Polyelectrolyte-coated gold nanorods and their biomedical applications
Dakrong
Pissuwan
*a and
Takuro
Niidome
*b
aMaterials Science and Engineering Program, Multidisciplinary Unit, Faculty of Science, Mahidol University, Thailand. E-mail: dakrong.pis@mahidol.ac.th
bDepartment of Applied Chemistry and Biochemistry, Graduate School of Science and Technology, Kumamoto University, Japan. E-mail: niidome@kumamoto-u.ac.jp
First published on 3rd November 2014
Abstract
Gold nanorods (GNRs) have been extensively used in biomedical applications, because of their favourable optical properties. Their longitudinal surface plasmon resonance can be tuned, providing a strong near-infrared (NIR) extinction coefficient peak within the tissue transparency window. However, the modification of the surface of GNRs is essential before they can be used for biomedical applications. The number of GNRs taken up by cells and their biodistribution depend on their surface modification. Here, we review the recent advances in modifying GNR surfaces with polyelectrolytes for biomedical applications. Major polyelectrolytes used to coat GNR surfaces over the past few years and the biocompatibility of polyelectrolyte-coated GNRs are discussed.
1. Introduction
Gold nanorods (GNRs) have attracted much attention for therapeutic applications, because of their excellent optical properties. It is well-known that GNRs exhibit two plasmon resonance bands, called the transverse plasmon resonance and longitudinal plasmon resonance. The latter provides a very high extinction coefficient at near infrared (NIR) wavelengths, in which tissue is transparent. Furthermore, the position of the longitudinal band can be tuned by controlling the GNR shape.With these properties, GNRs have a great potential in biomedical applications. GNRs are typically synthesised using hexadecyl-trimethylammonium bromide (CTAB), a cationic surfactant that is toxic. The resulting CTAB bilayer on the GNR surface causes cell toxicity.1 Many approaches have been used to modify the GNR surface to yield low toxic alternatives. The stability of the GNRs after surface modification must also be considered. The surface of CTAB-GNRs has been modified with polyelectrolytes (PEs),2 lipids,3 silica,4 and gels,5 which rendered the GNRs suitable for biomedical applications. PEs have been most commonly used, perhaps because of their use in various biological systems.6 Different PE coatings on GNR surfaces have different effects on cellular uptake.7,8 The distribution and accumulation of surface-modified GNRs in cells and tissues also depend on the coating,9,10 so it is important to tailor the surface of GNRs with suitable PEs to the specific biomedical application.
Herein, we review the recent use of PE-coated GNRs in biomedical applications. We also review the effect of PE-coated GNRs on cellular uptake, cell interaction, and biocompatibility (Scheme 1).
2. Cellular uptake and cell interaction of PE-coated GNRs
The surface modification of GNRs is essential before their use in biomedical applications, and various approaches have been used to render the GNR surface more biocompatible.11 Cationic and anionic polymers (normally called PEs) have been used to coat the surface, and therefore lower the toxicity of CTAB-GNRs. In 2005, Gole and Murphy coated the surface of CTAB-GNRs with polystyrene sulfonate (PSS),2 and this approach is now common practice. Multilayer coatings have also recently been applied to GNR surfaces.2,12 This involved the electrostatic adsorption of combinations of anionic and cationic PEs to the GNR surface. For example, PSS was applied to the surface of GNRs to render them negatively charged. The cationic PE poly(diallyldimethylammonium chloride (PDAC) was then deposited on the PSS-GNRs.2 Coating GNRs with PSS and poly(acrylic acid, sodium salt) (PAA, an anionic PE) allowed the GNRs to be suspended in organic solvents with high stability. Coating GNRs with PAA and then the cationic PE; polyallylamine hydrochloride (PAH), also suppressed aggregation of the GNRs in polar organic solvents.13 Polyethyleneimine (PEI, a cationic polyelectrolyte) is also commonly used to modify the surface of GNRs.14,15 Interestingly, different PEs result in different cellular uptake of the GNRs, because of their different chemical structures and physical properties. The cellular uptake of PSS-GNRs was reportedly lower than that of PDAC-GNRs and PAH-GNRs.7,8 Remarkably, it was reported that a double PSS layer on the GNR surface provided a higher uptake into cells and had a higher zeta potential, than a single PSS layer.7Charge is not the only factor effecting cellular uptake. Hauck et al.7 reported that GNRs coated with PSS and then with a second layer of PDAC or PAH exhibited differing cellular uptake. The PDAC-GNRs contained quaternary and PAH-GNRs contained primary amines on their surfaces, respectively, and the latter were less internalised by cells. The serum in the medium also affects the cellular uptake of PE-coated GNRs. The same research group also reported that the serum could enhance the cellular uptake of the GNRs, and interacted with the PE coating.7 Alkilany et al.9 reported that the toxicities of PAA-GNRs and PAH-GNRs on endothelial cells were comparable, despite these GNRs possessing different charges. Remarkably, the PAA-GNRs and PAH-GNRs were found to provide the same negative surface charge within the culture medium, accounting for their similar effect on cell viability.
