrero, L. S. Andrés, A. E. Lea1-Orozco, J. M. Padrón, J. M. Cornejo-Bravo and F. Leon, Tetrahedron Lett., 2013, 54, 4479–4482 CrossRef PubMed.
- M. Ukiya, T. Kawaguchi, K. Ishii, E. Ogihara, Y. Tachi, M. Kurita, Y. Ezaki, M. Fukatsu, Y. Kushi and T. Akihisa, Chem. Biodiversity, 2013, 10, 1260–1268 CAS.
- X.-C. Huang, M. Wang, Y.-M. Pan, G.-Y. Yao, H. S. Wang, X.-Y. Tian, J.-K. Qin and Y. Zhang, Eur. J. Med. Chem., 2013, 67, 508–520 CrossRef PubMed.
- H.-C. Huang, M. Wang, Y.-M. Pan, X.-Y. Tain, H.-S. Wang and Y. Zhang, Bioorg. Med. Chem. Lett., 2013, 23, 5283–5289 CrossRef PubMed.
- Z. Zhou, Z.-X. Lin, D. Liang and J.-Q. Hu, Tetrahedron, 2013, 69, 43–49 CrossRef CAS PubMed.
- G.-Y. Yao, M.-Y. Ye, R.-Z. Huang, Y.-J. Li, Y.-T. Zhu, Y.-M. Pan, Z.-X. Liao and H. S. Wang, Bioorg. Med. Chem. Lett., 2013, 23, 6755–6758 CrossRef CAS PubMed.
- O. Burmistrova, M. F. Simões, P. Rijo, J. Quintana, J. Bermejo and F. Estevez, J. Nat. Prod., 2013, 76, 1413–1423 CrossRef CAS PubMed.
- M. J. Hatfield, L. G. Tsurkan, J. L. Hyatt, C. C. Edwards, A. Lemoff, C. Jeffries, B. Yan and P. M. Potter, J. Nat. Prod., 2013, 76, 36–44 CrossRef CAS PubMed.
- I. Khan, M. Nisar, A. Zarrelli, G. D. Fabio, F. Gul, S. N. Gilani, M. R. Shaah, A. Z. Khai and H. Amin, Med. Chem. Res., 2013, 22, 5809–5813 CrossRef CAS.
- H. Gao, S. Chen, X. Rao, S. Shang and Z. Song, Bioorg. Med. Chem. Lett., 2013, 23, 2254–2259 CrossRef CAS PubMed.
- A. Obase, A. Kageyama, Y. Manabe, T. Ozawa, T. Araki, H. Yokoe, M. Kanematsu, M. Yoshida and K. Shishido, Org. Lett., 2013, 15, 3666–3669 CrossRef CAS PubMed.
- G. Ma, J. Yuan, H. Wu, L. Cao, X. Zhang, L. Xu, H. Wei, L. Wu, Q. Zheng, L. Li, J. Yang and X. Xu, J. Nat. Prod., 2013, 76, 1025–1031 CrossRef CAS PubMed.
- G. Ma, J.-Q. Yuan, L. Cao, J.-S. Yang and X.-D. Xu, Nat. Prod. Res., 2013, 27, 818–823 CrossRef CAS PubMed.
- Q.-J. Tian, Y.-H. Ou, X.-B. He, B. Liu and Y.-D. Jiang, Nat. Prod. Res., 2013, 27, 537–540 CrossRef CAS PubMed.
- Y. Zheng, S.-W. Zhang, H.-J. Cong, Y.-J. Huang and L.-J. Xuan, J. Nat. Prod., 2013, 76, 2210–2218 CrossRef CAS PubMed.
- C. O. Ochieng, L. A. O. Manguro, P. O. Owuor and H. Akala, Bioorg. Med. Chem. Lett., 2013, 23, 3088–3095 CrossRef CAS PubMed.
- J. Zhang, W. M. Abdel-Mageed, M. Liu, P. Huang, W. He, L. Li, F. Song, H. Dai, X. Liu, J. Liang and L. Zhang, Org. Lett., 2013, 15, 4726–4729 CrossRef CAS PubMed.
- J. J. Manríquez-Torres, J. M. Torres-Valencia, R. Velázquez-Jiménez, A. Valdez-Calderón, J. G. Alvarado-Rodríguez, C. M. García-Rojas and P. Joseph-Nathan, Org. Lett., 2013, 15, 4658–4661 CrossRef PubMed.
- K. Ravindar, P.-Y. Caron and P. Deslongchamps, Org. Lett., 2013, 15, 6270–6273 CrossRef CAS PubMed.
- B. Yang, G.-Y. Chen, X.-P. Song, L.-Q. Yang, X.-Y. Wu, C.-R. Han, W.-H. Chen, B.-Y. Zou and X.-M. Li, Bioorg. Med. Chem. Lett., 2013, 23, 5748–5751 CrossRef CAS PubMed.
- J.-Q. Zhao, J.-J. Lv, Y.-M. Wang, M. Xu, H.-T. Zhu, D. Wang, C.-R. Yang, Y.-F. Wang and Y.-J. Zhang, Tetrahedron Lett., 2013, 54, 4670–4674 CrossRef CAS PubMed.
- L.-Y. Wang, J. Wu, Z. Yang, X.-J. Wang, Y. Fu, S.-Z. Liu, H.-M. Wang, W.-L. Zhu, H.-Y. Zhang and W.-M. Zhao, J. Nat. Prod., 2013, 76, 745–749 CrossRef CAS PubMed.
- P. Seephonkai, S. G. Pyne, A. C. Willis and W. Lie, Tetrahedron Lett., 2013, 54, 2085–2088 CrossRef CAS PubMed.
- X.-D. Wu, J. He, X.-Y. Li, L.-B. Dong, X. Gong, L.-D. Song, Y. Li, L.-Y. Peng and Q.-S. Zhao, J. Nat. Prod., 2013, 76, 1032–1038 CrossRef CAS PubMed.
- Z.-Y. Jiang, C.-G. Huang, H.-B. Xiong, K. Tian, W.-X. Liu, Q.-F. Hu, H.-B. Wang, G.-Y. Yang and X.-Z. Huang, Tetrahedron Lett., 2013, 54, 3886–3888 CrossRef CAS PubMed.
- A.-W. Salae and N. Boonnak, Tetrahedron Lett., 2013, 54, 1356–1359 CrossRef CAS PubMed.
- F. de Jesus Cortez, D. Lapointe, A. M. Hamlin, E. M. Simmons and R. Sarpong, Tetrahedron, 2013, 69, 5665–5676 CrossRef PubMed.
- C. Thommen, C. K. Jana, M. Neuburger and K. Gademann, Org. Lett., 2013, 15, 1390–1393 CrossRef CAS PubMed.
- J. Deng, R. Li, Y. Luo, J. Li, S. Zhou, Y. Li, J. Hu and A. Li, Org. Lett., 2013, 15, 2022–2025 CrossRef CAS PubMed.
- M. Ozeki, M. Satake, T. Toizume, S. Fukutome, K. Arimitsu, S. Hosoi, T. Kajimoto, H. Iwasaki, N. Kojima, M. Node and M. Yamashita, Tetrahedron, 2013, 69, 3841–3846 CrossRef CAS PubMed.
- S. Ceunen and J. M. G. Guens, J. Nat. Prod., 2013, 76, 1201–1228 CrossRef CAS PubMed.
- C. Lohoelter, M. Weckbecker and S. R. Waldvoge1, Eur. J. Org. Chem., 2013, 5539–5554 CrossRef CAS.
- (a) P. Montoro, I. Molfetta, M. Maldini, L. Ceccarini, S. Piacente, C. Pizza and M. Macchia, Food Chem., 2013, 141, 745–753 CrossRef CAS PubMed; (b) S. Ceunen and J. M. C. Geuns, Phytochemistry, 2013, 89, 32–38 CrossRef CAS PubMed.
- S. Li, W. Li, Q.-Y. Xiao and Y. Xia, Food Chem., 2013, 138, 2064–2069 CrossRef CAS PubMed.
- O. E. Hutt, T. L. Doan and G. I. Georg, Org. Lett., 2013, 15, 1602–1605 CrossRef CAS PubMed.
- O. Wonganan, C. Tocharus, C. Puedsing, S. Homvisasevongsa, O. Sukcharoen and A. Suksamrarn, Eur. J. Med. Chem., 2013, 62, 771–776 CrossRef CAS PubMed.
- M. Ukiya, S. Sawada, T. Kikuchi, Y. Kushi, M. Fukatsu and T. Akihisa, Chem. Biodiversity, 2013, 10, 177–188 CAS.
- S.-L. Zhu, Y. Wu, C.-J. Liu, C.-Y. Wei, J.-C. Tao and H.-M. Liu, Eur. J. Med. Chem., 2013, 65, 70–82 CrossRef CAS PubMed.
- S.-L. Zhu, Y. Wu, C.-J. Liu, C.-Y. Wei, J.-C. Tao and H.-M. Liu, Bioorg. Med. Chem. Lett., 2013, 23, 1343–1346 CrossRef CAS PubMed.
- S. K. V. Bertolucci, A. B. D. Pereira, J. E. B. P. Pinto, A. B. Olivera and F. C. Braga, Chem. Biodiversity, 2013, 10, 288–295 CAS.
- W. M. Alarif, A. Abdel-Lateff, A. M. Al-Abd, S. A. Basaif, F. A. Badria, M. Shams and S. N. Ayyad, Eur. J. Med. Chem., 2013, 66, 204–210 CrossRef CAS PubMed.
- C.-X. Zhou, L.-R. Sun, F. Feng, J.-X. Mo, H. Zhu, B. Yang, Q.-J. He and L.-S. Gan, Helv. Chim. Acta, 2013, 96, 656–662 CrossRef CAS.
- H. Zheng, Q. Chen, M. Zhang, Y. Lai, L. Lei, P. Shu, J. Zhang, Y. Xue, Z. Luo, Y. Li, G. Yao and Y. Zhang, J. Nat. Prod., 2013, 76, 2253–2262 CrossRef CAS PubMed.
- R. Batista, P. A. García, M. A. Castro, J. M. Migue1 del Corral, N. L. Speziali, F. de P. Varotti, R. C. de Paula, L. F. García-Fernández, A. Francesch, A. San Feliciano and A. B. de Oliveira, Eur. J. Med. Chem., 2013, 62, 168–176 CrossRef CAS PubMed.
- (a) X. Deng, Y. Shen, J. Yang, J. He, Y. Zhao, L.-Y. Peng, Y. Leng and Q.-S. Zhao, Eur. J. Med. Chem., 2013, 65, 403–414 CrossRef CAS PubMed; (b) E. González-Burgos, M. E. Carretero and M. P. Gómez-Serranillos, Phytochemistry, 2013, 93, 116–123 CrossRef PubMed.
- P.-C. Kuo, M.-L. Yang, T.-L. Hwang, Y.-Y. Lai, Y.-C. Li, T. D. Thang and T.-S. Wu, J. Nat. Prod., 2013, 76, 230–236 CrossRef CAS PubMed.
- R. Bai, Y. Zhou, S. Deng, P.-P. Dong, B. J. Zhang, S. S. Huang, C. Y. Wang, H.-L. Zhang, Y.-Y. Zhao, L. Wang and X.-C. Ma, J. Asian Nat. Prod. Res., 2013, 15, 1107–1111 CrossRef CAS PubMed.
- X. Pang, L.-P. Kang, H.-S. Yu, Y. Zhao, C.-Q. Xiong, J. Zhang and B.-P. Ma, Nat. Prod. Res., 2013, 27, 1202–1207 CrossRef CAS PubMed.
- W. Liu, B.-L. Fan, H.-Y. Guo, Y.-L. Yuan, H.-J. Liang and S.-P. Bai, Nat. Prod. Res., 2013, 27, 1388–1392 CrossRef CAS PubMed.
- Y.-H. Gao, Z.-X. Wei, Y. Cheng, T.-H. Wang, L. Ni, X.-H. Zhou and L.-Y. Zhang, Chem. Biodiversity, 2013, 10, 1487–1493 CAS.
- R. Zhan, X.-N. Li, X. Du, W.-G. Wang, K. Dong, J. Su, Y. Li, J.-X. Pu and H.-D. Sun, J. Nat. Prod., 2013, 76, 1267–1277 CrossRef CAS PubMed.
- H.-Y. Jiang, W.-G. Wang, M. Zhou, H.-Y. Wu, R. Zhan, X.-N. Li, X. Du, Y. Li, J.-X. Pu and H.-D. Sun, J. Nat. Prod., 2013, 76, 2113–2119 CrossRef CAS PubMed.
- L. Lin, D. Zhu, L. Zou, B. Yang and M. Zhao, Food Chem., 2013, 139, 902–909 CrossRef CAS PubMed.
- D. Li, S. Xu, H. Cai, L. Pei, H. Zhang, L. Wang, H. Yao, X. Wu and J. Xu, Eur. J. Med. Chem., 2013, 59, 215–221 CrossRef PubMed , 322–328.
- C. Ding, Y. Zhang, H. Chen, C. Wild, T. Wang, M. A. White, Q. Shen and J. Zhou, Org. Lett., 2013, 15, 3718–3721 CrossRef CAS PubMed.
- E. C. Cherney, J. C. Green and P. S. Baran, Angew. Chem., Int. Ed., 2013, 52, 9019–9022 CrossRef CAS PubMed.
- P. Lu, Z. Gu and A. Zakarian, J. Am. Chem. Soc., 2013, 135, 14552–14555 CrossRef CAS PubMed.
- N. Ungur, V. Kulcitki, O. Chetraru, M. Grinco and P. F. Vlad, Helv. Chim. Acta, 2013, 96, 864–871 CrossRef CAS.
- B. M. Fraga, V. González-Vallejo, C. Bressa, R. Guillermo and S. Suárez, Tetrahedron, 2013, 69, 3002–3012 CrossRef CAS PubMed.
- M. J. P. Lange, A. Liebrandt, L. Arnold, S.-M. Chmielewska, A. Felsberger, E. Freier, M. Heuer, D. Zur and T. Lange, Phytochemistry, 2013, 90, 62–69 CrossRef PubMed.
- B. M. Fraga, V. González-Vallejo, R. Guillermo and J. M. Amaro-Luis, Phytochemistry, 2013, 89, 39–46 CrossRef CAS PubMed.
- (a) T. Urbanová, D. Tarkowská, O. Novák, P. Heddon and M. Stand, Talanta, 2013, 112, 85–94 CrossRef PubMed; (b) S. Liu, W. Chen, L. Qu, Y. Gai and X. Jiang, Anal. Bioanal. Chem., 2013, 405, 1257–1266 CrossRef CAS PubMed.
- K. H. Kim, S. U. Choi, M. W. Son, S. Z. Choi, J. Clardy and K. R. Lee, J. Nat. Prod., 2013, 76, 1376–1379 CrossRef CAS PubMed.
- M. Zhang, Y. Zhu, G. Zhan, P. Shu, R. Sa, L. Lei, M. Xiang, Y. Xue, Z. Luo, Q. Wan, G. Yao and Y. Zhang, Org. Lett., 2013, 15, 3094–3097 CrossRef CAS PubMed.
- W.-G. Wang, Z.-Y. Wu, R. Chen, H.-Z. Li, H.-M. Li, Y.-D. Li, R.-T. Li and H.-R. Luo, Chem. Biodiversity, 2013, 10, 1061–1071 CAS.
- Y.-P. Li, X.-N. Li, L.-H. Gao, H.-Z. Li, G.-X. Wu and R.-T. Li, J. Agric. Food Chem., 2013, 61, 7219–7224 CrossRef CAS PubMed.
- Y. Li, Y.-B. Liu, J.-J. Zhang, Y.-H. Li, J.-D. Jiang, S.-S. Yu, S.-G. Ma, J. Qu and H.-N. Lv, Org. Lett., 2013, 15, 3074–3077 CrossRef CAS PubMed.
- A. Meguroa, T. Tomita, M. Nishiyama and T. Kuzuyama, ChemBioChem, 2013, 14, 316–321 CrossRef PubMed.
- J. Arens, B. Engels, S. Klopries, S. Jennewein, C. Ottmann and F. Schulz, Chem. Commun., 2013, 49, 4337–4339 RSC.
- M. Todorova, A. Trendafilova, N. Javsmaa, S. Altansetseg and S. Shatar, J. Asian Nat. Prod. Res., 2013, 15, 1060–1063 CrossRef CAS PubMed.
- (a) A. Saitman, S. D. E. Sullivan and E. A. Theodorakis, Tetrahedron Lett., 2013, 54, 1612–1615 CrossRef CAS PubMed; (b) A. Saitman and E. A. Theodorakis, Org. Lett., 2013, 15, 2410–2413 CrossRef CAS PubMed.
- W.-W. Tao, J.-A. Duan, Y.-P. Tang, N.-Y. Yang, J.-P. Li and Y.-F. Qian, Phytochemistry, 2013, 94, 249–253 CrossRef CAS PubMed.
- Y. Li, F. Qin, S.-M. Wang, R.-X. Guo, Y.-F. Zhang, Y. C. Gu and Q.-W. Shi, Chem. Biodiversity, 2013, 10, 1729–1753 CAS.
- S. Hirai, N. Urushizako, M. Miyano, T. Fujii and M. Nakada, Tetrahedron Lett., 2013, 54, 1888–1892 CrossRef CAS PubMed.
- C. W. Sabandar, N. Ahmat, F. M. Jaafar and I. Sahidin, Phytochemistry, 2013, 85, 7–29 CrossRef CAS PubMed.
- (a) B.-B. Zhang, Q. Jiang, Z.-X. Liao, C. Liu, S.-J. Liu, L.-J. Ji and H.-F. Sun, Chem. Biodiversity, 2013, 10, 1887–1893 CrossRef CAS PubMed; (b) Y. Tian, W. Xu, C. Zhu, S. Lin, Y. Guo and J. Shi, J. Nat. Prod., 2013, 76, 1039–1046 CrossRef CAS PubMed.
- (a) M. Jadranin, M. Pešić, I. S. Aljančić, S. M. Milosović, N. M. Todorović, A. Podolski-Renić, J. Banković, N. Tanić, I. Marković, V. E. Vajs and V. V. Tešić, Phytochemistry, 2013, 86, 208–217 CrossRef CAS PubMed; (b) D. S. Yang, Y.-L. Zhang, W.-B. Peng, L.-Y. Wang, Z.-L. Li, X. Wang, K.-C. Liu, Y.-P. Yang, H.-L. Li and X.-L. Li, J. Mater. Prod., 2013, 76, 265–269 CAS.
- S. Kawakami, H. Toyoda, L. Harinantenaina, K. Matsunami, H. Otsuka, T. Shinzato, Y. Takeda, M. Kawahata and K. Yamaguchi, Chem. Pharm. Bull., 2013, 61, 411–418 CrossRef CAS PubMed.
- X.-L. Zhang, L. Wang, F. Li, K. Yu and M.-K. Wang, J. Nat. Prod., 2013, 76, 858–864 CrossRef CAS PubMed.
- Y. Asada, A. Sukemori, T. Watanabe, K. J. Malla, T. Yoshikawa, W. Li, X. Kuang, K. Koike, C.-H. Chen, T. Akiyama, K. Qian, K. Nakagawa-Goto, S. L. Morris-Natschke, Y. Lu and K.-H. Lee, J. Nat. Prod., 2013, 76, 852–857 CrossRef CAS PubMed.
- R. Wang, J.-Y. Li, H.-Y. Qi and Y.-P. Shi, J. Asian Nat. Prod. Res., 2023, 15, 502–506 CrossRef PubMed.
- F. Li, Q. Sun, L. Hong, L. Li, Y. Wu, M. Xia, T. Ikejima, Y. Peng and S. Song, Bioorg. Med. Chem. Lett., 2013, 23, 2500–2504 CrossRef CAS PubMed.
- K. K. Bang, C.-Y. Yun, C. Lee, Q. Jin, J. W. Lee, S.-H. Jung, D. Lee, M. K. Lee, J. T. Hong, Y. Kim and B. Y. Hwang, Bioorg. Med. Chem. Lett., 2013, 23, 3334–3337 CrossRef CAS PubMed.
- X.-D. Wu, L.-C. Zhang, J. He, G.-T. Li, L.-F. Ding, X. Gao, L.-B. Dong, L.-D. Song, Y. Li and Q.-S. Zhao, J. Asian Nat. Prod. Res., 2013, 15, 151–157 CrossRef CAS PubMed.
- S. M. Ghanadian, A. M. Ayatollahi, M. A. Mesaik and O. M. Abdalla, Nat. Prod. Res., 2013, 27, 246–254 CrossRef CAS PubMed.
- H.-B. Wang, L.-P. Liu and X.-Y. Wang, Magn. Reson. Chem., 2013, 51, 580–592 CrossRef CAS PubMed.
- R. K. Devappa, C. C. Malakar, H. P. S. Makkar and K. Becker, Nat. Prod. Res., 2013, 27, 1459–1462 CrossRef CAS PubMed.
- X. Liang, G. Grue-Sørensen, K. Månsson, P. Vedsø, A. Soor, M. Stahlhut, M. Bertelsen, K. M. Engell and T. Högberg, Bioorg. Med. Chem. Lett., 2013, 23, 5624–5629 CrossRef CAS PubMed.
- A. J. Catino, A. Sherlock, P. Shieh, J. S. Wzorek and D. A. Evans, Org. Lett., 2013, 15, 3330–3333 CrossRef CAS PubMed.
- H. Takekawa, K. Tanaka, E. Fukushi, K. Matsuo, T. Nehira and M. Hashimoto, J. Nat. Prod., 2013, 76, 1047–1051 CrossRef CAS PubMed.
- C. Wang, D. Wang and S. Gao, Org. Lett., 2013, 15, 4402–4405 CrossRef CAS PubMed.
- Y.-G. Wang, J. Ren, A.-G. Wang, J.-B. Yang, T.-F. Li, Q.-G. Ma, J. Tian and Y.-L. Su, J. Nat. Prod., 2013, 76, 2074–2079 CrossRef CAS PubMed.
- J. M. H. Lu, M. V. Perkins and H. J. Griesser, Tetrahedron, 2013, 69, 6468–6473 CrossRef CAS PubMed.
- B. Yang, G.-Y. Chen, X.-P. Song, L.-Q. Yang, C.-R. Han, X.-Y. Wu, C.-J. Zheng, X. Ran and R.-F. Tang, Tetrahedron Lett., 2013, 54, 6434–6436 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |