Recent progress in magnetic iron oxide–semiconductor composite nanomaterials as promising photocatalysts
Wei
Wu
*ab,
Changzhong Jiang
c and
Vellaisamy A. L.
Roy
*b
aLaboratory of Printable Functional Nanomaterials and Printed Electronics, School of Printing and Packaging, Wuhan University, Wuhan 430072, P. R. China. E-mail: weiwu@whu.edu.cn; val.roy@cityu.edu.hk
bDepartment of Physics and Materials Science, City University of Hong Kong, Hong Kong SAR, P. R. China
cKey Laboratory of Artificial Micro- and Nano-structures of Ministry of Education, School of Physics and Technology, Wuhan University, Wuhan 430072, P. R. China
First published on 30th October 2014
Abstract
Photocatalytic degradation of toxic organic pollutants is a challenging tasks in ecological and environmental protection. Recent research shows that the magnetic iron oxide–semiconductor composite photocatalytic system can effectively break through the bottleneck of single-component semiconductor oxides with low activity under visible light and the challenging recycling of the photocatalyst from the final products. With high reactivity in visible light, magnetic iron oxide–semiconductors can be exploited as an important magnetic recovery photocatalyst (MRP) with a bright future. On this regard, various composite structures, the charge-transfer mechanism and outstanding properties of magnetic iron oxide–semiconductor composite nanomaterials are sketched. The latest synthesis methods and recent progress in the photocatalytic applications of magnetic iron oxide–semiconductor composite nanomaterials are reviewed. The problems and challenges still need to be resolved and development strategies are discussed.
1 Introduction
Our surrounding environment continues to become more polluted, and the traditional chemical methods that deal with environmental pollution have been unable to meet the requirements of modern energy-saving themes and environmental protection. Environmental problems induced by toxic and hardly-degradable organic pollutants (such as halides, dioxins, pesticides, dyes, etc.) have posed a grave menace to human well-being and development in the 21st century. Photocatalysis refers to the rate of photoreactions (oxidation/reduction) brought on by the activation of a catalyst, usually a semiconductor oxide, through illumination under ultraviolet (UV) or visible light. Use of semiconductor oxide nanomaterials-based photocatalysts to degrade organic pollutants is recognized as one of the most promising areas of research and application.1–3 On this regard, photocatalysts are regularly used in solid–liquid reaction systems, especially for the treatment of toxic waste.However, the main restriction factor of large scale practical applications of semiconductor oxide photocatalysts are as follows: (1) high recombination rate of electronic–hole pairs resulting in low quantum yield for single-component semiconductor oxide photocatalysts. For instance, Sun and Bolton have reported less than 5% primary quantum yield of ˙OH radical generation in a TiO2 suspension.4 (2) The limitation in the harvesting of visible light. Generally, wide bandgap semiconductor oxides are employed as photocatalysts, for example, the bandgap value of anatase TiO2 is 3.2 eV, and the corresponding absorption wavelength is 387.5 nm, resulting in limited light absorption of the UV region. Unfortunately the solar spectrum consists of only 5–7% of UV light, while 46% and 47% of the spectrum consists of visible light and infrared radiation, respectively.5 (3) Poor selective adsorption and the complexity of intermediate products. For example, photodegradation reaction products such as CO2 and H2O are easily adsorbed on the surface of TiO2 in the gas–solid photocatalyst system due to its super-hydrophilicity and active sites.6 The high oxidization potential energy of OH radicals can induce many backward reactions, such as oxidizing the intermediates and products converted from the as-adsorbed CO2.7,8 (4) The photocatalytic treatment of a high concentration of organic pollutants from industrial waste poisons the photocatalyst resulting in deactivation. In addition, it is difficult to separate a pure semiconductor oxide photocatalyst from the waste water treating system,9 which further deactivates the photocatalysts. (5) High cost of photocatalyst industrialization. This factor limits the industrial applications of photocatalysts, hence research and development of low-cost, high-performance, and recyclable photocatalysts have became an important issue.10,11
As a hot issue, photocatalysis has witnessed a sea of change over the past two decades with significant advancements being made in the preparation of novel materials and nanostructures, and the design of efficient processes for the photodegradation of pollutants and the generation of energy. Thus, the development of a simple recyclable photocatalyst can not only prevent excessive use of photocatalysts, but also the recovery of deactivated photocatalysts, thereby reducing the total cost, and further lowering the overall usage of the photocatalytic material. Since visible light constitutes a large fraction of solar energy, one of the great challenges of photocatalyst study is to devise new catalysts that exhibit high activity under illumination by visible light.
Combining the magnetic iron oxide nanomaterials with semiconductor nanomaterials to form a magnetic iron oxide–semiconductor composite photocatalyst system becomes a simple and effective method. In an iron oxide–semiconductor system, iron oxide has many advantages, for example, low cost, high stability and compatibility, it not only plays the role of separating the photocatalyst from the solution, but also it can degrade organic pollutants.12,13 The common metal oxide semiconductors like titanium dioxide (TiO2), zinc oxide (ZnO), tungsten oxide (WO3), and tin oxide (SnO2) are proven to be dynamic photocatalysts for organic dyes and pollutants, these semiconductors not only destroy the conjugated chromophoric system, but also breakdown the molecular structure of the organic dyes and pollutants into harmless CO2 and H2O. As magnetic recovery photocatalytic materials, the iron oxide–semiconductor oxide photocatalyst system can effectively break through the bottleneck of low activity under visible light and demanding recycling processes from the products, and eventually become a potential visible light responsive MRPs in the future.
Therefore, the design of an iron oxide–semiconductor photocatalytic system is an essential prerequisite for both basic and applied research. If the system focuses on the magnetic recovery properties, the saturation magnetization value of used iron oxides should be no less than 1 emu g−1, in order to separate via an external magnetic field for further reusing and regeneration. If the system focuses on the photocatalytic performance, the used iron oxides should possess a relatively narrow bandgap value. For instance, goethite and hematite are often studied as photocatalysts in recent years because of their low band gap (2.2 eV). There are reported techniques to improve the photocatalytic performance of an iron oxide–semiconductor system, such as a composite heterostructure with a narrow/wide bandgap, p–n heterojunctions, noble metal loading, plasmonic structure, graphene loading, etc.14–16 Overall, an optimal iron oxide–semiconductor photocatalytic system design should meet the following requirements. First, the synthesis and preparation process should be both simple, and facile with high-yield. Second, the composite system should exhibit an enhanced photocatalytic performance remarkably superior to existing naked iron oxide and pure semiconductor materials. Third, the compositephotocatalyst should be recycled by the external magnetic field that facilitates easy reuse and regeneration. Finally, the composite photocatalyst should possess good photocorrosion resistance ability and be stable at room temperature for months.
In this review, we will first describe the structure and mechanism of a magnetic iron oxide–semiconductor photocatalysis system. We discuss different synthesis methods and recent advances in magnetic iron oxide–semiconductor nanomaterials. The potential of magnetic iron oxide–semiconductor based materials as photocatalysts is also examined. Finally, we discuss future prospects in realizing this technology and further research directions.
2 Structure and mechanism for magnetic iron oxide–semiconductor composite photocatalysts
2.1 Magnetic iron oxide nanomaterials
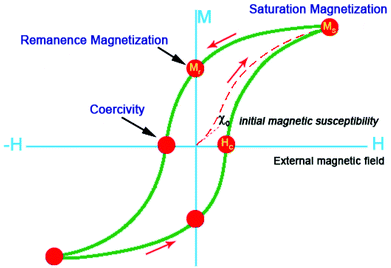
As a common compound, iron oxide is widely distributed in nature and can be synthesized on a large-scale. The application of small iron oxide nanoparticles has been practised in in vitro diagnostics for more than 60 years.17 Over the past few decades, magnetic iron oxide nanoparticles with various morphologies and structures are widely fabricated because of their importance in basic research. On the other hand, magnetic iron oxides are of great interest for researchers due to wide range of applications, including pigments, magnetic fluids, catalysis, targeted drug delivery, biosensor, magnetic resonance imaging, data storage, and environmental remediation.13,18,19 Iron oxides are composed of Fe together with O. There are eight iron oxides known.20 Among the iron oxides, hematite (α-Fe2O3), magnetite (Fe3O4) and maghemite (γ-Fe2O3) are promising and popular candidates due to their polymorphism involving temperature induced phase transition. These three different crystalline iron oxides have unique biochemical, magnetic, catalytic, and other properties that make them suitable for specific technical and biomedical applications.Magnetic measurements of α-Fe2O3 show obvious weak ferromagnetism and its saturation magnetization is often less than 1 emu g−1 at room temperature. However, γ-Fe2O3 and Fe3O4 show saturation magnetization values up to 92 emu g−1.21 More importantly, the magnetic properties of iron oxide nanoparticles are related to their size and shape. For example, Demortière and co-workers have investigated the size-dependence of iron oxide nanocrystals on their structural and magnetic properties by fine tuning the size within the nanometer scale (diameters range from 2.5 to 14 nm). The evolution of magnetic behavior with nanoparticle size clearly emphasizes the influence of the surface, especially on the saturation magnetization (Ms) and the magneto-crystalline anisotropy. Dipole interactions and thermal dependence have also been taken into account in the study of nanoscale size-effects on magnetic properties.22 More recently, we have reported a comparative study on the magnetic behavior of single and tubular clustered Fe3O4 nanoparticles. The results reveal that the coercivity of small iron oxide nanoparticles could be enhanced by the competition between the demagnetization energy of the morphology and magneto-crystalline anisotropy energy.23 Choi and co-workers have prepared Fe3O4 nanoparticles with different shapes, including solid nanospheres and solid/hollow nanoellipsoids. All these structures were obtained by either adding the appropriate amount of sodium acetate (NaOAc) or using the anion exchange of β-FeOOH. All magnetite nanoparticles exhibited ferromagnetic behaviour with different values for the saturation magnetization (Ms) and coercivity (Hc), and these values were highly dependent on the shape due to their grain size, spin disorder, shape, and surface anisotropy.24 In general, iron oxide nanoparticles become superparamagnetic at room temperature when the size of the iron oxide nanoparticles are below ca. 15 nm, meaning that the thermal energy can overcome the anisotropy energy barrier of a single nanoparticle. If the semiconductor is coated on the surface of iron oxide, the Ms value would decrease. There are a number of magnetic properties for the characterization of naked iron oxides nanoparticles and iron oxide–semiconductor composite nanomaterials. The most important properties are the type and magnetization which can be determined from the hysteresis loops (M–H) and zero-field cooled/field cooled (ZFC/FC, M–T) curves. As shown in Fig. 1, the saturation magnetization (MS), remanence magnetization (Mr), and coercivity (HC) can be obtained from the hysteresis loop. When the naked iron oxide nanoparticles and iron oxide–semiconductor composite nanomaterials exhibit superparamagnetic, the M–H curve should show no hysteresis at a certain temperature (T > TB, blocking temperature). The forward and backward magnetization curves overlap completely.23,25
The general strategy for preparing magnetic iron oxide nanoparticles in solution is to separate the nucleation and growth of nanocrystals. Numerous synthetic methods have been developed to synthesize magnetic iron oxide NPs, including co-precipitation,26–28 high-temperature thermal decomposition,29–31 hydrothermal and solvothermal reaction,32–34 sol–gel reactions and polyol method,25,35,36 microemulsion synthesis,37–39 sonochemical reaction,40–44 microwave-assisted synthesis45–48 and biosynthesis.49–51 Other than the above-mentioned methods, alternative chemical or physical methods can also be used to synthesize magnetic iron oxide nanoparticles, such as the electrochemical methods,52–54 flow injection synthesis,55 and aerosol/vapor methods,56–58etc. In literature, there are many reports on the fabrication of magnetic iron oxide NPs. Here, we briefly review the recent advances on the synthesis of magnetic iron oxide–semiconductor composite nanomaterials.
2.2 Semiconductor nanomaterials
Semiconductor oxides (e.g., TiO2, ZrO2, ZnO, WO3, MoO3, SnO2, α-Fe2O3, etc.) and semiconductor sulfides (e.g., ZnS, CdS, CdSe, WS2, MoS2, etc.) can be used as catalysts for photoinduced chemical reactions due to their intrinsic electronic structure that consists of a filled valence band (VB) and an empty conduction band (CB).59–64 When a photon with energy (hv) matches or exceeds the band gap energy (Eg) of the semiconductor, a photogenerated electron (e−) in the valence band is excited into the conduction band, leaving a positive hole (h+) in the valence band. The photoinduced charge carriers play a key role in the photocatalytic degradation process. The holes mediate the oxidation of organic compounds through the formation of hydroxyl radicals (˙OH), and the electrons mediate redox reactions through the formation of superoxide radicals (˙O2). However, the photoinduced charge carriers in the excited states are unstable and can easily recombine, converting the input energy to heat and thus leading to the low activity of a photocatalyst.65 An ideal photocatalyst should be stable, inexpensive, non-toxic and, of course, highly photoactive.On the basis of thermodynamic requirements, the VB and CB of the semiconductor photocatalyst should be located in such a way that the oxidation potential of the hydroxyl radicals (E0(H2O/˙OH) = 2.8 V vs. NHE) and the reduction potential of superoxide radicals (E0(O2/˙O2−) = −0.28 V vs. NHE), lie well within the band gap. In other words, the redox potential of the VB holes must be sufficiently positive to produce hydroxyl radicals. On the other hand, the CB electrons must be sufficiently negative to produce superoxide radicals.66Fig. 2 shows the bandgap energy and band edge positions of common semiconductor oxides and semiconductor sulfides, along with selected redox potentials. Obviously, the bandgap energy and band edge positions of TiO2, ZnO, SnO2, Fe2O3, WO3 and ZrO2 are relatively good. As already mentioned, such semiconductor materials are prone to be applied in photocatalysis due to their inherently filled VB and empty CB. When these semiconducting solids absorb photons and hv ≥ Eg, an e− is excited from the VB to the CB. This can be expressed in the following formula: hv + semiconductor → h+ + e−. Then the electron of the semiconductor can be transferred to an adjacent compound.
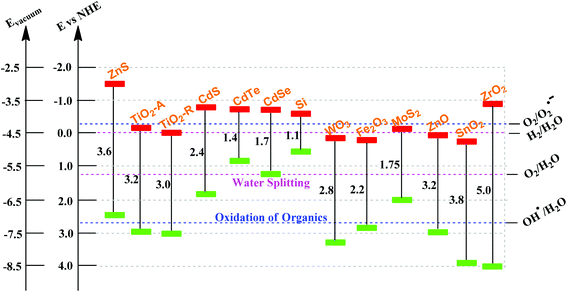 | ||
| Fig. 2 Band gap energy, VB and CB for a range of semiconductors on a potential scale (V) versus the normal hydrogen electrode (NHE). | ||
Additionally, the choice of semiconductor materials for photocatalytic applications rely on the consideration of photocorrosion resistance ability. For instance, CdS and ZnO only have a stable valence of +2, and can be decomposed by photogenerated holes from the VB. Furthermore, ZnO is prone to be deactivated due to the generation of Zn(OH)2 on its surface.10 Currently, there are many methods to inhibit or delay the deactivation caused by photocorrosion, and the common method is to combine with other materials and to form composite nanomaterials.67,68 As compared to other materials, the oxidation state of Ti in TiO2 can be reversibly changed (from +4 and +3), thereby TiO2 is more stable and suitable for photocatalytic applications. Additionally, the anatase phase TiO2 (Eg = 3.2 eV) is more active for photocatalysis applications, even though the rutile phase TiO2 (Eg = 3.0 eV) possesses a smaller band gap, revealing the possibility of absorption of long wavelength radiation. The CB of anatase TiO2 is more negative compared to rutile.
In addition to the aforementioned factors, other requirements such as low-cost, a non-toxic nature (environmentally benign) and easy preparation should also be taken into consideration for photocatalytic degradation reactions.
2.3 The structure of iron oxide–semiconductor composite nanomaterials
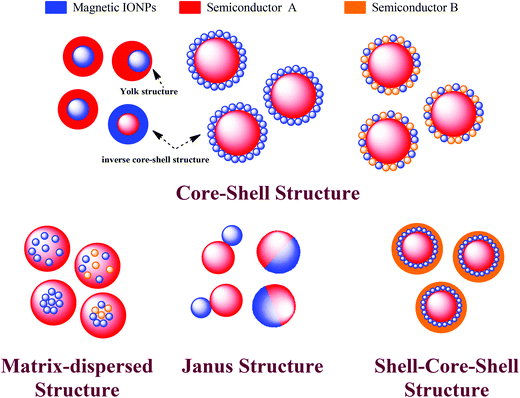
In order to increase the range of applications of iron oxide nanoparticles, some functional materials have been introduced and formed as newly composite nanostructures. Comparing with single-component nanomaterials, multiple-component nanomaterials have become the subject of extensive research due to the synergistic interaction effects between each component, which could improve the final catalytic performance. Currently, wide band gap semiconductors with good photocatalytic properties have been used to functionalize magnetic iron oxides. In a iron oxide–semiconductor composite system, the magnetic iron oxide can not only separate and recover the photocatalyst, but can also form narrow/wide band gap semiconductor heterostructures. The narrow/wide band gap semiconductor heterostructures can promote the separation of electron and hole pairs efficiently, consequently increasing the visible light utilization and finally improving the photocatalytic efficiency.As shown in Fig. 3, if iron oxide nanoparticles are always the core, the structure of iron oxide–semiconductor composite nanomaterials can be simply divided into four structures: core–shell, matrix-dispersed, Janus and shell–core–shell structures.
More importantly, understanding the relationship between the photocatalytic performance and the microstructure is a prerequisite for widespread application. Therefore, design and controllable synthesis of the nanostructured photocatalysts, and further optimization of the microstructure and photocatalytic performance are still under broad investigation.76 A prerequisite for every possible applied structure is the proper surface properties of the magnetic composite NPs, which determine their interaction with the environment. These interactions ultimately affect the colloidal stability and photocatalytic efficiency of the composite particles.
2.4 Charge transfer mechanism
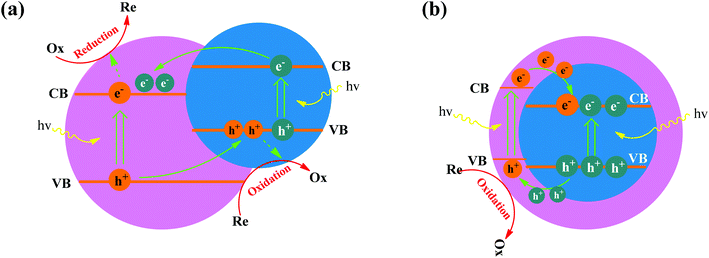
The charge separation mechanism in both capped semiconductor systems and coupled semiconductor systems involves the photoinduced electrons in one semiconductor being injected into the lower lying CB of the second semiconductor. Therefore, coupling semiconductors techniques do not always improve the photocatalytic performance by charge separation. The design of coupling semiconductor photocatalysts depends on the band structures of each component. Generally, photogenerated electrons on the CB of a higher level semiconductor are injected into the CB of a lower level semiconductor. As such coupled semiconductor photocatalytic systems bear great hope for next-generation solar energy harvesting and advancing environmental remediation techniques. Governments and researchers have devoted considerable interests and resources to such fabrication, characterization, and optimization.77In the iron oxide–semiconductor system, iron oxides can be narrow band gap semiconductors, with a band gap value for Fe2O3 of 2.2 eV, they also absorb visible light. For example, the work function (ϕ) of α-Fe2O3 is 5.88 eV, which is higher than most common wide band gap semiconductors (TiO2 is 3.87 eV, ZnO is 4.35 eV, SnO2 is 4.3 eV, WO3 is 5.24 eV, etc.). As shown in Fig. 4a, the band configuration and photogenerated charge carrier separation at the interface of iron oxide–semiconductor (wide band gap) under light irradiation are proposed. Under light irradiation, the photoinduced electrons and holes are separated at the interface of the iron oxide–semiconductor, the photoinduced electrons in the CB of iron oxide tend to transfer to that the CB of the semiconductor due to the decreased potential energy, and hence the coupling structure reduces the electron–hole recombination probability and increases the electron mobility. Thereby the electrons and holes were transferred to the surface of the iron oxide and semiconductor, respectively, and finally form hydroxyl radicals (˙OH). The superoxygen radicals (˙O2) are formed by the combination of electrons with O2 adsorbed on the surface of the semiconductor. As a powerful oxidant, ˙OH can degrade many pollutes, such as organic dyes, wastewater, and plastics. However, capped semiconductors on the other hand have a core and shell geometry, as shown in Fig. 4b. The electrons are injected into the energy levels of the core semiconductor (on condition that it has a conduction band potential which is lower than that of the shell). Hence, the electrons are trapped within the core particle, and is not readily accessible for the reduction reaction.78
The introduction of an interlayer into the iron oxide–semiconductor heterojuction for tailoring the photocatalytic efficiency is another option. As shown in Fig. 5, when the insulating SiO2 layer is introduced, the photogenerated electrons in the CB of iron oxide are not able to transfer to the CB of the semiconductor. However, from our previous reports and Christopher's reports, the photogenerated electrons can still transfer if the thickness of the SiO2 is less than 5 nm.79,80 Therefore, the thickness of SiO2 is a key factor and responsible for the photocatalytic abilities of iron oxide/SiO2/semiconductor systems. As an alternative, photogenerated electrons in the CB of iron oxide can transfer to the CB of the semiconductor via a carbon interlayer, which behaves as an electron conductor to enhance the electron–hole separation. For example, Hou and co-workers have reported an interlayer of graphene that transfers the electrons from the CB of a BiV1−xMoxO4 shell to the CB of the Fe2O3 core in α-Fe2O3 nanorod/graphene/BiV1−xMoxO4 core–shell heterojunction due to band alignment and potential difference, which provides a direct path for electron transport.81
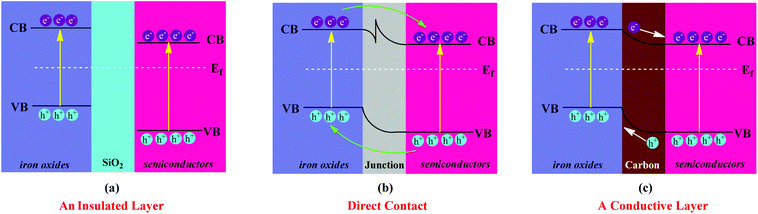 | ||
| Fig. 5 Schematic diagram showing the photogenerated charge transfer of (a) iron oxide/SiO2/semiconductors, (b) iron oxide–semiconductors and (c) iron oxide/C/semiconductors heterojunctions. | ||
As a classical heterostructure, the iron oxide–semiconductor system has many advantages. First of all, the built-in potential at the interface of iron oxide and semiconductor can promote the separation and transport of photoinduced charge carriers. Second, iron oxides with relatively smaller band gaps sensitize the wide band gap semiconductors. Third, semiconductor metal oxides and metal sulfides such as RuO2, NiO and IrO2, MoS2 and cobalt phosphates, can also act as effective co-catalysts to facilitate the surface electrochemical reaction. These co-catalysts improve charge separation, suppress the recombination of photogenerated charge carriers and lower the potential for electrochemical reaction.14 Although α-Fe2O3 is stable, it is prone to photocorrosion. Its photocatalytic degradation efficiency for organic dyes needs to be improved. Therefore, as an outer layer, semiconductors such as TiO2 with excellent electrochemical- and photochemical-stability can be used on the surface of iron oxide to improve the stability of the catalysts.
3 Synthesis of magnetic iron oxide–semiconductor composite nanomaterials
3.1 Seed-mediated growth strategy
As shown in Fig. 6a, the seed-mediated growth strategy is the most common method for synthesizing high-quality magnetic iron oxide–semiconductor composite nanomaterials, especially the preparation of core–shell heterostructures. A typical growth protocol involves the addition of magnetic iron oxide nanoparticles, as seeds, to the bulk semiconductor growth. The growth solution is obtained by the reduction of semiconductor precursors. In this protocol, seeds are sequentially added to the growth solution in order to control the rate of heterogeneous deposition and thereby the rate of crystal growth. Many wet-chemical approaches have been used to generate iron oxide–semiconductor composite heterostructures, such as the co-precipitation, hydrothermal, and solvothermal methods, etc.82–85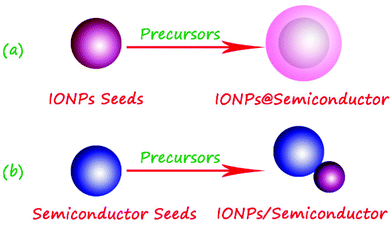 | ||
| Fig. 6 Scheme of the preparation of iron oxide–semiconductor composite nanomaterials by the seed-mediated growth method. | ||
For example, Chiu and co-workers have reported the synthesis of Fe3O4/ZnO core–shell nanoparticles by the seed-meditated growth method under nonhydrolytic conditions. Control over the thermal pyrolysis of zinc acetate give the option to overgrow the ZnO layer on the surface of Fe3O4 seeds. These core–shell nanocrystals were magnetically separated by a 0.6 T magnet, which shows high potential for using such nanocrystals as recoverable catalyst materials.86 We proposed a facile pathway to prepare three different types of magnetic iron oxide/TiO2 hybrid nanoparticles by the seed-mediated method. The hybrid nanoparticles are composed of spindle, hollow, and ultrafine iron oxide nanoparticles as seeds and 3-aminopropyltriethyloxysilane as the linker between the magnetic cores and TiO2 layers, respectively. About 50% to 60% of MB was decomposed in 90 min in the presence of magnetic hybrid nanoparticles, which is higher than pure TiO2 nanoparticles. The synthesized magnetic hybrid nanoparticles display high photocatalytic efficiency and can be used for cleaning polluted water with the help of magnetic separation.87 Recently, Li and co-workers have reported a versatile kinetics-controlled coating approach to fabricate homogeneous porous TiO2 shells for multi-functional core–shell nanostructures. By simply controlling the kinetics of the hydrolysis and condensation of tetrabutyl titanate (TBOT) in ethanol–ammonia mixtures, the core–shell heterostructure with homogeneous porous TiO2 shells were fabricated with variable diameter, geometry, and composition as seeds (e.g., α-Fe2O3 ellipsoids, Fe3O4 spheres, SiO2 spheres, graphene oxide sheets, and carbon spheres). This approach exhibits many advantages, such as facile, reproducible and the thickness of TiO2 shells can be tailored from 0 to 25, 45, and 70 nm.88 Yuan and co-workers have prepared Fe3O4@TiO2 nanoparticles (the size is 6.7 ± 2.9 nm) by a modified sol–gel method. The TiO2 shell was formed by gradually adding TiCl4 to the iron oxide nanoparticle gel. The as-prepared composite nanoparticles are used for targeted drug delivery.89
In addition, the semiconductor nanoparticles can also be seeds for the synthesis of iron oxide–semiconductor composite nanomaterials, and this strategy is often employed to fabricate the iron oxide–semiconductor with a Janus structure (Fig. 6b). For instance, Buonsanti and co-workers developed a colloidal seeded-growth strategy to synthesize all-oxide semiconductor/magnetic hybrid nanocrystals in various topological arrangements, in which the dimensions of the constituent material domains were controlled independently over a wide range. The FexOy/TiO2 composite nanorods were synthesized by using the brookite TiO2 nanorods as seeds and Fe(CO)5 as the iron precursor via a high-temperature thermal decomposition method. The preliminary magnetic and photocatalytic investigations had highlighted that the creation of bonding heterojunctions leads to significantly modified or even unexpected physical–chemical behaviour, relative to that offered by brookite TiO2 and FexOy alone.90,91 Zeng and co-workers first synthesized TiO2 nanoparticles with a diameter of about 5–10 nm with ferric acetylacetonate as an iron source, the multifunctional Fe3O4/TiO2 nanocomposites with a Janus structure were prepared by the solvent–thermal method.73 Liu and Gao first prepared the sheet-like TiO2 seeds by hydrothermal treatment of TiO2 nanoparticles. Then the α-Fe2O3/TiO2 composite nanosheets were fabricated by hydrothermal treatment of ferric nitrate and hydroxylamine. Results showed that the photocatalytic activities of α-Fe2O3 make the MB degradation efficient under visible light irradiation.92 Wang and co-workers synthesized one-dimensional (1D) heterostructures of uniform CdS nanowires separately decorated with hematite (α-Fe2O3) nanoparticles or magnetite (Fe3O4) microspheres via a two-step solvothermal deposition method. Each CdS nanowire had a uniform diameter of 40–50 nm and a length ranging to several tens of micrometers. Quasicubic α-Fe2O3 nanoparticles with edge lengths up to 30 nm, and Fe3O4 microspheres with diameters of about 200 nm produced 1D dimer-type CdS/α-Fe2O3 semiconductor heterostructures or CdS/Fe3O4 semiconductor magnetic functionally assembled heterostructures. In comparison with the bare CdS nanowires and commercial anatase TiO2, enhanced photocatalytic activity was observed in CdS/α-Fe2O3 heterostructures.93
3.2 Step-by-step deposition strategy
Step-by-step deposition strategy is mainly used to prepare iron oxide–semiconductor composite multi-shell structures.94–96 In fact, the need for a better control over surface properties or to protect the iron oxide itself, an interlayer was introduced in the magnetic iron oxide–semiconductor system to form a multi-shell structure, as shown in Fig. 7.97–99 The most commonly used interlayer materials are the SiO2 and carbon, respectively. Additionally, the interlayer can be removed by chemical corrosion or calcination.70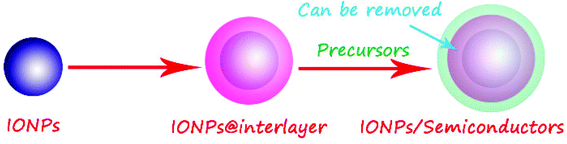 | ||
| Fig. 7 Scheme of the preparation of iron oxide–semiconductor composite nanomaterials by layer-by-layer deposition method. | ||
Silica coating can enhance dispersion in solution because the silica layer can screen the magnetic dipolar attraction between magnetic iron oxide nanoparticles, and hence increase the stability of iron oxide nanoparticles and protect them in acidic environments. Silica has become the most used interlayer material.100,101 For example, Cheng and co-workers have synthesized Fe3O4@SiO2@CeO2 microspheres with a magnetic core and mesoporous shell by a step-by-step deposition strategy. Such multifunctional materials were utilized to capture phosphopeptides and catalyze the dephosphorylation simultaneously, thereby labeling the phosphopeptides for rapid identification.102 Sarkar and co-workers synthesized Fe3O4 nanoparticles with a diameter of 20–40 nm by co-precipitation method, and then a SiO2 interlayer was deposited on the surface of Fe3O4 nanoparticles by classical Stöber method. The Fe3O4/SiO2/ZrO2 composite nanoparticles were finally fabricated by reducing the ZrOCl2 precursor. The thickness of ZrO2 was about 8–10 nm, and the BET surface area of the composite nanoparticles was up to 107 m2g−1 due to the mesoporous ZrO2 shell.103 More recently, Chi and co-workers have prepared Fe3O4@SiO2@TiO2/Ag nanocomposites by a step-by-step deposition strategy, the as-prepared microspheres show a number of important features as a recyclable photocatalyst: a high field-responsive magnetic Fe3O4 core for efficient magnetic separation, a SiO2 interlayer for protecting the Fe3O4 core from chemical- and photocorrosion, and a TiO2 nano-shell with well dispersed Ag nanoparticles for enhanced photocatalytic activity.104
Like the SiO2 interlayer, hydrophilic carbon coating on an iron oxide nanoparticle core also endows better dispersibility and stability. More importantly, carbon coated iron oxide nanoparticles have recently triggered enormous research activity due to the good chemical and thermal stability. The intrinsic high electrical conductivity of the carbon interlayer helps to transfer electrons.105–107 For instance, Qi and co-workers first deposited a carbon interlayer on the surface of Fe3O4 seeds by the hydrothermal reaction of glucose, and then deposited SnO2 on the surface of Fe3O4/C, they successfully obtained Fe3O4/C/SnO2 composite nanoparticles.108 Shi and co-workers have prepared a core/multi-shell-structured Fe3O4/C/TiO2 magnetic photocatalyst by the vapor phase hydrolysis process, and the photocatalytic abilities for degradation of methylene blue are studied. Compared with commercial anatase TiO2, Fe3O4/C/TiO2 with low TiO2 content (37%) exhibited a relatively higher photocatalytic activity. The C interlayer prevented the photocorrosion of Fe3O4 effectively, and the composite nanoparticles present a good magnetic recycling property due to magnetic core materials.109 Liu and co-workers have fabricated one-dimensional Fe3O4/C/CdS coaxial nanochains by a magnetic field-induced assembly and microwave-assisted deposition method. First, one-dimensional pearl chain-like Fe3O4/C core–shell nanocables were successfully assembled via the hydrothermal reaction of nanoscale Fe3O4 spheres with glucose in water in the presence of an external magnetic field. The carbonaceous layer was about 10 nm in thickness, and it acted as the stabilizer for the Fe3O4 nanochains. Afterwards, CdS nanoparticles were deposited on Fe3O4/C nanochains by a rapid microwave-irradiation route to generate Fe3O4/C/CdS coaxial nanochains. The subsequent photocatalytic test for organic pollutants demonstrated that these magnetic composites possess enhanced photocatalytic activity as MRPs under visible light irradiation. The decolorization fraction using a sample of microwave irradiation was up to 94.7% in 20 min, and the photocatalytic performance was still stable after 12 cycles of degradation of RhB, the results revealed that this MRP possessed excellent stability.107
3.3 Other strategies
Except the above two conventional strategies, some new synthesis or preparation methods have also been used to fabricate iron oxide–semiconductor composite nanomaterials, such as ion implantation method, spray pyrolysis, microwave, and sonochemical method.110–112As shown in Fig. 8, we first dropped the hematite seeds onto the surface of a clean slide and implanted Ti ions. and Magnetic monodispersed TiO2 grains filled into spindle-like hematite bi-component nanoparticles were successfully synthesized.113 The different implanted energy and magnetic properties of the bi-component α-Fe2O3/TiO2 nanoparticles were investigated. The results illustrate that the α-Fe2O3/TiO2 composite nanoparticles could be obtained by Ti ion implantation with different energies, and the saturation magnetization (MS) of the samples after ion implantation were significantly enhanced.114 Li and co-workers prepared a novel core–shell α-Fe2O3/SnO2 heterostructure by one-step flame-assisted spray pyrolysis of an iron and tin precursor. The effect of the SnO2 component was investigated for the evolution of the phase composition and morphology in detail. It was found that the doping of SnO2 in Fe2O3 could effectively promote the phase transition from γ-Fe2O3 to α-Fe2O3 during flame synthesis. The unique morphology composed of tin doped α-Fe2O3 core and SnO2 as a shell was attributed to the solubility, segregation and second-phase surface nucleation of SnO2 in Fe2O3.115
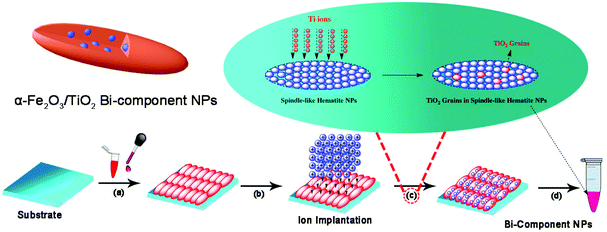 | ||
| Fig. 8 Scheme depicting α-Fe2O3/TiO2 (TiO2 grains in spindle-like α-Fe2O3) bi-component NP synthesis by ion implantation method.113 | ||
4 Progress on magnetic iron oxide–semiconductor composite photocatalysts
4.1 Magnetic iron oxide–metal oxide–semiconductor composite photocatalysts
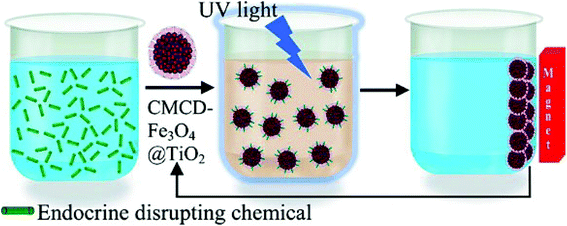 | ||
| Fig. 9 Scheme of the reuse of cyclodextrin-functionalized Fe3O4@TiO2 for photocatalytic degradation of endocrine-disrupting chemicals in water supplies.121 | ||
On the other hand, α-Fe2O3 has often been introduced into the magnetic iron oxide/TiO2 composite photocatalyst in order to use its narrow band gap properties, and to obtain magnetic iron oxide/TiO2 composite heterostructures.92,122–124 For example, Peng and co-workers have synthesized Fe2O3/TiO2 heterostructural photocatalysts by impregnation of Fe3+ on the surface of TiO2 and annealing at 300 °C, the composites possess different mass ratios of Fe2O3vs. TiO2. The photocatalytic activities of Fe2O3/TiO2 heterocomposites, pure Fe2O3 and TiO2 were studied by the photocatalytic degrading of Orange II dye in aqueous solution under visible light (λ > 420 nm) irradiation. The Fe2O3/TiO2 heterogeneous photocatalysts exhibited an enhanced photocatalytic ability for Orange II, higher than either pure Fe2O3 or TiO2. The best photocatalytic performance for Orange II could be obtained when the mass ratio in Fe2O3/TiO2 is 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The results illustrate that the generation of heterojunctions between Fe2O3 and TiO2 is key for improving movement and restraining the recombination of photoinduced charge carriers, and finally improving the photocatalytic performance of Fe2O3/TiO2 composites.125 Recently, Palanisamy and co-workers have prepared Fe2O3/TiO2 (10, 30, 50, 70 and 90 wt% Fe2O3) photocatalysts by a sol–gel process. Mesoporous Fe2O3/TiO2 composites exhibited excellent photocatalytic degradation ability for 4-chlorophenol in aqueous solution under sunlight irradiation. The author claimed that the photogenerated electrons in the VB of TiO2 are transferred to Fe(III) ions resulting in the reduction of Fe(III) ions to Fe(II) ions. Thus the photoinduced holes in the VB of Fe2O3/TiO2 cause an oxidation reaction and decompose the 4-chlorophenol to CO2 and H2O. Meanwhile the transferred electrons in Fe(III) ions could trigger the reduction reaction.126
3. The results illustrate that the generation of heterojunctions between Fe2O3 and TiO2 is key for improving movement and restraining the recombination of photoinduced charge carriers, and finally improving the photocatalytic performance of Fe2O3/TiO2 composites.125 Recently, Palanisamy and co-workers have prepared Fe2O3/TiO2 (10, 30, 50, 70 and 90 wt% Fe2O3) photocatalysts by a sol–gel process. Mesoporous Fe2O3/TiO2 composites exhibited excellent photocatalytic degradation ability for 4-chlorophenol in aqueous solution under sunlight irradiation. The author claimed that the photogenerated electrons in the VB of TiO2 are transferred to Fe(III) ions resulting in the reduction of Fe(III) ions to Fe(II) ions. Thus the photoinduced holes in the VB of Fe2O3/TiO2 cause an oxidation reaction and decompose the 4-chlorophenol to CO2 and H2O. Meanwhile the transferred electrons in Fe(III) ions could trigger the reduction reaction.126
On the one hand is the fabrication of iron oxide/SnO2 heterostructures, for instance, Niu and co-workers have prepared branched SnO2/α-Fe2O3 semiconductor nanoheterostructures (SNHs) of high purity by a low-cost and environmentally friendly hydrothermal strategy, through crystallographic-oriented epitaxial growth of SnO2 nanorods on α-Fe2O3 nanospindles and nanocubes, respectively (Fig. 10). SnO2/α-Fe2O3 SNHs exhibited excellent visible light or UV photocatalytic ability, remarkably superior to their α-Fe2O3 precursors, mainly owing to the effective electron–hole separation at the SnO2/α-Fe2O3 interface.127 Recently, Zhang and co-workers have also synthesized three-dimensional SnO2/α-Fe2O3 semiconductor hierarchical nanoheterostructures via crystallographic-oriented epitaxial growth of SnO2 onto the surface of flower-like three-dimensional iron oxide hierarchical nanostructures. For this photocatalyst, visible-light-active flower-like Fe2O3 hierarchical nanostructures were employed as a medium to harvest the visible light and generate photoinduced charge carriers, and SnO2 layer was employed as a charge collector to transport the photoinduced charge carriers. The SnO2/α-Fe2O3 semiconductor hierarchical heterostructures present admirable visible-light photodegradation ability for methylene blue, which could be assigned to the wide visible-light absorption range, high surface area, and efficient charge carrier separation of the SnO2-α-Fe2O3 heterostructures.128 Zhu and co-workers have synthesized core–shell structured α-Fe2O3@SnO2 shuttle-like composites via a facile solvothermal approach. The photocatalytic activities of the as-synthesized α-Fe2O3@SnO2 core–shell shuttle-like composites were studied by the photodegradation of RhB dye under UV light irradiation (λ = 365 nm), and the absorption peak of RhB diminished gradually as the exposed time extended and completely disappeared after 70 min. Compared with uncoated α-Fe2O3 shuttle-like nanorods, SnO2 nanoparticles, and the mixture of α-Fe2O3 nanorods and SnO2 particles, as-synthesized core–shell shuttle-like composites exhibited enhanced photodegradation abilities, suggesting that the synergistic effect of α-Fe2O3 and SnO2 was beneficial to improve the photocatalytic activity.129
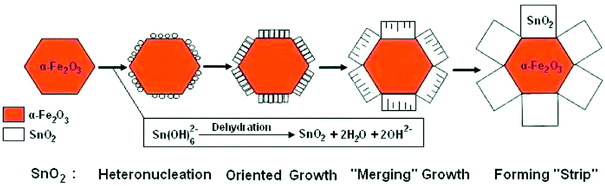 | ||
| Fig. 10 Schematically illustrated formation process of hierarchically assembled SnO2/α-Fe2O3 heterostructures based on α-Fe2O3 nanospindle precursor.127 | ||
On another hand, fabrication of the magnetically recoverable iron oxide/SnO2 composite photocatalyst system is also attractive for various reasons. As shown in Fig. 11, we have successfully synthesized the spindle-like and spherical iron oxide/SnO2 composite nanoparticles via a seed-mediated growth strategy recently, and the as-prepared iron oxides/SnO2 core–shell heterostructures displayed enhanced visible light and UV photodegradation activity for RhB, which is significant higher than the uncoated a-Fe2O3 seeds and commercially available SnO2 products. Significantly, the composite nanoparticles can be magnetically separated from the dispersion after photocatalytic degradation.130,131 Zhang and co-workers have fabricated superparamagnetic iron oxide (SPIO)@SnO2 yolk–shell heterostructures (YSHs) by a facile template approach, the as-obtained SnO2 shell is mesoporous, the thickness of the shell layer and void spaces are both tailorable. Under UV light irradiation for 1 h, the photodegradation activity of the as-obtained SPIO@SnO2 YSHs and commercial P25 TiO2 for RhB were about 75% and 97%, respectively. Because SPIO cores are not photocatalytically active, the mass of the SnO2 component in 25 mg of SPIO@SnO2 YSHs would be less than the photocatalyst component in the same mass of P25 TiO2.132
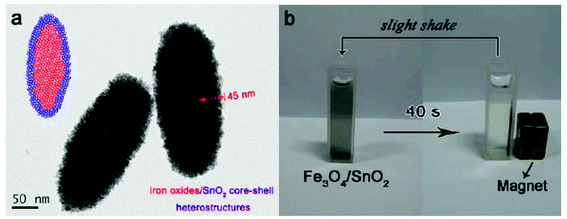 | ||
| Fig. 11 TEM image (a) and photograph of magnetic separation of spindle-like iron oxide/SnO2 composite nanoparticles.130 | ||
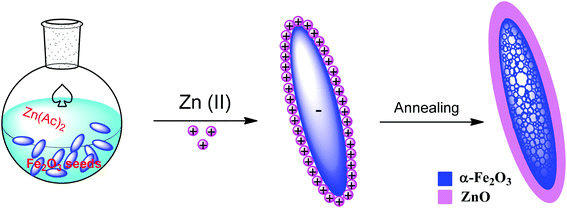 | ||
| Fig. 12 Synthetic route and formation mechanism for fabricating the mesoporous hematite/ZnO core–shell composite particles.137 | ||
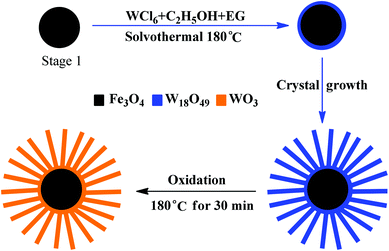 | ||
| Fig. 13 Formation of the Fe3O4/WO3 core–shell structures: (a) polycrystalline Fe3O4 microspheres; (b) Fe3O4 microspheres coated with a thin layer of W18O49; (c) Fe3O4/W18O49 core–shell structures; (d) Fe3O4/WO3 core–shell structures obtained by oxidizing Fe3O4/W18O49 in air.140 | ||
Recently, new semiconductor oxides have been used in photocatalytic applications, such as V2O5,143 Nb2O5,144,145 Ta2O5,146,147 CeO2,148,149 Ga2O3,150etc.151 However, reports on the synthesis of magnetic iron oxide–semiconductor oxides are very scarce so far, and it is worth studying various ways to introduce semiconductor oxides into iron oxide–semiconductor systems and to improve their photocatalytic ability.
4.2 Magnetic iron oxide–metal chalcogenides semiconductor composite photocatalysts
Currently, the proportion of using semiconductor oxides in photocatalysts is large, however, the semiconductor sulfides such as ZnS, CdS, Bi2S3, SnS2, ZnSe, etc. have also been attractive due to the importance of their special quantum confinement effect.93,152–156 As a competitive alternative, the application of magnetic iron oxide–semiconductor sulfides is a relatively new technology. The optical properties and photocatalytic performance of semiconductor sulfides could be different from their oxide counterparts.For example, Liu and co-workers have reported the preparation of Fe3O4/CdS nanocomposites via a sonochemical route in an aqueous solution. These Fe3O4/CdS nanocomposites displayed fluorescence and exhibited excellent magnetic properties at room temperature. Photocatalytic activity studies confirmed that the as-prepared nanocomposites had high photocatalytic activity towards the photodegradation of methyl orange in aqueous solution. Furthermore, the photodecomposition rate decreased slightly after 12 cycles of photocatalysis (89% of MO is decomposed in the last cycle).157 Their subsequent studies revealed that the Fe3O4/ZnS had high photocatalytic activity towards the photodegradation of eosin Y in aqueous solution. The catalytic efficiency only decreased by 5% after 15 cycles.112 As shown in Fig. 14, Shi and co-workers have synthesized α-Fe2O3/CdS corn-like nanocomposites via CdS nanoparticles by a simple one-step wet-chemical route, in which preformed single-crystaline α-Fe2O3 nanorods were used as substances for growing CdS nanoparticles. The corn-like nanocomposites exhibited superior photocatalytic performance under visible light irradiation (86.7% of MB was degraded in 120 min) over pure α-Fe2O3 nanorods and CdS nanoparticles. The enhanced performance is attributed to the larger surface area of the corn-like structure, the crystalline nature of the materials and the synergy in light absorption and charge separation between α-Fe2O3 and CdS.158 Luo and co-workers have developed a facile and rapid synthesis of urchin-shaped Fe3O4@Bi2S3 core–shell hierarchical structure through a sonochemical method. The as-prepared Fe3O4@Bi2S3 hierarchical core–shell structures showed excellent photocatalytic efficiency for the degradation of RhB and retained the photocatalytic activity after being recycled for five times with the help of an external magnetic field.159
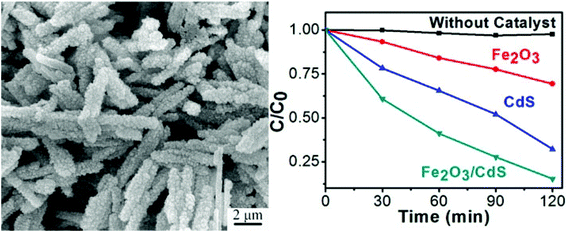 | ||
| Fig. 14 SEM image and photocatalytic performances of α-Fe2O3/CdS corn-like nanorods under visible light irradiation.158 | ||
Additionally, some ternary semiconductor sulfides have been used in the application of photocatalysts, such as ZnxCd1−xS, ZnIn2S4, CuInS2, etc.160–165 However, there is no literature report on iron oxide–ternary semiconductor sulfide composite photocatalytic systems. More importantly, the toxicity of the semiconductor sulfides should also be considered in practical application.
4.3 Multiple semiconductor shell photocatalysts
The synergetic effects of multiple semiconductor photocatalysts have been extensively observed in photocatalytic degradations. In core–shell–shell structures, the first semiconductor shell layer can offer special active-sites for the adsorption/reaction of reactant/reaction intermediates. The second semiconductor shell layer could also influence the overall band configuration via altering the bandgap absorption and to separate the photoinduced charge carriers.166–168 Moreover, semiconductors with a narrow band gap can expand the spectral response range. As shown in Fig. 15, by introducing semiconductor heterojunction on the surface of magnetic iron oxide nanomaterials, magnetically recoverable photocatalysts are obtained.14,169–171 | ||
| Fig. 15 Scheme of the preparation of iron oxide–multiple semiconductor layer composite photocatalysts. | ||
For example, Chen and co-workers have synthesized three types of ellipsoidal complex hollow structures with the shells assembled from anatase TiO2 nanosheets with exposed (001) facets by utilizing silica-coated hematite (α-Fe2O3) nanospindles as the starting templates. As shown in Fig. 16, the α-Fe2O3/SiO2/SnO2/TiO2 composite can be prepared by hydrothermal deposition of a SnO2 layer on the surface of SiO2. The Fe3O4@SnO2@TiO2 nanorattles manifested a much higher degradation efficiency compared to Degussa P25 nanoparticles, as well as their analogous Fe3O4@TiO2 core–shell nanomaterial without the exposed (001) high-energy facets.172 Dong and co-workers have prepared the CdS modified TiO2/Fe3O4 photocatalysts by sol–gel and immersion methods. The CdS-TiO2/Fe3O4 composites exhibited higher photocatalytic activity than pure TiO2 and TiO2/Fe3O4 for the degradation of Reactive Brilliant Red X-3B dye (X-3B) under simulated sunlight. In addition, a gradual loss of photocatalytic activity was observed in the reusability test of CdS-TiO2/Fe3O4 composites, and degradation of X-3B reached 78.9% after five runs.173 Obviously, photocatalysts with multiple semiconductor shells can effectively improve the photocatalytic abilities.
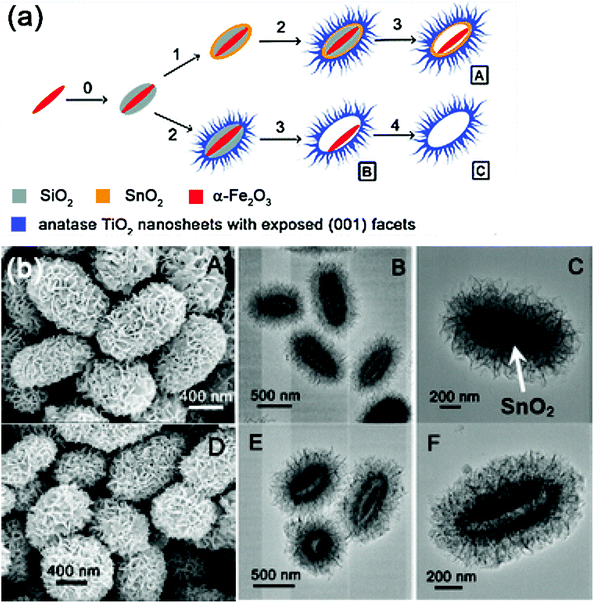 | ||
| Fig. 16 Synthetic route (a) and SEM, and TEM images for fabricating the ellipsoidal α-Fe2O3/SiO2/SnO2/TiO2 composite photocatalytic nanomaterials (b).172 | ||
5 Summary and perspectives
Several fundamental issues must be addressed before photocatalysts are economically viable for large scale industrial applications. Apart from offering easy separation of the photocatalysts from the reaction system, the magnetic iron oxide–semiconductor photocatalytic system, which interfaces chemistry with materials science, possesses a unique position in the advancement of heterogeneous photocatalysis. Table 1 depicts the representative magnetic iron oxide–semiconductor photocatalysts and their photocatalytic performances. Though a lot of effort has been made in design and fabricating magnetic iron oxide–semiconductor composite photocatalytic system, it is still a field of research in modern photocatalysis and following issues are still need to be addressed.| Structure | Materials | Pollutants | Light resource | Rate constant k (10−2 min−1) | Stability performance | Ref. |
|---|---|---|---|---|---|---|
| a Photocatalytic degradation of BPA is complete within 60 min. b Photocatalytic degradation of MB is complete within 120 min. c Photocatalytic degradation of Eosin Y is complete within 37 min. d Photocatalytic degradation of MB is 70% within 120 min. | ||||||
| Binary structure | Fe3O4@TiO2 | Bisphenol A | UV light | —a | 90% after 10 cycles | 121 |
| α-Fe2O3@ TiO2 | RhB | Visible light | 0.81 | — | 197 | |
| γ-Fe2O3@SnO2 | RhB | UV light | 0.68 | — | 131 | |
| α-Fe2O3@ZnO | RhB | UV + visible light | 2.4 | — | 137 | |
| Fe3O4@WO3 | MB | Visible light | —b | No obvious decrease after 3 cycles | 140 | |
| Fe3O4/ZnS | Eosin Y | UV light | —c | 95% after 15 cycles | 112 | |
| α-Fe2O3/CdS | MB | Visible light | 1.68 | — | 158 | |
| Ternary structure | Fe3O4@SiO2@TiO2 | Methyl orange | UV light | — | 91% after 6 cycles | 120 |
| Graphene/TiO2/Fe3O4 | RhB | UV light | 16 | No obvious decrease after 5 cycles | 177 | |
| α-Fe2O3@SnO2@Cu2O | RhB | UV + visible light | 3.29 | 85% after 8 cycles | 198 | |
| Multiple layers structure | α-Fe2O3/Ag/SiO2/SnO2 | RhB | UV light | 0.13 | — | 176 |
| Visible ligth | 0.41 | — | ||||
| UV + visible light | 7.21 | 96% after 8 cycles | ||||
| α-Fe2O3/SiO2/SnO2/TiO2 | MB | UV light | 0.29d | — | 172 | |
(1) In order to improve the photocatalytic activities of photocatalysts, extension of the excitation wavelength, reduced charge carrier recombination, and the promotion of active sites around the surface should be considered. Therefore, if noble metal nanomaterials are introduced in the magnetic iron oxide–semiconductor system, the photocatalytic efficiency could be enhanced. The photogenerated charge carriers in the noble metal can be separated by the metal/semiconductor heterojunction. Additionally, the separated electron and hole can take part in the chemical reactions on the surface of metal and semiconductor, respectively. The absorbed photons can excite the valence electrons of noble metals due to the surface plasmon resonance (SPR) effect. The energy of photoinduced electrons is higher than the Schottky barrier resulted in crossing the interface and transferring to the VB of the semiconductor. Numerous literature reports are dedicated to the metal–semiconductor composite photocatalytic system. However, reports on magnetic iron oxide/noble metal/semiconductors ternary photocatalysts are scarce and need to be strengthened.174 Owing to the SPR effect, solution processed metal nanoparticles coated onto the surface of iron oxide or semiconductors is an effective method to enhance the absorption of visible light. However, the metal nanoparticles can also act as recombination centres resulting in inferior photocatalytic performance due to the incorporation of chemically synthesized metal nanoparticles in the iron oxide–semiconductor composite system. Many factors can cause undesirable exciton quenching and decrease the plasmonic effect. Indeed, coating of metal nanoparticles with insulating materials can prevent such recombination centres.175 More recently, we have reported a novel iron oxide/noble metal/semiconductor ternary multilayer hybrid structure that was prepared by template synthesis and subsequent layer-by-layer deposition method. Three different morphologies of α-Fe2O3/Ag/SiO2/SnO2 heterosturctures were obtained, the thickness of the insulating SiO2 interlayer was tailored to control the coupling of noble metal silver with tin oxide. The as-obtained α-Fe2O3/Ag/SiO2/SnO2 nanocomposites exhibited enhanced catalytic abilities under UV or visible light irradiation, higher than the commercially available pure SnO2, naked α-Fe2O3 seeds and α-Fe2O3/SnO2 binary nanocomposites. Moreover, α-Fe2O3/Ag/SiO2/SnO2 exhibited significant stability and recyclability because of its photodegradation rate maintains at 96% after 8 cycles.176
(2) The fusion of catalysis with nanotechnology continues to generate better materials and improve their functions. Graphene and its use in photodegradation is one of the latest examples. Its interesting electrical and mechanical properties, and high surface area make graphene a novel substrate for forming hybrid structures with a variety of nanomaterials. The use of graphene to enhance the efficiency of photocatalysts has attracted much attention. Utilization of single-layer graphene sheets can not only provide a high quality two-dimensional photocatalyst support, but also a two-dimensional circuit board, with an attractive potential to harness their perfect electrical and redox properties. There are few literature reports on composites of graphene with magnetic iron oxide–semiconductors photocatalytic system.177
(3) At present, a lot of fundamental and applied research of photocatalysis are focused on the synthesis and modification of new photocatalysts, nevertheless, with those endeavours, the effect of photocatalyst microstructure on their photocatalytic performance still cannot be understood, understanding the relationship between these two parts is a prerequisite for the broad application of composite nanomaterials in photocatalysis. However, the understanding of interface effects, the coupling mechanism, photocatalyst life, deactivation and the regeneration mechanism are still relatively weak.178 As a heterogeneous catalytic reaction system, semiconductor photocatalytic materials would be deactivated in practical application, such as the photocatalytic efficiency of P25 TiO2 becomes very low after 3 cycles under sunlight. Therefore, deactivation and the regeneration mechanism of semiconductor photocatalysts should be reinforced.
As a key issue for practical applications, the facile method to increase the photocorrosion suppression ability, the life and stability of magnetic iron oxide–semiconductor composite photocatalytic system must be further developed and improved. To date, the underlying photocorrosion mechanism for the iron oxide–semiconductor composite photocatalyst is not clear, and systematic studies are necessary. Many methods have been used to reduce the photocorrosion of pure semiconductors, such as graphene composites,179 graphene oxide,180 quantum dot,181etc., and these materials can be introduced to the magnetic iron oxide–semiconductor composite photocatalytic system. Moreover, recycling and regeneration is also an effective method to extend the life of a deactivated photocatalyst. However, reports on the above mentioned issues are scarce so far.
(4) Although a great effort has been made in the past years to unravel the mechanisms of bi/ternary and multiple composite photocatalysts, it is still a challenge for various researchers. To develop an efficient heterostructural photocatalysis system for large-scale industrialization, understanding of the kinetics and mechanisms of these charge transfer processes is very important.182 More efforts on photo-inducing charge carrier generation, trapping, recombination, and transporting are needed to further to strengthen and improve it. Apart from the traditional characterization techniques, more and more photoelectrochemical methods and techniques have been used to study the kinetics and mechanisms of heterostructural photocatalysis systems. For instance, photoelectron spectroscopy (PES) is used to measure band bending in semiconductors, femtosecond transient reflecting grating (TRG) method is used to detect the photogenerated ultrafast relaxation dynamic at solid/liquid interfaces, O2 photostimulated desorption (PSD) and electronstimulated desorption (ESD) are used to study the surface photoreactions induced by the photo-excited electrons and holes in the semiconductor, etc.183–186 At present, charge transfer kinetics on a short duration is well studied, while the charge transfer on a more extended timescale is still unclear. Therefore, unravelling the mechanisms that play an important and key role in magnetic iron oxide–semiconductor composite photocatalytic system is necessary. On this regard, there are several mechanisms that are still not fully understood and many works need to be carried out.
(5) In fact, the photodegradation of pollutants is mainly used in a suspension of semiconductor nanomaterials in this field. However, from a practical point of view, there are many limitations of using a photocatalyst suspension, such as requirement of large photo-reactors, hard to filtrate the nanoscale photocatalyst, etc. As the catalytic mechanism of these synthesized photocatalysts is very complicated, the pure semiconductors and α-Fe2O3/semiconductor composite photocatalytic systems are hardly recycled by external magnetic fields due to their weak magnetic response. As shown in Fig. 17, these photocatalysts can be printed on rigid or flexible substrates as photocatalytic arrays or patterns, including screen printing,187–189 offset printing,190 inkjet printing,191–193 gravure printing,194etc.195,196 These printed patterns with semiconductor photocatalysts can also be recycled. Therefore, combining and developing more practical methods to use these composite photocatalytic systems should be further reinforced.
 | ||
| Fig. 17 Depiction of various semiconductor solution deposition methods by printing.196 | ||
Acknowledgements
The authors thank the Hong Kong Scholars Program, NSFC (51471121, 51201115, 51171132), Young Chenguang Project of Wuhan City (2013070104010011), China Postdoctoral Science Foundation (2014M550406), Hubei Provincial Natural Science Foundation (2014CFB261) and the Fundamental Research Funds for the Central Universities.References
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- S. Liu, J. Yu and M. Jaroniec, Chem. Mater., 2011, 23, 4085–4093 CrossRef CAS.
- L. Sun and J. R. Bolton, J. Phys. Chem., 1996, 100, 4127–4134 CrossRef CAS.
- T. Bak, J. Nowotny, M. Rekas and C. C. Sorrell, Int. J. Hydrogen Energy, 2002, 27, 991–1022 CrossRef CAS.
- A. C. Papageorgiou, N. S. Beglitis, C. L. Pang, G. Teobaldi, G. Cabailh, Q. Chen, A. J. Fisher, W. A. Hofer and G. Thornton, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 2391–2396 CrossRef PubMed.
- L. Liu and Y. Li, Aerosol Air Qual. Res., 2014, 14, 453–469 CAS.
- J. Zhang, S. Chen, L. Qian, X. Tao, L. Yang, H. Wang, Y. Li, E. Zhang, J. Xi and Z. Ji, J. Am. Ceram. Soc., 2014 DOI:10.1111/jace.13187.
- D. J. Cole-Hamilton, Science, 2003, 299, 1702–1706 CrossRef CAS PubMed.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- S. G. Kumar and L. G. Devi, J. Phys. Chem. A, 2011, 115, 13211–13241 CrossRef CAS PubMed.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed.
- Y. Qu and X. Duan, Chem. Soc. Rev., 2013, 42, 2568–2580 RSC.
- P. Xu, G. M. Zeng, D. L. Huang, C. L. Feng, S. Hu, M. H. Zhao, C. Lai, Z. Wei, C. Huang, G. X. Xie and Z. F. Liu, Sci. Total Environ., 2012, 424, 1–10 CrossRef CAS PubMed.
- Y. Wang, Q. Wang, X. Zhan, F. Wang, M. Safdar and J. He, Nanoscale, 2013, 5, 8326–8339 RSC.
- W. Wei, Q. G. He and C. Hong, Prog. Chem., 2008, 20, 265–272 Search PubMed.
- W. Wu, Q. G. He and C. Z. Jiang, Nanoscale Res. Lett., 2008, 3, 397–415 CrossRef CAS PubMed.
- L. Zhou, J. Yuan and Y. Wei, J. Mater. Chem., 2011, 21, 2823–2840 RSC.
- R. M. Cornell and U. Schwertmann, The Iron Oixdes: Structures, Properties, Reactions, Occurrences and Uses, Wiley-VCH, 2003 Search PubMed.
- M. Yamaura, R. L. Camilo, L. C. Sampaio, M. A. Macedo, M. Nakamura and H. E. Toma, J. Magn. Magn. Mater., 2004, 279, 210–217 CrossRef CAS PubMed.
- A. Demortière, P. Panissod, B. P. Pichon, G. Pourroy, D. Guillon, B. Donnio and S. Bégin-Colin, Nanoscale, 2011, 3, 225–232 RSC.
- W. Wu, X. H. Xiao, F. Ren, S. F. Zhang and C. Z. Jiang, J. Low Temp. Phys., 2012, 168, 306–313 CrossRef CAS.
- J. Choi, J. Cha and J.-K. Lee, RSC Adv., 2013, 3, 8365–8371 RSC.
- W. Wu, X. H. Xiao, S. F. Zhang, H. Li, X. D. Zhou and C. Z. Jiang, Nanoscale Res. Lett., 2009, 4, 926–931 CrossRef CAS PubMed.
- R. Massart, IEEE Trans. Magn., 1981, 17, 1247–1248 CrossRef.
- W. Wu, Q. G. He, R. Hu, J. K. Huang and H. Chen, Rare Met. Mater. Eng., 2007, 36, 238–243 CAS.
- S. K. Suh, K. Yuet, D. K. Hwang, K. W. Bong, P. S. Doyle and T. A. Hatton, J. Am. Chem. Soc., 2012, 134, 7337–7343 CrossRef CAS PubMed.
- J. Park, K. An, Y. Hwang, J. G. Park, H. J. Noh, J. Y. Kim, J. H. Park, N. M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS PubMed.
- J. Park, E. Lee, N. M. Hwang, M. Kang, S. C. Kim, Y. Hwang, J. G. Park, H. J. Noh, J. Y. Kim and J. H. Park, Angew. Chem., Int. Ed., 2005, 117, 2932–2937 CrossRef.
- D. Amara, J. Grinblat and S. Margel, J. Mater. Chem., 2012, 22, 2188–2195 RSC.
- W. Wu, X. H. Xiao, S. F. Zhang, J. A. Zhou, L. X. Fan, F. Ren and C. Z. Jiang, J. Phys. Chem. C, 2010, 114, 16092–16103 CAS.
- W. Wu, X. H. Xiao, S. F. Zhang, T. C. Peng, J. Zhou, F. Ren and C. Z. Jiang, Nanoscale Res. Lett., 2010, 5, 1474–1479 CrossRef CAS PubMed.
- Y. Tian, B. Yu, X. Li and K. Li, J. Mater. Chem., 2011, 21, 2476–2481 RSC.
- W. T. Dong and C. S. Zhu, J. Mater. Chem., 2002, 12, 1676–1683 RSC.
- H. Qi, B. Yan, W. Lu, C. Li and Y. Yang, Curr. Nanosci., 2011, 7, 381–388 CrossRef CAS.
- K. Wongwailikhit and S. Horwongsakul, Mater. Lett., 2011, 65, 2820–2822 CrossRef CAS PubMed.
- L.-H. Han, H. Liu and Y. Wei, Powder Technol., 2011, 207, 42–46 CrossRef CAS PubMed.
- R. Ladj, A. Bitar, M. Eissa, Y. Mugnier, R. Le Dantec, H. Fessi and A. Elaissari, J. Mater. Chem. B, 2013, 1, 1381–1396 RSC.
- R. Abu Mukh-Qasem and A. Gedanken, J. Colloid Interface Sci., 2005, 284, 489–494 CrossRef CAS PubMed.
- R. Abu-Much and A. Gedanken, J. Phys. Chem. C, 2008, 112, 35–42 CAS.
- A. L. Morel, S. I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois, A. Brisson and M. Simonoff, ACS Nano, 2008, 2, 847–856 CrossRef CAS PubMed.
- W. Wu, Q. G. He, H. Chen, J. X. Tang and L. B. Nie, Nanotechnology, 2007, 18, 145609 CrossRef.
- S. G. Zhang, Y. Zhang, Y. Wang, S. M. Liu and Y. Q. Deng, Phys. Chem. Chem. Phys., 2012, 14, 5132–5138 RSC.
- X. Hu, J. C. Yu, J. Gong, Q. Li and G. Li, Adv. Mater., 2007, 19, 2324–2329 CrossRef CAS.
- L. H. Wu, H. B. Yao, B. Hu and S. H. Yu, Chem. Mater., 2011, 23, 3946–3952 CrossRef CAS.
- Z. Ai, K. Deng, Q. Wan, L. Zhang and S. Lee, J. Phys. Chem. C, 2010, 114, 6237–6242 CAS.
- G. Qiu, H. Huang, H. Genuino, N. Opembe, L. Stafford, S. Dharmarathna and S. L. Suib, J. Phys. Chem. C, 2011, 115, 19626–19631 CAS.
- H. Vali, B. Weiss, Y. L. Li, S. K. Sears, S. S. Kim, J. L. Kirschvink and L. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16121–16126 CrossRef CAS PubMed.
- A. Scheffel, M. Gruska, D. Faivre, A. Linaroudis, J. M. Plitzko and D. Schuler, Nature, 2006, 440, 110–114 CrossRef CAS PubMed.
- D. A. Bazylinski and R. B. Frankel, Nat. Rev. Microbiol., 2004, 2, 217–230 CrossRef CAS PubMed.
- C. Pascal, J. L. Pascal, F. Favier, M. L. Elidrissi Moubtassim and C. Payen, Chem. Mater., 1998, 11, 141–147 CrossRef.
- M. Starowicz, P. Starowicz, J. Żukrowski, J. Przewoźnik, A. Lemański, C. Kapusta and J. Banaś, J. Nanopart. Res., 2011, 13, 7167–7176 CrossRef CAS PubMed.
- G. Wang, Y. Ling, D. A. Wheeler, K. E. N. George, K. Horsley, C. Heske, J. Z. Zhang and Y. Li, Nano Lett., 2011, 11, 3503–3509 CrossRef CAS PubMed.
- G. Salazar-Alvarez, M. Muhammed and A. A. Zagorodni, Chem. Eng. Sci., 2006, 61, 4625–4633 CrossRef CAS PubMed.
- H. Y. Huang, Y. T. Shieh, C. M. Shih and Y. K. Twu, Carbohydr. Polym., 2010, 81, 906–910 CrossRef CAS PubMed.
- I. Morjan, R. Alexandrescu, F. Dumitrache, R. Birjega, C. Fleaca, I. Soare, C. Luculescu, G. Filoti, V. Kuncer and L. Vekas, J. Nanosci. Nanotechnol., 2010, 10, 1223–1234 CrossRef CAS PubMed.
- G. Martínez, A. Malumbres, R. Mallada, J. Hueso, S. Irusta, O. Bomatí-Miguel and J. Santamaría, Nanotechnology, 2012, 23, 425605 CrossRef PubMed.
- T. Thurston and J. Wilcoxon, J. Phys. Chem. B, 1999, 103, 11–17 CrossRef CAS.
- W. Wu, S. F. Zhang, J. Zhou, X. H. Xiao, F. Ren and C. Z. Jiang, Chem. – Eur. J., 2011, 17, 9708–9719 CrossRef CAS PubMed.
- I. Shakir, M. Shahid and D. J. Kang, Chem. Commun., 2010, 46, 4324–4326 RSC.
- W. Wu, X. H. Xiao, T. C. Peng and C. Z. Jiang, Chem. – Asian J., 2010, 5, 315–321 CrossRef CAS PubMed.
- W. Wu, X. H. Xiao, S. F. Zhang, J. A. Zhou, F. Ren and C. Z. Jiang, Chem. Lett., 2010, 39, 684–685 CrossRef CAS.
- D. Chen, F. Huang, G. Ren, D. Li, M. Zheng, Y. Wang and Z. Lin, Nanoscale, 2010, 2, 2062–2064 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- R. Vinu and G. Madras, J. Indian Inst. Sci., 2010, 90, 189–230 CAS.
- L. Zhang, H. Cheng, R. Zong and Y. Zhu, J. Phys. Chem. C, 2009, 113, 2368–2374 Search PubMed.
- T. T. Vu, L. del Río, T. Valdés-Solís and G. Marbán, Appl. Catal., B, 2013, 140–141, 189–198 CrossRef CAS PubMed.
- J. Liu, J. Xu, R. Che, H. Chen, M. Liu and Z. Liu, Chem. – Eur. J., 2013, 19, 6746–6752 CrossRef CAS PubMed.
- W. Li, Y. Deng, Z. Wu, X. Qian, J. Yang, Y. Wang, D. Gu, F. Zhang, B. Tu and D. Zhao, J. Am. Chem. Soc., 2011, 133, 15830–15833 CrossRef CAS PubMed.
- Y. Luo, J. Luo, J. Jiang, W. Zhou, H. Yang, X. Qi, H. Zhang, H. J. Fan, D. Y. W. Yu, C. M. Li and T. Yu, Energy Environ. Sci., 2012, 5, 6559–6566 CAS.
- C. Wang, L. Yin, L. Zhang, L. Kang, X. Wang and R. Gao, J. Phys. Chem. C, 2009, 113, 4008–4011 CAS.
- L. Zeng, W. Ren, L. Xiang, J. Zheng, B. Chen and A. Wu, Nanoscale, 2013, 5, 2107–2113 RSC.
- F. Mou, L. Xu, H. Ma, J. Guan, D.-r. Chen and S. Wang, Nanoscale, 2012, 4, 4650–4657 RSC.
- C. Wang, C. Xu, H. Zeng and S. Sun, Adv. Mater., 2009, 21, 3045–3052 CrossRef CAS PubMed.
- W. Wu, L. Liao, S. F. Zhang, J. Zhou, X. H. Xiao, F. Ren, L. L. Sun, Z. G. Dai and C. Z. Jiang, Nanoscale, 2013, 5, 5628–5636 RSC.
- R. Mohamed, D. McKinney and W. Sigmund, Mater. Sci. Eng., R, 2012, 73, 1–13 CrossRef CAS PubMed.
- I. Bedja and P. V. Kamat, J. Phys. Chem., 1995, 99, 9182–9188 CrossRef CAS.
- J. Zhou, F. Ren, S. Zhang, W. Wu, X. Xiao, Y. Liu and C. Jiang, J. Mater. Chem. A, 2013, 1, 13128–13138 CAS.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS PubMed.
- Y. Hou, F. Zuo, A. Dagg and P. Feng, Nano Lett., 2012, 12, 6464–6473 CrossRef CAS PubMed.
- J. Zhang, X. H. Liu, L. W. Wang, T. L. Yang, X. Z. Guo, S. H. Wu, S. R. Wang and S. M. Zhang, Nanotechnology, 2011, 22, 185501 CrossRef PubMed.
- W. Yan, H. Fan and C. Yang, Mater. Lett., 2011, 65, 1595–1597 CrossRef CAS PubMed.
- L. Peng, T. Xie, Y. Lu, H. Fan and D. Wang, Phys. Chem. Chem. Phys., 2010, 12, 8033–8041 RSC.
- X. Yu, J. Wan, Y. Shan, K. Chen and X. Han, Chem. Mater., 2009, 21, 4892–4898 CrossRef CAS.
- W. Chiu, P. Khiew, M. Cloke, D. Isa, H. Lim, T. Tan, N. Huang, S. Radiman, R. Abd-Shukor, M. A. A. Hamid and C. Chia, J. Phys. Chem. C, 2010, 114, 8212–8218 CAS.
- W. Wu, X. H. Xiao, S. F. Zhang, F. Ren and C. Z. Jiang, Nanoscale Res. Lett., 2011, 6, 533 CrossRef PubMed.
- W. Li, J. Yang, Z. Wu, J. Wang, B. Li, S. Feng, Y. Deng, F. Zhang and D. Zhao, J. Am. Chem. Soc., 2012, 134, 11864–11867 CrossRef CAS PubMed.
- Y. Yuan, S. Chen, T. Paunesku, S. C. Gleber, W. C. Liu, C. B. Doty, R. Mak, J. Deng, Q. Jin, B. Lai, K. Brister, C. Flachenecker, C. Jacobsen, S. Vogt and G. E. Woloschak, ACS Nano, 2013, 7, 10502–10517 CrossRef CAS PubMed.
- R. Buonsanti, E. Snoeck, C. Giannini, F. Gozzo, M. Garcia-Hernandez, M. A. Garcia, R. Cingolani and P. D. Cozzoli, Phys. Chem. Chem. Phys., 2009, 11, 3680–3691 RSC.
- R. Buonsanti, V. Grillo, E. Carlino, C. Giannini, F. Gozzo, M. Garcia-Hernandez, M. A. Garcia, R. Cingolani and P. D. Cozzoli, J. Am. Chem. Soc., 2010, 132, 2437–2464 CrossRef CAS PubMed.
- H. Liu and L. Gao, J. Am. Ceram. Soc., 2006, 89, 370–373 CrossRef CAS PubMed.
- L. Wang, H. Wei, Y. Fan, X. Gu and J. Zhan, J. Phys. Chem. C, 2009, 113, 14119–14125 CAS.
- G. Schneider and G. Decher, Nano Lett., 2004, 4, 1833–1839 CrossRef CAS.
- Y. Wang, A. S. Angelatos and F. Caruso, Chem. Mater., 2007, 20, 848–858 CrossRef.
- S. Srivastava and N. A. Kotov, Acc. Chem. Res., 2008, 41, 1831–1841 CrossRef CAS PubMed.
- Y. Li, J. S. Wu, D. W. Qi, X. Q. Xu, C. H. Deng, P. Y. Yang and X. M. Zhang, Chem. Commun., 2008, 564–566 RSC.
- S. Abramson, L. Srithammavanh, J.-M. Siaugue, O. Horner, X. Xu and V. Cabuil, J. Nanopart. Res., 2009, 11, 459–465 CrossRef CAS.
- C. Wang, L. Yin, L. Zhang, L. Kang, X. Wang and R. Gao, J. Phys. Chem. C, 2009, 113, 4008–4011 CAS.
- T. A. Gad-Allah, K. Fujimura, S. Kato, S. Satokawa and T. Kojima, J. Hazard. Mater., 2008, 154, 572–577 CrossRef CAS PubMed.
- X. Huang, G. Wang, M. Yang, W. Guo and H. Gao, Mater. Lett., 2011, 65, 2887–2890 CrossRef CAS PubMed.
- G. Cheng, J.-L. Zhang, Y.-L. Liu, D.-H. Sun and J.-Z. Ni, Chem. Commun., 2011, 47, 5732–5734 RSC.
- A. Sarkar, S. K. Biswas and P. Pramanik, J. Mater. Chem., 2010, 20, 4417–4424 RSC.
- Y. Chi, Q. Yuan, Y. Li, L. Zhao, N. Li, X. Li and W. Yan, J. Hazard. Mater., 2013, 262, 404–411 CrossRef CAS PubMed.
- H. Wang, L. Sun, Y. Li, X. Fei, M. Sun, C. Zhang, Y. Li and Q. Yang, Langmuir, 2011, 27, 11609–11615 CrossRef CAS PubMed.
- Y. Jing, C. Shi-Hai and L. Hong-Zhen, Chin. J. Inorg. Chem., 2013, 29, 2043–2048 Search PubMed.
- Y. Liu, L. Zhou, Y. Hu, C. Guo, H. Qian, F. Zhang and X. W. D. Lou, J. Mater. Chem., 2011, 21, 18359–18364 RSC.
- D. W. Qi, J. Lu, C. H. Deng and X. M. Zhang, J. Phys. Chem. C, 2009, 113, 15854–15861 CAS.
- F. Shi, Y. Li, Q. Zhang and H. Wang, Int. J. Photoenergy, 2012, 2012, 365401 CrossRef PubMed.
- H.-P. Peng, R.-P. Liang, L. Zhang and J.-D. Qiu, Electrochim. Acta, 2011, 56, 4231–4236 CrossRef CAS PubMed.
- Z. Wang, L. Wu, M. Chen and S. Zhou, J. Am. Chem. Soc., 2009, 131, 11276–11277 CrossRef CAS PubMed.
- X. Liu, Q. Hu, X. Zhang, Z. Fang and Q. Wang, J. Phys. Chem. C, 2008, 112, 12728–12735 CAS.
- L. L. Sun, W. Wu, S. F. Zhang, J. Zhou, G. X. Cai, F. Ren, X. H. Xiao, Z. G. Dai and C. Z. Jiang, AIP Adv., 2012, 2, 032179 CrossRef PubMed.
- L. L. Sun, W. Wu, S. F. Zhang, Y. C. Liu, X. H. Xiao, F. Ren, G. X. Cai and C. Z. Jiang, J. Nanosci. Nanotechnol., 2013, 13, 5428–5433 CrossRef CAS PubMed.
- Y. Li, Y. Hu, H. Jiang, X. Hou and C. Li, CrystEngComm, 2013, 15, 6715–6721 RSC.
- S. Xuan, W. Jiang, X. Gong, Y. Hu and Z. Chen, J. Phys. Chem. C, 2008, 113, 553–558 Search PubMed.
- Q. Yuan, N. Li, W. C. Geng, Y. Chi and X. T. Li, Mater. Res. Bull., 2012, 47, 2396–2402 CrossRef CAS PubMed.
- Y. Z. Wang, X. B. Fan, S. L. Wang, G. L. Zhang and F. B. Zhang, Mater. Res. Bull., 2013, 48, 785–789 CrossRef CAS PubMed.
- B. Cui, H. X. Peng, H. Q. Xia, X. H. Guo and H. L. Guo, Sep. Purif. Technol., 2013, 103, 251–257 CrossRef CAS PubMed.
- Z. Wang, L. Shen and S. Zhu, Int. J. Photoenergy, 2012, 2012, 202519 Search PubMed.
- R. Chalasani and S. Vasudevan, ACS Nano, 2013, 7, 4093–4104 CrossRef CAS PubMed.
- W. Zhou, H. Fu, K. Pan, C. Tian, Y. Qu, P. Lu and C.-C. Sun, J. Phys. Chem. C, 2008, 112, 19584–19589 CAS.
- F.-X. Chen, W.-Q. Fan, T.-Y. Zhou and W.-H. Huang, Acta Phys. Chim. Sin., 2013, 29, 167–175 CAS.
- W. S. Tung and W. A. Daoud, ACS Appl. Mater. Interfaces, 2009, 1, 2453–2461 CAS.
- L. L. Peng, T. F. Xie, Y. C. Lu, H. M. Fan and D. J. Wang, Phys. Chem. Chem. Phys., 2010, 12, 8033–8041 RSC.
- B. Palanisamy, C. M. Babu, B. Sundaravel, S. Anandan and V. Murugesan, J. Hazard. Mater., 2013, 252–253, 233–242 CrossRef CAS PubMed.
- M. T. Niu, F. Huang, L. F. Cui, P. Huang, Y. L. Yu and Y. S. Wang, ACS Nano, 2010, 4, 681–688 CrossRef CAS PubMed.
- S. W. Zhang, J. X. Li, H. H. Niu, W. Q. Xu, J. Z. Xu, W. P. Hu and X. K. Wang, ChemPlusChem, 2013, 78, 192–199 CrossRef CAS.
- L.-P. Zhu, N.-C. Bing, D.-D. Yang, Y. Yang, G.-H. Liao and L.-J. Wang, CrystEngComm, 2011, 13, 4486–4490 RSC.
- W. Wu, S. F. Zhang, F. Ren, X. H. Xiao, J. Zhou and C. Z. Jiang, Nanoscale, 2011, 3, 4676–4684 RSC.
- S. F. Zhang, F. Ren, W. Wu, J. Zhou, X. H. Xiao, L. L. Sun, Y. Liu and C. Z. Jiang, Phys. Chem. Chem. Phys., 2013, 15, 8228–8236 RSC.
- X. Zhang, H. Ren, T. Wang, L. Zhang, L. Li, C. Wang and Z. Su, J. Mater. Chem., 2012, 22, 13380–13385 RSC.
- A. Hernandez, L. Maya, E. Sanchez-Mora and E. M. Sanchez, J. Sol-Gel Sci. Technol., 2007, 42, 71–78 CrossRef CAS PubMed.
- W. Chiu, P. Khiew, M. Cloke, D. Isa, H. Lim, T. Tan, N. Huang, S. Radiman, R. Abd-Shukor and M. A. A. Hamid, J. Phys. Chem. C, 2010, 114, 8212–8218 CAS.
- J. H. Sui, J. Li, Z. G. Li and W. Cai, Mater. Chem. Phys., 2012, 134, 229–234 CrossRef CAS PubMed.
- Y. Liu, L. Yu, Y. Hu, C. F. Guo, F. M. Zhang and X. W. Lou, Nanoscale, 2012, 4, 183–187 RSC.
- W. Wu, S. F. Zhang, X. H. Xiao, J. Zhou, F. Ren, L. L. Sun and C. Z. Jiang, ACS Appl. Mater. Interfaces, 2012, 4, 3602–3609 CAS.
- D. Bi and Y. Xu, Langmuir, 2011, 27, 9359–9366 CrossRef CAS PubMed.
- H. Tong, S. Ouyang, Y. Bi, N. Umezawa, M. Oshikiri and J. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- G. Xi, B. Yue, J. Cao and J. Ye, Chem. – Eur. J., 2011, 17, 5145–5154 CrossRef CAS PubMed.
- S.-K. Li, F.-Z. Huang, Y. Wang, Y.-H. Shen, L.-G. Qiu, A.-J. Xie and S.-J. Xu, J. Mater. Chem., 2011, 21, 7459–7466 RSC.
- Y. Wang, S. Li, X. Xing, F. Huang, Y. Shen, A. Xie, X. Wang and J. Zhang, Chem. – Eur. J., 2011, 17, 4802–4808 CrossRef CAS PubMed.
- C. Zou, Y. Rao, A. Alyamani, W. Chu, M. Chen, D. A. Patterson, E. A. Emanuelsson and W. Gao, Langmuir, 2010, 26, 11615–11620 CrossRef CAS PubMed.
- A. G. Prado, L. B. Bolzon, C. P. Pedroso, A. O. Moura and L. L. Costa, Appl. Catal., B, 2008, 82, 219–224 CrossRef CAS PubMed.
- S. Ge, H. Jia, H. Zhao, Z. Zheng and L. Zhang, J. Mater. Chem., 2010, 20, 3052–3058 RSC.
- T. Murase, H. Irie and K. Hashimoto, J. Phys. Chem. B, 2004, 108, 15803–15807 CrossRef CAS.
- S.-T. Bae, H. Shin, S. Lee, D. W. Kim, H. S. Jung and K. S. Hong, React. Kinet., Mech. Catal., 2012, 106, 67–81 CrossRef CAS.
- L. Xu and J. Wang, Environ. Sci. Technol., 2012, 46, 10145–10153 CAS.
- N. Wetchakun, S. Chaiwichain, B. Inceesungvorn, K. Pingmuang, S. Phanichphant, A. I. Minett and J. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 3718–3723 CAS.
- H. Yang, R. Shi, J. Yu, R. Liu, R. Zhang, H. Zhao, L. Zhang and H. Zheng, J. Phys. Chem. C, 2009, 113, 21548–21554 CAS.
- A. Di Paola, E. García-López, G. Marcì and L. Palmisano, J. Hazard. Mater., 2012, 211, 3–29 CrossRef PubMed.
- Y. Lei, S. Song, W. Fan, Y. Xing and H. Zhang, J. Phys. Chem. C, 2009, 113, 1280–1285 CAS.
- Y. Hu, Y. Liu, H. Qian, Z. Li and J. Chen, Langmuir, 2010, 26, 18570–18575 CrossRef CAS PubMed.
- T. Wu, X. Zhou, H. Zhang and X. Zhong, Nano Res., 2010, 3, 379–386 CrossRef CAS PubMed.
- Y. C. Zhang, J. Li, M. Zhang and D. D. Dionysiou, Environ. Sci. Technol., 2011, 45, 9324–9331 CrossRef CAS PubMed.
- F. Cao, W. Shi, L. Zhao, S. Song, J. Yang, Y. Lei and H. Zhang, J. Phys. Chem. C, 2008, 112, 17095–17101 CAS.
- X. Liu, Z. Fang, X. Zhang, W. Zhang, X. Wei and B. Geng, Cryst. Growth Des., 2008, 9, 197–202 Search PubMed.
- Y. Shi, H. Li, L. Wang, W. Shen and H. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 4800–4806 CAS.
- S. Luo, F. Chai, L. Zhang, C. Wang, L. Li, X. Liu and Z. Su, J. Mater. Chem., 2012, 22, 4832–4836 RSC.
- W. Li, D. Li, W. Zhang, Y. Hu, Y. He and X. Fu, J. Phys. Chem. C, 2010, 114, 2154–2159 CAS.
- X. Xu, R. Lu, X. Zhao, S. Xu, X. Lei, F. Zhang and D. G. Evans, Appl. Catal., B, 2011, 102, 147–156 CrossRef CAS PubMed.
- J. Shi, H. n. Cui, Z. Liang, X. Lu, Y. Tong, C. Su and H. Liu, Energy Environ. Sci., 2011, 4, 466–470 CAS.
- W. Zhang and X. Zhong, Inorg. Chem., 2011, 50, 4065–4072 CrossRef CAS PubMed.
- Z. Chen, D. Li, W. Zhang, Y. Shao, T. Chen, M. Sun and X. Fu, J. Phys. Chem. C, 2009, 113, 4433–4440 CAS.
- Y. Chen, S. Hu, W. Liu, X. Chen, L. Wu, X. Wang, P. Liu and Z. Li, Dalton Trans., 2011, 40, 2607–2613 RSC.
- H. Yu and X. Quan, Prog. Chem., 2009, 21, 406–419 CAS.
- Z. Zhang and J. T. Yates, Chem. Rev., 2012, 112, 5520–5551 CrossRef CAS PubMed.
- W. Y. Teoh, J. A. Scott and R. Amal, J. Phys. Chem. Lett., 2012, 3, 629–639 CrossRef CAS.
- Y. Shaogui, Q. Xie, L. Xinyong, L. Yazi, C. Shuo and C. Guohua, Phys. Chem. Chem. Phys., 2004, 6, 659–664 RSC.
- Y. Liao, H. Li, Y. Liu, Z. Zou, D. Zeng and C. Xie, J. Comb. Chem., 2010, 12, 883–889 CrossRef CAS PubMed.
- H.-i. Kim, J. Kim, W. Kim and W. Choi, J. Phys. Chem. C, 2011, 115, 9797–9805 CAS.
- J. S. Chen, C. Chen, J. Liu, R. Xu, S. Z. Qiao and X. W. Lou, Chem. Commun., 2011, 47, 2631–2633 RSC.
- X.-L. Dong, X.-Y. Mou, H.-C. Ma, X.-X. Zhang, X.-F. Zhang, W.-J. Sun, C. Ma and M. Xue, J. Sol-Gel Sci. Technol., 2013, 66, 231–237 CrossRef CAS.
- Y. Zhang, X. Yu, Y. Jia, Z. Jin, J. Liu and X. Huang, Eur. J. Inorg. Chem., 2011, 2011, 5096–5104 CrossRef CAS.
- E. Stratakis and E. Kymakis, Mater. Today, 2013, 16, 133–146 CrossRef CAS PubMed.
- L. L. Sun, W. Wu, S. L. Yang, J. Zhou, M. Q. Hong, X. H. Xiao, F. Ren and C. Z. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 1113–1124 CAS.
- Y. Lin, Z. Geng, H. Cai, L. Ma, J. Chen, J. Zeng, N. Pan and X. Wang, Eur. J. Inorg. Chem., 2012, 2012, 4439–4444 CrossRef CAS.
- Y. C. Tang, C. HU, Y. Z. Wang, H. P. Zhang and X. H. Huang, Prog. Chem., 2005, 17, 225–230 CAS.
- Z. Chen, N. Zhang and Y.-J. Xu, CrystEngComm, 2013, 15, 3022–3030 RSC.
- Y. Zhang, Z. Chen, S. Liu and Y.-J. Xu, Appl. Catal., B, 2013, 140–141, 598–607 CrossRef CAS PubMed.
- P. Sheng, W. Li, J. Cai, X. Wang, X. Tong, Q. Cai and C. A. Grimes, J. Mater. Chem. A, 2013, 1, 7806–7815 CAS.
- L. Zhang, H. H. Mohamed, R. Dillert and D. Bahnemann, J. Photochem. Photobiol., C, 2012, 13, 263–276 CrossRef CAS PubMed.
- Y. Gao, Mater. Sci. Eng., R, 2010, 68, 39–87 CrossRef PubMed.
- M. M. Waegele, X. Chen, D. M. Herlihy and T. Cuk, J. Am. Chem. Soc., 2014, 136, 10632–10639 CrossRef CAS PubMed.
- V. Zhukov, V. Tyuterev, E. Chulkov and P. Echenique, Int. J. Photoenergy, 2014, 2014, 738921 CrossRef PubMed.
- N. G. Petrik and G. A. Kimmel, J. Phys. Chem. Lett., 2010, 1, 1758–1762 CrossRef CAS.
- P. S. Marcos, J. Marto, T. Trindade and J. Labrincha, J. Photochem. Photobiol., A, 2008, 197, 125–131 CrossRef CAS PubMed.
- D. Tsoukleris, A. Kontos, P. Aloupogiannis and P. Falaras, Catal. Today, 2007, 124, 110–117 CrossRef CAS PubMed.
- E. Rego, J. Marto, P. S. Marcos and J. Labrincha, Appl. Catal., A, 2009, 355, 109–114 CrossRef CAS PubMed.
- S. Nishimoto, A. Kubo, K. Nohara, X. Zhang, N. Taneichi, T. Okui, Z. Liu, K. Nakata, H. Sakai and T. Murakami, Appl. Surf. Sci., 2009, 255, 6221–6225 CrossRef CAS PubMed.
- P. Dzik, M. Vesely and J. Chomoucka, J. Adv. Oxid. Technol., 2010, 13, 172–183 CAS.
- M. Arin, P. Lommens, N. Avci, S. C. Hopkins, K. De Buysser, I. M. Arabatzis, I. Fasaki, D. Poelman and I. Van Driessche, J. Eur. Ceram. Soc., 2011, 31, 1067–1074 CrossRef CAS PubMed.
- M. Morozova, P. Kluson, J. Krysa, P. Dzik, M. Vesely and O. Solcova, Sens. Actuators, B, 2011, 160, 371–378 CrossRef CAS PubMed.
- T. Kawahara, K. Doushita and H. Tada, J. Sol-Gel Sci. Technol., 2003, 27, 301–307 CrossRef CAS.
- K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2012, 13, 169–189 CrossRef CAS PubMed.
- R. M. Pasquarelli, D. S. Ginley and R. O'Hayre, Chem. Soc. Rev., 2011, 40, 5406–5441 RSC.
- Y. Xia and L. Yin, Phys. Chem. Chem. Phys., 2013, 15, 18627–18634 RSC.
- Q. Tian, W. Wu, L. Sun, S. Yang, M. Lei, J. Zhou, Y. Liu, X. Xiao, F. Ren, C. Jiang and V. A. L. Roy, ACS Appl. Mater. Interfaces, 2014, 6, 13088–13097 CAS.
| This journal is © The Royal Society of Chemistry 2015 |