In the case of PEI coating, the viability of HEK293 cells increased after treating with PEI-GNRs for 24 and 48 h, compared with cells treated with the original CTAB-GNRs.14 Coating GNRs with PEI could both reduce the toxicity arising from CTAB, and increase the cellular uptake of the GNRs.16 Besides different PE coatings, the concentration of the PE-coated GNRs used in treating cells is also important. PSS-GNRs have been used at a concentration of 50 μg mL−1, without any impact on cell proliferation and function. However, PDAC-GNRs (25 μg mL−1) exhibited a high toxicity on macrophages.8
The effect of PE-coated GNRs on percutaneous absorption by the skin was also investigated by Lee et al.17 They reported that negatively charged PSS-GNRs could penetrate through a human skin equivalent model more readily than positively charged PDAC-PSS-GNRs. The reason for the greater penetration of PSS-GNRs, which had the same charge as skin, was not clear. Further investigation is required to understand the detailed skin penetration of both PE-coated GNRs.
3. Biomedical applications of PE-coated GNRs
3.1 Photothermal therapy
GNRs strongly absorb light, which can then be converted to heat by electron–phonon and phonon–phonon processes.18 This makes GNRs attractive candidates for photothermal therapy. PSS (molecular weight ∼18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was used to coat the surface of GNRs before conjugating with an antibody (anti-epidermal growth factor (anti-EGFR) monoclonal antibodies), which was specific to EGFR strongly expressed on malignant cell surfaces. The anti-EGFR-conjugated PSS-GNRs were incubated with malignant cells for 30 min. Dead malignant cells were observed at low power density irradiation (∼10 W cm−2), owing to heating by the GNRs. In contrast, a higher power density was required to destroy the nonmalignant cells incubated with anti-EGFR-conjugated PSS-GNRs, because of their lower GNR uptake.19 PAA-GNRs have also been used for cellular targeting. For example, PAA was coated on the surface of CTAB-GNRs. The carboxyl group of PAA allowed the PAA-GNRs to bind with the targeting agent, A33 single chain antibody (A33scFv). The resulting particles were used to destroy colorectal cancer, by irradiation with an 808 nm laser.20
000) was used to coat the surface of GNRs before conjugating with an antibody (anti-epidermal growth factor (anti-EGFR) monoclonal antibodies), which was specific to EGFR strongly expressed on malignant cell surfaces. The anti-EGFR-conjugated PSS-GNRs were incubated with malignant cells for 30 min. Dead malignant cells were observed at low power density irradiation (∼10 W cm−2), owing to heating by the GNRs. In contrast, a higher power density was required to destroy the nonmalignant cells incubated with anti-EGFR-conjugated PSS-GNRs, because of their lower GNR uptake.19 PAA-GNRs have also been used for cellular targeting. For example, PAA was coated on the surface of CTAB-GNRs. The carboxyl group of PAA allowed the PAA-GNRs to bind with the targeting agent, A33 single chain antibody (A33scFv). The resulting particles were used to destroy colorectal cancer, by irradiation with an 808 nm laser.20
Wang et al.21 recently reported the use of PAH-GNRs conjugated with rose bengal (RB, an anionic photosensitiser) in photothermal oral cancer therapy. This conjugation occurred via the electrostatic interaction of RB (presenting negative-charge from carboxylic and aromatic hydroxyl groups) with the positively charged PAH-GNR surface (Fig. 1).22 The RB molecules specific to oral cancer cells (Cal-27 cancer cells) were used for cancer targeting. Cells incubated with RB-PAH-GNRs and then irradiated with NIR wavelengths for 5 min were mostly dead. A similar effect was observed in an in vivo experiment, using a hamster cheek pouch as a model. After NIR irradiation for 60 s, the temperature of the tumour increased from 33.5 to 43.9 °C (Fig. 2). The tumour injected with solely phosphate buffer saline exhibited a temperature of ∼38 °C after 60 s irradiation.21
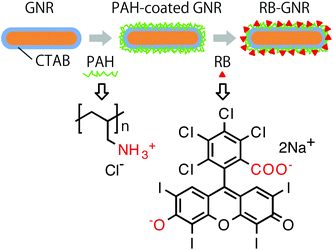 | ||
| Fig. 1 Schematic showing the preparation of RB-GNR conjugates (redrawn with permission from ref. 22). | ||
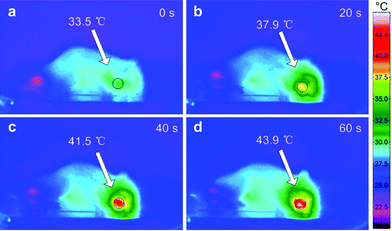 | ||
| Fig. 2 Infrared thermal images of a 7,12-dimethylbenz[α]anthracene-induced hamster tumour (a) nonirradiated and (b–d) irradiated at 810 nm for 20 to 60 s (reproduced and adapted with permission from ref. 21). | ||
Another example, PEI-PSS-GNRs were conjugated with methoxypolyethylene glycol thiol (mPEG-SH), and the resulting conjugates formed a complex with Al(III) phthalocyanine chloride tetrasulfonic acid (AlPcS4). A1PcS4 adsorbed on the PEI branches, and acted as a photosensitiser for photodynamic therapy. After exposure to light, this photosensitiser converted endogenous oxygen to singlet oxygen, which is toxic to MCF-7 cancer cells. The distance between the A1PcS4 and GNRs of the complex was ∼2 nm. A1PcS4 showed no phototoxicity in the circulatory system before release from the complex. The complex concentration used to treat the cancer cells was 5 nM. Approximately 90% of MCF-7 cells were destroyed after irradiating at 80 mW cm−2 for 0.5 min and then at 25 mW cm−2 for 1.5 min (Fig. 3).15
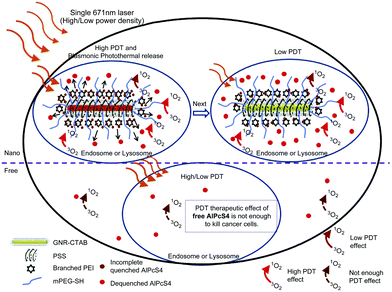 | ||
| Fig. 3 Schematic showing a modified GNR-AlPcS4 complex subjected to high/low power density irradiation for cancer cell destruction (reproduced with permission from ref. 15). | ||
The use of PE-coated GNRs to treat cancer was also reported by Wang et al.23 PAA-GNRs were prepared and then covalently bound to phage displayed peptides, by activating carboxylic groups of PAA using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide. Following this, the conjugated GNRs were treated with the HER2 overexpressed human mammary carcinoma cell line (SK-BR-3). The peptide on the surface of the PAA-GNRs was specific to the HER2 antigen, therefore the cellular internalisation of particles was higher than that of nonspecific cells. The cells were irradiated at 808 nm using a laser scanning confocal microscope, and were finally destroyed by hyperthermia.
3.2 Vaccine, drug and gene delivery
GNRs can be used as carriers for loading with therapeutic materials to treat diseases.24–26 In 2012, PDAC-GNRs and PEI-GNRs were used as adjuvants to enhance the immunogenicity of HIV-Env plasmid DNA. After attaching the Env plasmid DNA to PDAC-GNRs and PEI-GNR (referred to here as PDAC-GNR-Env and PEI-GNR-Env, respectively), these particles were intradermally injected into mice. The secretion of IFN-γ (a representative for the cellular immunity response) was significantly enhanced, compared with the injection of plasmid DNA alone. A very low immune response was observed in mice injected with CTAB-GNR-Env and PEI-Env (PEI attached with Env plasmid DNA without GNRs). Since PDAC-GNR-Env and PEI-GNR-Env strongly enhanced the immune response, they can be candidates for vaccine adjuvants.14PAH-PAA-GNRs containing the fusion protein (F) (the major virus protective material) were prepared by Stone et al.27 The respiratory syncytial virus (RSV) F protein was covalently conjugated to the PAH-PAA-GNRs using EDC, to form an amide bond between the PAH-PAA-GNRs and F. F-PAH-PAA-GNRs were treated with the co-culture system between human primary dendritic cells and human T cells. F-PAH-PAA-GNRs were then taken up by dendritic cells, and later induced T cells to produce an immune response against viral proteins. The high proliferation of T cells confirmed that the conjugation of F with PAH-PAA-GNRs had no toxic effect on T cells.
PSS-GNRs coated with cationic neopentyl glycol diglycidyl ether-6 (NPGDE-6) via electrostatic interactions were used for gene delivery. In this case, NPGDE-6-PSS-GNRs were attached to the DNA (luciferase gene PGL3). Later, MIA-PaCa-2 cells were treated with PGL3-NPGDE-6-PSS-GNRs, and the expression of luciferase was observed within cells. This confirmed that NPGDE-6-PSS-GNRs had attached to the DNA, and could be used for transgene delivery and expression.28 Huang et al.29 used PSS-GNRs wrapped with a cationic PE (ethyleneglycol diglycidyl ether reacted with 3,3′-diamino-N-methyl dipropylamine, EDGE-3,3′), to deliver plasmid DNA to prostate cancer cells (PC3-PSMA). Plasmid DNA was attached to EDGE-3,3′-PSS-GNRs and then DNA-EDGE-3,3′-PSS-GNRs were transfected into PC3-PSMA cells. Their results showed that EDGE-3,3′-PSS-GNRs enhanced the transfection of plasmid DNA into cells, and increased the stability of the GNRs. This increased GNR stability helped avoid low transfection efficiency.30,31 The intracellular delivery of antisense oligonucleotides (ASODNs) could also be performed using PEI-PSS-GNRs electrostatically conjugated with ASODNs.32
GNRs containing layer-by-layer coatings have also been prepared. Four layers were achieved, by repeatedly coating with PSS and PDAC. The coated GNRs were then attached to bovine serum albumin (BSA) to yield BSA-PE-GNRs. BSA on the GNR surface might enhance the cellular uptake of the GNRs, possibly via the endocytic pathway. The BSA layer also increased the stability of the particles.33,34 Following this, the anticancer drug doxorubicin (DOX) was electrostatically adsorbed on the BSA-PE-GNRs. The BSA-PE-GNRs@DOX particles were incubated with MCF-7 cells for 60 min, after which intense red fluorescence was observed in treated cells. Combining PE-coated GNRs, BSA, and anti-cancer drugs is a potential drug delivery system (Fig. 4).34
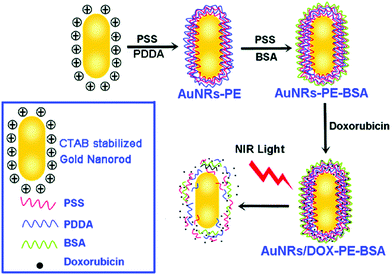 | ||
| Fig. 4 Schematic showing the preparation of BSA-PE-GNRs@DOX particles. PDDA indicated in this figure is identical to PDAC (reprinted with permission from ref. 34). | ||
In another example, PE-coated GNRs were conjugated with the model drugs fluorescein isothiocyanate (FITC) or paclitaxel (PTX).35 After conjugation, the GNR-drug conjugates were embedded within PEs to form GNRs/FITC@PAA and GNRs/PTX@PDAC, respectively. FITC and PTX were released from the complex following NIR irradiation. In this case, the heat energy strongly affected the drug release. The drug was released from the conjugate by the change in conjugate morphology, which occurred upon the conversion of absorbed energy into heat. This technique has also been used to confirm that particles were internalised within cells, and that the drugs were released to the target area. The GNRs/FITC@PAA complex was incubated with MCF-7 cells. The green fluorescence of FITC was detected around the centre of cell nuclei, both before and after irradiation. This implied that FITC reacted with amine groups of nucleus proteins within cells.35 The cell viability of MCF-7 decreased after treating cells with GNRs/PTX@PDAC and subsequent NIR irradiation.
3.3 Imaging
PE-coated GNRs have also been used in bioimaging. In 2007, Ding et al. coated GNRs with negatively charged poly(3,4-ethylenedioxythiophene) (PEDT)/PSS and then with positively charged PDAC. The resulting PDAC-PEDT/PSS-GNRSs were electrostatically attached to transferrin (Tf). The Tf-PEDT/PSS-GNRSs were incubated with HeLa cells, which were over-expressed in transferrin receptors. Fig. 5c shows that orange/red light scattering spots were observed in HeLa cells treated with Tf-PDAC-PEDT/PSS-GNRSs. These spots originated from the strong longitudinal surface plasmon oscillation of the GNRs. This scattering was more intense than the emission of most fluorophores.36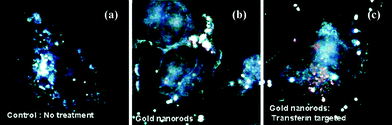 | ||
| Fig. 5 Dark field images of (a) HeLa cells untreated and treated with different GNRs. (b) Cells treated with GNRs did not show strong orange/red scattering and (c) cells treated with Tf-PDAC-PEDT/PSS-GNRs exhibited strong orange/red scattering from the surface plasmon enhancement of the GNRs (reprinted and adapted with permission from ref. 36). | ||
Ding et al. also used Tf-PDAC-PEDT/PSS-GNRs in a two-photon imaging technique. Human pancreatic carcinoma (Panc-1) cells overexpressed in transferrin receptors were incubated with Tf-PDAC-PEDT/PSS-GNRs for 3 h. Two-photon images were then recorded with a confocal system. The Panc-1 cells incubated with the Tf-PDAC-PEDT/PSS-GNRs exhibited stronger fluorescence than those subjected to other treatments. This strong fluorescence signal was enhanced by the GNRs attached on cells.37
Multilayered PE-coated GNRs have also been used as optical contrast agents for cancer cell imaging. Chen et al.38 reported multilayered GNRs coated with PSS and PAH. PAH-PSS-GNRs were stable and readily taken up by cells, via electrostatic interactions between the negative charge of cell membrane and the positive charge of PAH-PSS-GNRs. HeLa cells were treated with PAH-PSS-GNRs, after which red emission was detected within cells by dark field microscopy. This emission was from the light scattering of the GNRs. HeLa cells treated with CTAT-GNRs and PSS-GNRs did not exhibit the same emission as that from cells treated with PAH-PSS-GNRs (Fig. 6).
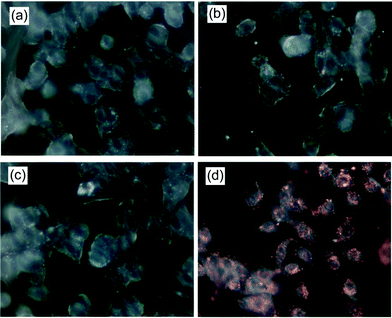 | ||
| Fig. 6 Dark field images of HeLa cells (a) untreated, (b) treated with CTAB-GNRs, (c) treated with PSS-GNRs, and (d) treated with PAH-PSS-GNRs (reprinted with permission from ref. 38). | ||
Molecules adsorbed on rough surfaces of noble nanomaterials can exhibit a huge enhancement in their Raman scattering, known as surface-enhanced Raman scattering (SERS). The SERS of GNRs has been used in various biological applications. This is because the longitudinal mode of the GNRs provides a large extinction coefficient and strong oscillation of electrons at the length of GNRs.39 This amplification of electromagnetic field means that SERS exhibits very high sensitivity for detecting analytes at low concentrations.40,41 Biocompatible PAH-PSS-GNRs have been incubated with HeLa cells, and a distinct SERS peak was observed at 435 cm−1. This peak was used as a characteristic peak to detect the distribution of PAH-PSS-GNRs on the cell surface.38 The SERS of PDAC-GNRs could be used to observe the distribution of intracellular SERS signals in macrophage cells as well.
The above results demonstrated that PE-coated GNRs could be used as SERS probes to detect biological molecules within cells.42 The SERS technique can be performed in another way using Raman reporters. For example, 4-mercaptobenzoic acid (MBA) has been used as a Raman reporter to prepare PAH-MBA-GNRs, which were used as a SERS probe to detect the epidermal growth factor receptor (EGFR) on single breast cancer cells (A431). The SERS signals of the probe with anti-EGFR differed from signals of the probe without anti-EGFR and the EGFR sample blocked with the free anti-EGFR antibody (Fig. 7).
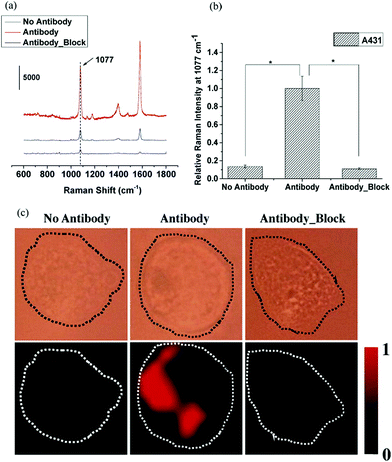 | ||
| Fig. 7 (a) SERS MBA probe spectra of anti-EGFR antibody (1077 and 1588 cm−1), no antibody and blocked antibody. (b) Raman intensity of the 1077 cm−1 peak from the antibody group treated on A431 cells, which was higher than those without antibody and blocked antibody (P < 0.001). (c) SERS mapping images of A431 cells incubated with SERS probes without the anti-EGFR antibody, SERS probes conjugated with the anti-EGFR antibody, and cells blocked with the anti-EGFR antibody before incubating with SERS probes conjugated with the anti-EGFR antibody (reprinted and adapted with permission from ref. 42). | ||
3.4 Therapeutic agents for antifibrotic therapy
PE-coated GNRs have been reported as agents for regulating cardiac fibrosis.43 It has been reported that the excess activity of myofibroblast cells can result in excess fibrous connective tissue, known as fibrosis. This fibrosis can lead to organ dysfunction;44 therefore, regulating fibrosis formation is important. Sisco et al.43 investigated the effect of GNRs on cell-mediated matrix remodelling. The GNR surface was modified in a layer-by-layer manner to yield PSS-PDAC-PSS-GNRs. Varying concentrations of these GNRs were mixed with type I collagen and cardiac fibroblast from neonatal rats, to form GNR-collagen-fibroblast composite gels. Gel contraction was inhibited when the high concentration of PSS-PDAC-PSS-GNR (5.6 × 109 GNR particles per gel) was incorporated into the composite. This reduced contraction did not arise from the death of fibroblast cells, confirming that the PSS-PDAC-PSS-GNRs were not toxic to cells. The enzyme matrix metalloproteinase 2 (MMP2) was reportedly produced by fibroblasts during gel contraction, which effected gel contraction and remodelling.45The PE-coated GNRs used in the above experiment did not affect the cells’ ability to produce MMP2 to degrade the extracellular matrix. The construction of collagen and PSS-PDAC-PSS-GNRs induced the expression of the β-actin gene. The GNRs in this construction reduced α-smooth muscle actin and type I collagen. The PSS-PDAC-PSS-GNRs obstructed the transition of fibroblasts to myofibroblast PSS-PDAC-PSS-GNRs are therefore candidates for future antifibrotic therapy.43
3.5 Natural PE-coated GNRs
Another aspect of interest, while synthetic PEs have been used to coat the surface of GNRs (Table 1), is the use of natural PEs for surface modification of GNRs. Recently, it has been reported that some natural PEs such as polysaccharide based polyelectrolytes (glycosaminoglycan) and polypeptides could be used to coat the surface of GNRs. In 2009, the surface of GNRs was modified by Wilson et al. using the natural polyanion sulfated glycosaminoglycans (heparin or chondroitin sulfate).46 In this case, the GNRs were firstly modified by PSS and PDAC to form PDAC-PSS-GNRs, and then polyanions (heparin or chondroitin) were added. These heparin-PDAC-PSS-GNRs and chondroitin-PDAC-PSS-GNRs could be used to promote cell function because both heparin and chondroitin sulfate molecules can bind with cellular ligands related to cell behavior.46,47 The surface modification of GNRs with natural PEs could present a lower toxicity than using synthetic PEs, especially at high concentration.8| Abbreviation | Name | Character | Application | Ref. |
|---|---|---|---|---|
| PSS | Polystyrene sulfonate | Anionic | Cellular uptake, Photothermal therapy, gene delivery, oligonucleotide delivery, drug delivery, imaging, antifibrotic therapy | 2,7,8,12,15,17,18,28–34,37,38,42,43,46 |
| PDAC (PDDA) | Poly(diallyldimethylammonium chloride) | Cationic | Cellular uptake, vaccine, drug delivery, imaging, antifibrotic therapy | 2,7,8,24–26,33–35,37,43,46 |
| PAA | Poly(acrylic acid) | Anionic | Cellular uptake, photothermal therapy, vaccine, drug delivery | 9,13,20,23,27,35 |
| PAH | Polyallylamine hydrochloride | Cationic | Cellular uptake, Photothermal therapy | 7–9,13,21,22,27,38,42 |
| PEI | Polyethyleneimine | Cationic | Cellular uptake, photothermal therapy, vaccine, oligonucleotide delivery | 14–16,24–26,32 |
| NPGDE-6 | Neopentyl glycol diglycidyl ether-6 | Cationic | Gene delivery | 28 |
| EDGE-3,3′ | Ethyleneglycol diglycidyl ether reacted with 3,3′-diamino-N-methyl dipropylamine | Cationic | Gene delivery | 29–31 |
| PEDT | Poly(3,4-ethylenedioxythiophene) | Anionic | Imaging | 36,37 |
4. Conclusions
PE-coated GNRs provide various benefits in biomedical applications. They can improve the efficiency of treatment and diagnosis techniques, especially in photothermal therapy, drug and gene delivery, vaccine delivery, bioimaging, and antifibrotic therapy. The PE-coating on the GNR surface is an important consideration, because different PE-coatings have different impacts on cellular uptake. These PEs can reduce the toxicity of CTAB on the original GNR surface, but the concentration of PE-coated GNRs must be considered. The careful design of PEs and GNRs will promote the use of PE-coated GNRs in future biomedical applications.Acknowledgements
This work was supported by the Talent Management Project and Materials Science and Engineering Program, Multidisciplinary Unit, Faculty of Science, Mahidol University, Thailand.References
- L. Wang, X. Jiang, Y. Ji, R. Bai, Y. Zhao, X. Wu and C. Chen, Nanoscale, 2013, 5, 8384 RSC.
- A. Gole and C. J. Murphy, Chem. Mater., 2005, 17, 1325 CrossRef CAS.
- E. T. Castellana, R. C. Gamez and D. H. Russell, J. Am. Chem. Soc., 2011, 133, 4182 CrossRef CAS PubMed.
- Z. Zhang, L. Wang, J. Wang, X. Jiang, X. Li, Z. Hu, Y. Ji, X. Wu and C. Chen, Adv. Mater., 2012, 24, 1418 CrossRef CAS PubMed.
- T. Kawano, Y. Niidome, T. Mori, Y. Katayama and T. Niidome, Bioconjugate Chem., 2009, 20, 209 CrossRef CAS PubMed.
- A. B. Scranton, B. Rangarajan and J. Klier, Biomedical applications of polyelectrolytes, In Biopolymers II, ed. N. Peppas and R. Langer, Springer Berlin Heidelberg, 1995, Vol. 122, pp 1 Search PubMed.
- T. S. Hauck, A. A. Ghazani and W. C. W. Chan, Small, 2008, 4, 153 CrossRef CAS PubMed.
- D. Pissuwan, Y. Kumagai and N. I. Smith, Part. Part. Syst. Char., 2013, 30, 427 CrossRef CAS.
- A. M. Alkilany, A. Shatanawi, T. Kurtz, R. B. Caldwell and R. W. Caldwell, Small, 2012, 8, 1270 CrossRef CAS PubMed.
- R. G. Rayavarapu, W. Petersen, L. Hartsuiker, P. Chin, H. Janssen, F. W. B. Leeuwen, C. Otto, S. Manohar and T. G. Leeuwen, Nanotechnology, 2010, 21, 145101 CrossRef PubMed.
- L. Tong, Q. Wei, A. Wei and J.-X. Cheng, Photochem. Photobiol., 2009, 85, 21 CrossRef CAS PubMed.
- C. G. Wilson, P. N. Sisco, F. A. Gadala-Maria, C. J. Murphy and E. C. Goldsmith, Biomater., 2009, 30, 5639 CrossRef PubMed.
- A. M. Alkilany, L. B. Thompson and C. J. Murphy, ACS Appl. Mater. Interfaces, 2010, 2, 3417 CAS.
- L. Xu, Y. Liu, Z. Chen, W. Li, Y. Liu, L. Wang, Y. Liu, X. Wu, Y. Ji, Y. Zhao, L. Ma, Y. Shao and C. Chen, Nano Lett., 2012, 12, 2003 CrossRef CAS PubMed.
- Z. Shi, W. Ren, A. Gong, X. Zhao, Y. Zou, E. M. B. Brown, X. Chen and A. Wu, Biomater., 2014, 35, 7058 CrossRef PubMed.
- H. A. Andersson, Y.-S. Kim, B. E. O'Neill, Z.-Zheng Shi and R. E. Serda, Vaccines, 2014, 2, 216 CrossRef PubMed.
- O. Lee, S. H. Jeong, W. U. Shin, G. Lee, C. Oh and S. W. Son, Skin Res. Technol., 2013, 19, e390 CrossRef PubMed.
- S. Link and M. A. El-Sayed, Int. Rev. Phys. Chem., 2000, 19, 409 CrossRef CAS.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115 CrossRef CAS PubMed.
- D. K. Kirui, S. Krishnan, A. D. Strickland and C. A. Batt, Macromol. Biosci., 2011, 11, 779 CrossRef CAS PubMed.
- B. Wang, J.-H. Wang, Q. Liu, H. Huang, M. Chen, K. Li, C. Li, X.-F. Yu and P. K. Chu, Biomater., 2014, 35, 1954 CrossRef CAS PubMed.
- J. H. Wang, B. Wang, Q. Liu, Q. Li, H. Huang, L. Song, T.-Y. Sun, H. Wang, X.-F. Yu, C. Li and P. K. Chu, Biomater., 2013, 34, 4274 CrossRef CAS PubMed.
- J. Wang, B. Dong, B. Chen, Z. Jiang and H. Song, Dalton Trans., 2012, 41, 11134 RSC.
- K. V. Chakravarthya, A. C. Bonoiud, W. G. Davisb, P. Ranjanb, H. Dingd, R. Hud, B. Bowzardb, E. J. Bergeyd, J. M. Katzb, P. R. Knighta, S. Sambharab and P. N. Prasadd, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10172 CrossRef PubMed.
- D. Pissuwan, T. Niidome and M. B. Cortie, J. Controlled Release, 2011, 149, 65 CrossRef CAS PubMed.
- J. Ramos, H. C. Huang and K. Rege, Delivery of Plasmid DNA to Mammalian Cells Using Polymer–Gold Nanorod Assemblies. In Cellular and Subcellular Nanotechnology, ed. V. Weissig, T. Elbayoumi and M. Olsen, Humana Press, 2013, 991, 81 Search PubMed.
- J. W. Stone, N. J. Thornburg, D. L. Blum, S. J. Kuhn, D. W. Wright and J. E. C. Jr., Nanotechnology, 2013, 24, 295102 CrossRef PubMed.
- L. Vu, J. Ramos, T. Potta and K. Rege, Theranostics, 2012, 2, 1160 CrossRef CAS PubMed.
- H. C. Huang, S. Barua, D. B. Kay and K. Rege, ACS Nano, 2009, 3, 2941 CrossRef CAS PubMed.
- F. Tang and J. A. Hughes, J. Controlled Release, 1999, 62, 345 CrossRef CAS.
- W. Yu, C. Liu, J. Ye, W. Zou, N. Zhang and W. Xu, Nanotechnology, 2009, 20, 215102 CrossRef PubMed.
- S. Chen, Y. Ji, Q. Lian, Y. Wen, H. Shen and N. Jia, Nano Biomed. Eng., 2010, 2, 15 CAS.
- A. M. Alkilany, P. K. Nagaria, C. R. Hexel, T. J. Shaw, C. J. Murphy and M. D. Wyatt, Small, 2009, 5, 701 CrossRef CAS PubMed.
- H. Chen, X. Chi, B. Li, M. Zhang, Y. Ma, S. Achilefu and Y. Gu, Biomater. Sci., 2014, 2, 996 RSC.
- T. R. Kuo, V. A. Hovhannisyan, Y. C. Chao, S. L. Chao, S. J. Chiang, S. J. Lin, C. Y. Dong and C. C. Chen, J. Am. Chem. Soc., 2010, 132, 14163 CrossRef CAS PubMed.
- H. Ding, K. T. Yong, I. Roy, H. E. Pudavar, W. C. Law, E. J. Bergey and P. N. Prasad, J. Phys. Chem. C, 2007, 111, 12552 CAS.
- J. Zhu, K. T. Yong, I. Roy, R. Hu, H. Ding, L. Zhao, M. T. Swihart, G. S. He, Y. Cui and P. N. Prasad, Nanotechnology, 2010, 21, 285106 CrossRef PubMed.
- L.-L. Chen, L. Jiang, Y.-L Wang, J. Qian and S. He, J. Zhejiang Univ. Sci. B., 2010, 11, 417 CrossRef CAS PubMed.
- D. Pissuwan, S. M. Valenzuela and M. B. Cortie, Biotechnol. Gen. Eng. Rev., 2008, 25, 93 CrossRef CAS.
- G. von Maltzahn, A. Centrone, J.-H. Park, R. Ramanathan, M. J. Sailor, T. A. Hatton and S. N. Bhatia, Adv. Mater., 2009, 21, 3175 CrossRef CAS PubMed.
- Y. Zhu, H. Kuang, L. Xu, W. Ma, C. Peng, Y. Hua, L. Wang and C. Xu, J. Mater. Chem., 2012, 22, 2387 RSC.
- L. Xiao, S. Harihar, D. R. Welch and A. Zhou, Anal. Chim. Acta, 2014, 843, 73 CrossRef CAS PubMed.
- P. N. Sisco, C. G. Wilson, E. Mironova, S. C. Baxter, C. J. Murphy and E. C. Goldsmith, Nano Lett., 2008, 8, 3409 CrossRef CAS PubMed.
- T. R. Cox and Janine T. Erler, Dis. Model. Mech., 2011, 4, 165 CrossRef CAS PubMed.
- K. T. Borg, W. Burgess, L. Terracio and T. K. Borg, Cardiovasc. Pathol., 1997, 6, 261 CrossRef CAS.
- G. C. Wilson, P. N. Sisco, C. E. Goldsmith and C. J. Murphy, J. Mater. Chem., 2009, 19, 6332 RSC.
- J. Luo, M. Kato, H. Wang, M. Bernfield and J. Bischoff, J. Cell. Biochem., 2001, 80, 522 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |