Thiol–bromo click polymerization for multifunctional polymers: synthesis, light refraction, aggregation-induced emission and explosive detection
Yiren
Zhang
a,
Gan
Chen
a,
Yiliu
Lin
a,
Lifang
Zhao
a,
Wang Zhang
Yuan
*a,
Ping
Lu
b,
Cathy K. W.
Jim
c,
Yongming
Zhang
*a and
Ben Zhong
Tang
*cd
aSchool of Chemistry and Chemical Engineering, Shanghai Key Lab of Electrical Insulation and Thermal Aging, Shanghai Jiao Tong University (SJTU), No. 800 Dongchuan Road, Minhang District, Shanghai 200240, China. E-mail: wzhyuan@sjtu.edu.cn; ymzsjtu@gmail.com
bState Key Laboratory of Supramolecular Structure and Materials, Jilin University, 2699 Qianjin Avenue, Changchun 130012, China
cDepartment of Chemistry, Institute of Molecular Functional Materials and Institutes for Advanced Study, The Hong Kong University of Science & Technology (HKUST), Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
dHKUST-Shenzhen Research Institute, No. 9 Yuexing 1st Road, South Area, Hi-tech Park, Nanshan, Shenzhen 518057, China
First published on 8th September 2014
Abstract
New multifunctional polymers PI and PII are synthesized from 4,4′-thiodibenzenethiol and 1,2-bis[4-(bromoalkoxy)phenyl]-1,2-diphenylethene through facile thiol–bromo click polymerization. The resulting polymers exhibit high refractive indices over a wide spectral region (300–1700 nm) and large Abbé numbers due to the presence of sulfur atoms and a large fraction of aromatic building blocks. The polymers also show high optical transparency in the visible region, rendering them ideal candidates for optical applications. Additionally, they demonstrate typical aggregation-induced emission (AIE) characteristics owing to the incorporation of propeller-like tetraphenylethene (TPE) moieties. The polymers are nonemissive in good solvents; however, they become highly fluorescent as aggregated suspensions whose emission is further effectively quenched by a representative explosive of picric acid (PA), exhibiting a significantly amplified superquenching effect, with the detection limit as low as 0.5 ppm. These results suggest a great promise of preparing multifunctional polymers by thiol–bromo click polymerization with a rational molecular design.
Introduction
There has been an increasing demand for functional polymers with high refractive indices (RI) and optical transparency for various advanced optical applications during the past few decades. As a result, scientists have devoted much effort to the fabrication of high-RI materials.1 According to the classic Lorentz–Lorenz equation, which is often used to explain the RI of materials, a general approach to increasing the RI of polymers is to introduce substituents with high molar refraction and low molar volume.2 Therefore, polymers containing numerous aromatic rings are supposed to possess higher refractivity than their conventional counterparts, whose RI values are often in the range of 1.30–1.70. Besides, the introduction of sulfur-containing moieties has also been widely adopted to increase the RI of common polymers.3 Many sulfur-containing functional polymers, including polythiourethanes,4 poly(S-alkylcarbamates),5 poly(thioether sulfones),6 epoxy- and episulfide-type polymers7 are reported to exhibit high refractivity. According to the synergistic effects, polymers combining both the abovementioned moieties might be promising candidates for various high-tech optical applications.One of the most convenient methods to prepare such sulfur-containing polymers is to use thiol as initiating monomers by click reactions. Proposed by Sharpless in 2001, click chemistry is increasingly becoming the method of choice for the design and synthesis of multifunctional macromolecules and advanced materials.8,9 In particular, thiol-based click chemistry, such as thiol–ene,10 thiol–yne,11,12 and thiol–isocyanate13 reactions, has been developed into powerful approaches for fabrication and modification strategies.14 These reactions can efficiently proceed at room temperature without expensive and potentially toxic catalysts. Among them, the thiol–bromo click reaction, though not a strict atom economical reaction like its analogues, shares their key features of high-efficiency transformations, simple reaction conditions and high tolerance of functional groups, etc.15–17 Therefore, it is envisaged that thiol–bromo click chemistry, first established by Percec and coworkers in 2009,15,16 can be developed into a new polymerization methodology to the construction of novel functional polymers.15–17
Among varying functional polymers, polymeric fluorescent chemosensors have been widely utilized in the detection of explosives,18,19 metal ions,20,21 biomolecules22 and other substances. Compared with molecular luminogens, polymers can be readily fabricated into large-area films through simple processing techniques at low cost. Moreover, they normally exhibit an amplified effect when utilized as sensors compared to their molecular counterparts. However, the sensing performance of many traditional polymer sensors is often deteriorated by their intrinsic aggregation-caused quenching (ACQ) features. Recently, a series of emitters, such as silole,23 tetraphenylethene (TPE),24 cyanostilbene (CSB)25 and triphenylacrylonitrile (TPAN)26 based luminogens, have been found to be weakly fluorescence in solutions while being highly emissive when aggregated. Such a phenomenon was termed as aggregation-induced emission (AIE). So far, AIE-active molecules have been widely utilized in the fabrication of various fluorescent sensors.27 The AIE strategy has helped to solve the thorny ACQ problem and moreover impart the luminogens with unique properties for practical applications.28–32
In this work, multifunctional polymers with high RI values and high solid-state efficiencies are prepared by facile thiol–bromo click polymerization, utilizing 4,4′-thiodibenzenethiol containing multiple sulfur atoms and phenyl blades, together with TPE-bearing bis(alkyl bromide). Introduction of sulfur atoms into polymer chains is expected to result in a large increase in the RI values. Meanwhile, TPE is chosen as a building block due to its advantages of AIE characteristic, facile synthesis, high stability, and versatile functionalization, which endow the resulting polymers with a hereditary AIE property and thus high solid-state efficiency.27 It is also noted that multiple aromatic components from the monomers can further improve the RI of the polymers. The highly emissive polymer suspensions are further adopted to detect the explosives, utilizing picric acid (PA) as a representative, with a superamplification quenching effect being observed.
Experimental
Materials
THF was distilled under normal pressure from sodium benzophenone ketyl under nitrogen immediately prior to use. Dichloromethane (DCM) was distilled under normal pressure over calcium hydride under nitrogen before use. Triethylamine (TEA) was distilled and dried over sodium hydroxide. 4-Hydroxylbenzophenone (3), 1,2-dibromoethane [4(2)], 1,6-dibromohexane [4(6)], PA, potassium carbonate (K2CO3), titanium tetrachloride (TiCl4), anhydrous sodium sulfate (Na2SO4), zinc dust and acetone were purchased from Aldrich and used as received.Instruments
1H and 13C NMR spectra were measured on a Bruker ARX 400 spectrometer using CDCl3 as the solvent and tetramethylsilane (TMS) as an internal standard. IR spectra were recorded by a Bruker Vector 22 spectrometer. Matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF) high-resolution mass spectra (HRMS) were recorded on a GCT premier CAB048 mass spectrometer. Thermogravimetric analysis (TGA) was conducted using a NETZSCH TG209 F3 instrument under nitrogen at a heating rate of 20 °C min−1. Absorption spectra were taken on a Milton Roy Spectronic 3000 Array spectrometer. Photoluminescence (PL) spectra were measured on a PerkinElmer LS 55 fluorescence spectrometer. Number- and weight-averaged molecular weights (Mn and Mw) and polydipersity index (PDI, Mw/Mn) of the polymers were estimated by a Waters Associates gel permeation chromatography (GPC) system. THF was used as eluent at a flow rate of 1.0 mL min−1. A set of monodisperse polystyrene standards covering the molecular weight range of 103–107 was used for the molecular weight calibration. PL quantum yields of the luminogens in solutions (Φs) were measured using quinine sulfate (Φs = 54% in 0.1 N H2SO4) as a standard, whereas those of their solid films (Φf) were determined using an integrating sphere.Synthesis of intermediates and monomers
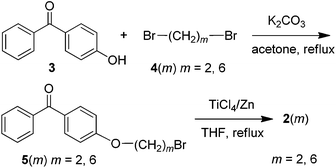
Intermediates [4-(2-bromoethoxy)phenyl](phenyl)methanone [5(2)] and {4-[(6-bromohexyl)oxy]phenyl}(phenyl)methanone [5(6)] were synthesized according to the route shown in Scheme 1. Detailed procedures and characterization data were given in our previous ref. 30,33. Monomers 2(m) (m = 2, 6) were prepared according to the following procedures.Preparation of PI and PII
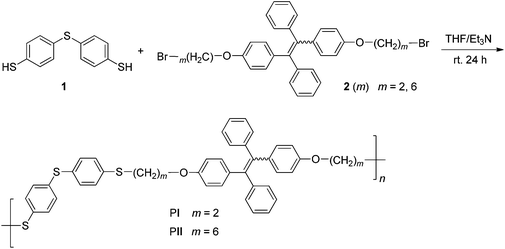
Polymers PI and PII were synthesized by thiol–bromo click polymerization between 1 and 2(m) (m = 2, 6), as shown in Scheme 2. The polymerization reactions and manipulations were carried out under nitrogen except for the purification of the polymers. A typical experimental procedure for the polymerization of monomer 1 with 2(6) is given below as an example. Into a baked 20 mL Schlenk tube were added 25 mg of 1 and 69.0 mg of 2(6). The tube was evacuated under vacuum for 5 min and flushed with nitrogen three times. Freshly distilled THF (4 mL) and TEA (1 mL) was injected into the tube under stirring. During the polymerization process, a white solid was precipitated from the solution. The reaction lasted for 24 h at room temperature (25 ± 0.5 °C). Then the mixture was diluted with 5 mL of THF, and added dropwise to 500 mL of hexanes through a cotton filter (to remove the insoluble salt) under stirring. The precipitate was allowed to stand overnight, and then filtered with a Gooch crucible. Product PII was isolated by washing with hexanes and drying in a vacuum oven at 40 °C to a constant weight. A white solid was obtained in 68.4% yield (Table 1, entry 8). Mw = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300, Mw/Mn = 1.44. 1H NMR (400 MHz, CDCl3, δ, ppm): 7.22, 7.10, 7.08, 7.06, 7.05, 7.04, 7.02, 7.01, 7.00, 6.94, 6.91, 6.90, 6.88, 6.63, 6.61, 6.60, 6.58, 3.86, 3.84, 3.83, 2.92, 2.90, 2.88, 1.72, 1.70, 1.68, 1.66, 1.64, 1.57, 1.46, 1.45, 1.43, 1.41, 1.40, 1.27. 13C NMR (100 MHz, CDCl3, δ, ppm): 157.42, 144.32, 139.61, 136.33, 136.24, 132.80, 132.56, 132.50, 131.40, 131.38, 129.30, 127.52, 127.51, 126.12, 113.60, 113.50, 67.52, 33.39, 31.57, 29.16, 29.05, 28.92, 28.54, 25.66, 22.64.
300, Mw/Mn = 1.44. 1H NMR (400 MHz, CDCl3, δ, ppm): 7.22, 7.10, 7.08, 7.06, 7.05, 7.04, 7.02, 7.01, 7.00, 6.94, 6.91, 6.90, 6.88, 6.63, 6.61, 6.60, 6.58, 3.86, 3.84, 3.83, 2.92, 2.90, 2.88, 1.72, 1.70, 1.68, 1.66, 1.64, 1.57, 1.46, 1.45, 1.43, 1.41, 1.40, 1.27. 13C NMR (100 MHz, CDCl3, δ, ppm): 157.42, 144.32, 139.61, 136.33, 136.24, 132.80, 132.56, 132.50, 131.40, 131.38, 129.30, 127.52, 127.51, 126.12, 113.60, 113.50, 67.52, 33.39, 31.57, 29.16, 29.05, 28.92, 28.54, 25.66, 22.64.
| Entry | Solvent | Yield (%) | M w | PDIb |
|---|---|---|---|---|
| a Carried out under an atmosphere of dry nitrogen for 24 h at room temperature (25 ± 0.5 °C) unless specified. b Determined by GPC in THF on the basis of a polystyrene calibration. c V THF = VDCM = 4VTEA; [1] = [2(m)] = 20 mM. d V THF = VDCM = 4VTEA; [1] = [2(m)] = 40 mM. e Polymerization temperature is 60 °C. | ||||
| PI | ||||
| 1c | DCM–TEA | 47.2 | 2600 | 1.57 |
| 2d | DCM–TEA | 60.2 | 2500 | 1.24 |
| 3c | THF–TEA | 56.3 | 3900 | 1.61 |
| 4d | THF–TEA | 62.2 | 5800 | 1.89 |
| 5d,e | THF–TEA | 76.3 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.66 |
| PII | ||||
| 6c | DCM–TEA | 63.7 | 3000 | 1.18 |
| 7d | DCM–TEA | 71.4 | 6500 | 1.79 |
| 8c | THF–TEA | 68.4 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.44 |
| 9d | THF–TEA | 83.6 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.42 |
| 10d,e | THF–TEA | 95.4 | 6300 | 1.58 |
Characterization data of PI: White solid, yield: 76.3%, Mw = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw/Mn = 3.66 (Table 1, entry 5). 1H NMR (400 MHz, CDCl3, δ, ppm): 7.91, 7.90, 7.60, 7.58, 7.28, 7.24, 7.21, 7.08, 7.03, 7.01, 6.906, 6.58, 4.06, 3.91, 3.60, 3.58, 3.57, 3.46, 3.23. 13C NMR (100 MHz, CDCl3, δ, ppm): 156.66 (Ar-O), 144.08, 143.98, 139.65, 136.83, 136.76, 134.96, 133.58, 132.52, 132.15, 131.48, 131.31, 130.82, 130.11, 128.52, 127.68, 127.56, 126.25, 113.75, 113.65 (Ar), 66.36 (OCH2), 32.73 (CH2S).
000, Mw/Mn = 3.66 (Table 1, entry 5). 1H NMR (400 MHz, CDCl3, δ, ppm): 7.91, 7.90, 7.60, 7.58, 7.28, 7.24, 7.21, 7.08, 7.03, 7.01, 6.906, 6.58, 4.06, 3.91, 3.60, 3.58, 3.57, 3.46, 3.23. 13C NMR (100 MHz, CDCl3, δ, ppm): 156.66 (Ar-O), 144.08, 143.98, 139.65, 136.83, 136.76, 134.96, 133.58, 132.52, 132.15, 131.48, 131.31, 130.82, 130.11, 128.52, 127.68, 127.56, 126.25, 113.75, 113.65 (Ar), 66.36 (OCH2), 32.73 (CH2S).
Measurement of refractive indices
RI values of PI and PII were measured on a J. A. Woollam variable-angle ellipsometry system with a wavelength tunable from 300 to 1700 nm. The Levenberg–Marquardt regression algorithm was employed to fit the acquired Ψ and Δ curves with the data obtained from the three-layered optical model, which consisted of a crystalline silicon substrate, a 2 nm SiO2 layer, and a uniform polymer film. And the Cauchy dispersion law was used to describe the polymer layer from the visible to IR spectral regions.Results and discussion
Polymers PI and PII were synthesized via the thiol–bromo click polymerization between monomer 1 and 2(m) (m = 2, 6) (Scheme 2). In all polymerizations, TEA was utilized as a catalyst and a scavenger to eliminate the formed hydrogen bromide, which would promote the polymerization processes. To prepare PI, we first attempted the polymerization in DCM–TEA at a monomer concentration of 20 mM, but only with oligomers (Mw = 2600) obtained in moderate yield (47.2%). Increasing the monomer concentration to 40 mM merely increases the yield (Mw = 2500, 60.2%). Better results were obtained when THF–TEA was utilized (Table 1, entries 3 and 4), wherein higher monomer concentration offers enhanced yield and improved molecular weight. Unfortunately, the resulting products are still low molecular weight oligomers. To further promote the polymerization, the reaction temperature was raised to 60 °C. To our delight, PI was attained in much higher yield (76.3%) and moreover with greatly improved Mw of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
The polymerization behaviors between 1 and 2(6) are quite similar to those between 1 and 2(2): while in DCM–TEA, only oligomers are produced, in THF–TEA, macromolecules with higher Mw and yield are achieved; in addition, higher concentration and temperature both contribute to the improvement of polymer yield. However, notably, with a higher temperature of 60 °C, Mw of PI increases from 5800 to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, whereas that of PII decreases from 15
000, whereas that of PII decreases from 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 to 6300. Such opposite trends may indicate the coexistence of polymerization and dissociation during the click reaction processes, whose kinetics can be simultaneously promoted by increased temperature. The final impact on molecular weight is thus dependent on the balance of these antagonistic effects.
200 to 6300. Such opposite trends may indicate the coexistence of polymerization and dissociation during the click reaction processes, whose kinetics can be simultaneously promoted by increased temperature. The final impact on molecular weight is thus dependent on the balance of these antagonistic effects.
The chemical structures of monomers and polymers were characterized by IR and NMR spectra, with satisfactory results obtained. For example, IR spectra of polymer PII and its monomers 1 and 2(6) are depicted in Fig. 1. The S–H and C–Br stretching vibrations of monomers 1 and 2(6) are observed at 2558 and 665 cm−1, respectively. However, these characteristic absorptions are completely absent in the spectra of PII, indicating that the mercapto groups of 1 and alkyl bromides of 2(6) have almost been consumed by the polymerization.
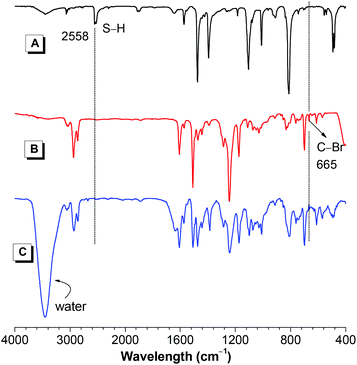 | ||
| Fig. 1 IR spectra of (A) monomer 1, (B) monomer 2(6) and (C) polymer PII. PII was taken from Table 1, entry 8. | ||
1H NMR spectra of polymer PII was further analyzed, and those of its monomers are given in the same figure for comparison. The protons of thiol and methylene bromide groups resonated at δ 3.46 (Fig. 2A, c) and 3.41 ppm (Fig. 2B, e) completely disappear in the spectrum of PII. Meanwhile, new resonance peaks assignable to the alkyl sulfide structures are observed at δ ∼2.90 ppm (Fig. 2C, e), thus suggesting the full consumption of thiol and bromide moieties. All other peaks can be rationally assigned to the resonances of appropriate protons of the monomers and polymers, and no unexpected peaks are found in the spectra (Fig. 2). These results are consistent with the IR results, thus verifying that the sulfur-containing polymers are successfully obtained by the facile thiol–bromo click polymerization.
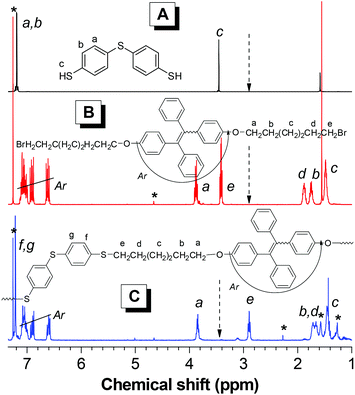 | ||
| Fig. 2 1H NMR spectra of (A) monomer 1, (B) monomer 2(6) and (C) polymer PII in CDCl3. PII was taken from Table 1, entry 8. | ||
The thermal stability of PI and PII is studied by TGA measurement. As depicted in Fig. 3, both PI and PII exhibit moderate thermal stability, with the degradation temperatures (Td, at which a sample loses its 5% weight) of 340 and 330 °C, respectively. Such reasonable stability makes them ready for practical applications.
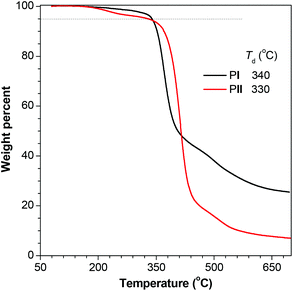 | ||
| Fig. 3 TGA thermograms of polymers PI and PII recorded under nitrogen at a scan rate of 20 °C min−1. PI and PII were taken from Table 1, entries 5 and 8, respectively. | ||
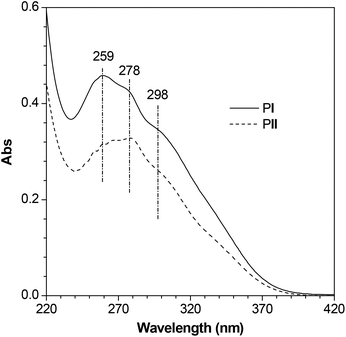
PI and PII show similar absorption profiles with peaks and/or shoulders at 259, 278, and 298 nm (Fig. 4), which should be ascribed to the overlapped absorptions (π–π* transitions) from phenyl sulfides and TPE units. Both polymers exhibit no absorption at wavelengths longer than 400 nm in THF solutions, indicating their high transparency in the visible spectral region, which is of crucial importance for their photonic applications. The excellent transparencies of the polymers may be attributed to the presence of alkyl and phenyl sulfides in the repeat units, which break the electronic communications between neighboring conjugated moieties without enhancing the effective conjugation length of the resulting polymer chains, thereby offering low or even no absorptivities for the polymers in the long wavelength region.
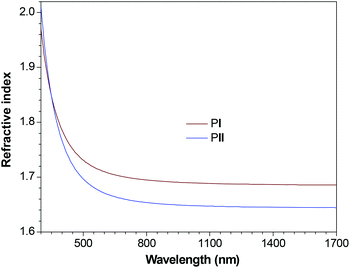
According to the Lorentz–Lorenz equation, the introduction of segments with high molar refraction, low molar volume or high density is effective in increasing light refractivity. The high fraction of sulfur and aromatic units and excellent transparency of the polymer films render them promising candidates as high RI materials. Indeed, as can be seen from Fig. 5, the thin film of PI displays high RI values (1.9710–1.6856) in the wide wavelength region of 300–1700 nm. Similarly high RI values are also observed in PII (2.0112–1.6444). The RI values of PI and PII at 632.8 nm are 1.7061 and 1.6663, respectively, which are much higher than those of the commercially important optical plastics [e.g., ∼1.49 for poly(methyl methacrylate) (PMMA) and ∼1.59 for polycarbonate (PC)].34 The RI data of polymers PI and PII are summarized in Table 2.
| n 632.8 | n 1064 | n 1319 | n 1550 | v ′D | |
|---|---|---|---|---|---|
| PI | 1.7061 | 1.6890 | 1.6869 | 1.6860 | 229.0 |
| PII | 1.6663 | 1.6475 | 1.6455 | 1.6447 | 230.5 |
At the telecommunication wavelength of 1550 nm, PI and PII show RI values of 1.6860 and 1.6447, respectively. The higher value of PI should be ascribed to its relatively larger fractions of sulfur and aromatic components owing to its much shorter alkyl chains. These results highlight the feature of high refractivity for our polymer system and manifest their manipulability through molecular engineering endeavors. It is also expected that polymers with much higher RI values can be obtained with less or even no alkyl chains between sulfur and TPE units.
Small chromatic aberration is a prerequisite for a material to be useful as an optical instrument, which can be reflected by the Abbé numbers. Generally, conventional polymers with high RI exhibit low Abbé numbers, that is, their chromatic dispersions are too large for practical applications. For PI and PII, we adopted a modified Abbé number (v′D) to evaluate their potential application as optical materials, using their RI values at the non-absorbing infrared wavelengths of 1064, 1319 and 1550 nm. The modified Abbé number is defined as: v′D = (n1319 − 1)/(n1064 − n1550), where n1319, n1064 and n1550 are the RI values at 1319, 1064 and 1550 nm, respectively. The n and v′D values for thin films of PI and PII are listed in Table 2. Clearly, both polymers exhibit simultaneously high RI values and large v′D up to ∼230. These results confirm the synergistic interplay between the sulfur groups and aromatic rings, which endow the polymers with high RI and low chromatic dispersions.
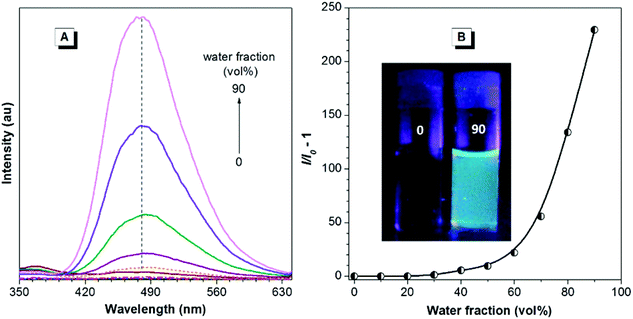
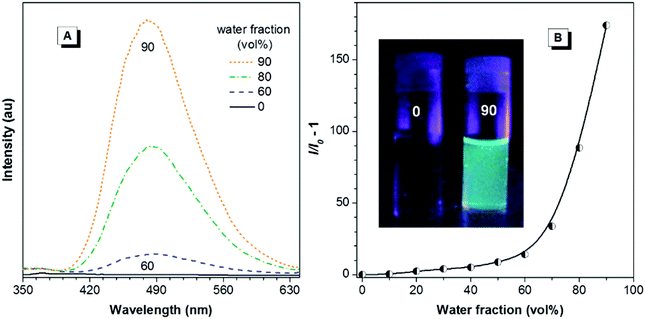
Besides high RI values and low chromatic dispersions, PI and PII are also expected to demonstrate unique AIE characteristics and high solid-state efficiencies because of the incorporation of TPE, which is an archetypal AIE-active moiety. To verify this, PL behaviors of both polymers in the aqueous mixtures with different water fractions were investigated. As demonstrated in Fig. 6, in the THF solution (0.008 mg mL−1), PI gives no visible emission upon excitation, indicating that it is nonluminescent when dissolved in good solvents. Fluorescence intensity remains almost unchanged in aqueous mixtures with water fraction less than 30%. The emission intensity, however, starts to increase with water fraction ≥40%. At the water fraction of 90%, the net increase of the PL peak intensity (I/I0 − 1) at 480 nm is as high as 229.5. Similar emission behaviors are also observed for PII (Fig. 7), whose PL peak intensity in 10/90 THF–water at 482 nm is increased by 174-fold compared to that in THF. Such emission behaviors reveal that both polymers are typical AIE molecules, which also can be seen from the vivid image contrast in Fig. 6B and 7B. To further quantify the AIE effects, quantum yields of PI and PII in both solution and solid film states were determined. Φs values of PI and PII in THF are merely 0.17% and 0.20%, whereas those of the solid films are boosted to 60.7% and 56.4%, giving AIE factors (α = Φf/Φs) as high as ∼357 and 282, respectively. Such AIE characteristics of the polymers should be ascribed to the restriction of intramolecular rotations of TPE units. In the solution state, the polymers are molecularly dissolved and the phenyl blades of TPE moieties can freely rotate, which effectively dissipates the exciton energy and thus makes the polymers nonemissive. Upon gradual addition of water or in the thin films, however, such intramolecular rotations are significantly impeded by the aggregate formation, which significantly blocks the nonradiative channels, thus making the polymers brightly luminescent.
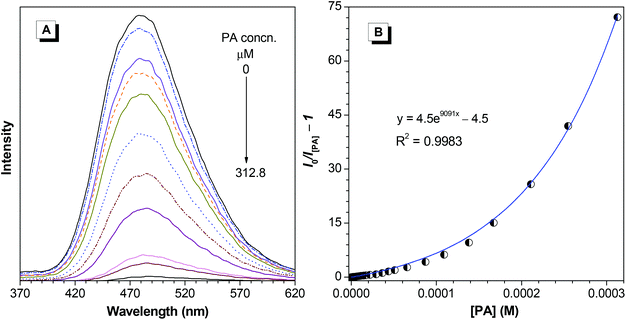
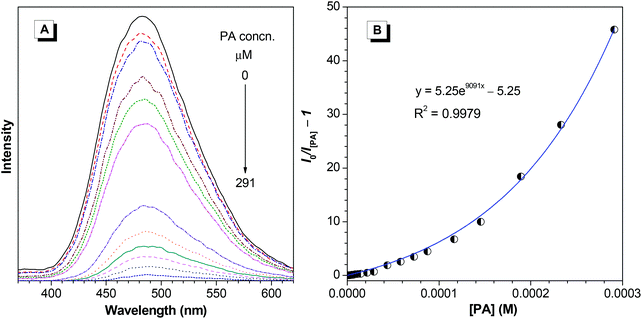
The high solid-state efficiency of the polymers prompted us to further explore its applications. Recently, explosives detection has been of great importance and been receiving intensive research interest due to its homeland security and anti-terrorism implications. We thus tried to use PI and PII as fluorescent chemosensors for explosives. Herein, PA is employed as a model explosive among varying nitroaromatic compounds because of its commercial availability. The nanoaggregates of PI and PII in 10/90 THF–water were used as explosive probes, whose emission intensities decrease with the increase of PA concentration (Fig. 8A and 9A). Notably, the detection limits can be as low as 0.5 and 0.8 ppm for PI and PII, respectively. The Stern–Volmer plot of relative PL intensity (I0/I[PA] − 1) versus the PA concentration ([PA]) gives a curve bending upward, instead of a linear line (Fig. 8B and 9B) that is normally observed for conventional probes. Such nonlinear Stern–Volmer curve can be well-fitted to the exponential equations of I/I0 = 4.5e9091[PA] − 3.5 (PI) and I/I0 = 5.25e9091[PA] − 4.25 (PII), which indicate that the PL quenching becomes more efficient with increasing quencher concentration, exhibiting an obvious superamplification quenching effect.35 When the AIE-active polymers aggregate in 10/90 THF–water mixture, a three-dimensional network is formed. Explosive molecules, such as PA, can penetrate into the network, and quench the emission of other luminogens nearby, thus leading to the superquenching effect.
Conclusions
In summary, thiol–bromo click polymerization, a new methodology to construct multifunctional polymers, is demonstrated. With rational molecular design, polymers consisting of AIE-active TPE units and sulfide linking groups, with high RI values and high solid-state efficiencies, are obtained through the facile thiol–bromo click polymerization. By taking advantage of the numerous sulfur atoms and phenyl rings in polymer chains, the resulting polymers PI and PII can possess high RI values and large Abbé numbers, making them promising for real-world optical applications. Meanwhile, they also exhibit typical AIE characteristics, with high solid-state efficiencies up to 60.7%. Amplified emission quenching occurred when PA solutions were added to the polymer suspensions in a 10/90 THF–water mixture, which renders the polymers useful as fluorescent chemosensors for the detection of explosives.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21104044 and 51473092), the National Basic Research Program of China (973 Program, 2013CB834701), the Ph.D. Programs Foundation of Ministry of Education of China (20110073120040). W.Z.Y. thanks the SMC-Chenxing Young Scholar Program of SJTU. Yiren Zhang thanks the Chun-Tsung Program of SJTU.Notes and references
- J.-G. Liu and M. Ueda, J. Mater. Chem., 2009, 19, 8907–8919 RSC.
- (a) T. Matsuda, Y. Funae, M. Yoshida and T. Takaya, J. Macromol. Sci., Part A: Pure Appl. Chem., 1999, 36, 1271–1288 CrossRef PubMed; (b) W. Z. Yuan, R. Hu, J. W. Y. Lam, N. Xie, C. K. W. Jim and B. Z. Tang, Chem. – Eur. J., 2012, 18, 2847–2856 CrossRef CAS PubMed.
- C. Gao, B. Yang and J. Shen, J. Appl. Polym. Sci., 2000, 75, 1474–1479 CrossRef CAS.
- T. Okubo, S. Kohmoto and M. Yamamoto, J. Appl. Polym. Sci., 1998, 68, 1791–1799 CrossRef CAS.
- T. Okubo, S. Kohmoto, M. Yamamoto and T. Nakahira, J. Mater. Sci., 1999, 34, 337–347 CrossRef CAS.
- R. Okutsu, Y. Suzuki, S. Ando and M. Ueda, Macromolecules, 2008, 41, 6165–6168 CrossRef CAS.
- Z. Cui, C. Lü, B. Yang, J. Shen, X. Su and H. Yang, Polymer, 2001, 42, 10095–10100 CrossRef CAS.
- H. C. Kolb, M. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249–1262 RSC.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2010, 49, 3415–3417 CrossRef CAS PubMed.
- A. B. Lowe, C. E. Hoyle and C. N. Bowman, J. Mater. Chem., 2010, 20, 4745–4750 RSC.
- H. Li, B. Yu, H. Matsushima, C. E. Hoyle and A. B. Lowe, Macromolecules, 2009, 42, 6537–6542 CrossRef CAS.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC.
- B. M. Rosen, G. Lligadas, C. Hahn and V. Percec, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3940–3948 CrossRef CAS.
- B. M. Rosen, G. Lligadas, C. Hahn and V. Percec, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3931–3939 CrossRef CAS.
- J. Xu, L. Tao, C. Boyer, A. B. Lowe and T. P. Davis, Macromolecules, 2010, 43, 20–24 CrossRef CAS.
- D. Gopalakrishnan and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 8357–8362 CrossRef CAS PubMed.
- R. Hu, J. W. Lam, J. Liu, H. H. Sung, I. D. Williams, Z. Yue, K. S. Wong, M. M. Yuen and B. Z. Tang, Polym. Chem., 2012, 3, 1481–1489 RSC.
- T. Han, X. Feng, B. Tong, J. Shi, L. Chen, J. Zhi and Y. Dong, Chem. Commun., 2011, 48, 416–418 RSC.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- M. Wang, G. Zhang, D. Zhang, D. Zhu and B. Z. Tang, J. Mater. Chem., 2010, 20, 1858–1867 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- H. Tong, Y. Hong, Y. Dong, M. Häußler, J. W. Lam, Z. Li, Z. Guo, Z. Guo and B. Z. Tang, Chem. Commun., 2006, 3705–3707 RSC.
- B. K. An, D. S. Lee, J. S. Lee, Y. S. Park, H. S. Song and S. Y. Park, J. Am. Chem. Soc., 2004, 126, 10232–10233 CrossRef CAS PubMed.
- (a) W. Z. Yuan, Y. Tan, Y. Gong, P. Lu, J. W. Y. Lam, X. Y. Shen, C. Feng, H. H. Y. Sung, Y. Lu, I. D. Williams, J. Z. Sun, Y. Zhang and B. Z. Tang, Adv. Mater., 2013, 25, 2837–2843 CrossRef CAS PubMed; (b) W. Z. Yuan, Y. Gong, S. Chen, X. Y. Shen, J. W. Y. Lam, P. Lu, Y. Lu, Z. Wang, R. Hu, N. Xie, H. S. Kwok, Y. Zhang, J. Z. Sun and B. Z. Tang, Chem. Mater., 2012, 24, 1518–1528 CrossRef CAS; (c) Y. Gong, Y. Tan, J. Liu, P. Lu, C. Feng, W. Z. Yuan, Y. Lu, J. Z. Sun, G. He and Y. Zhang, Chem. Commun., 2013, 49, 4009–4011 RSC; (d) Y. Gong, Y. Zhang, W. Z. Yuan, J. Z. Sun and Y. Zhang, J. Phys. Chem. C, 2014, 118, 10998–11005 CrossRef CAS; (e) Y. Gong, J. Liu, Y. Zhang, G. He, Y. Lu, W. B. Fan, W. Z. Yuan, J. Z. Sun and Y. Zhang, J. Mater. Chem. C, 2014, 2, 7552–7560 RSC.
- Y. Hong, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- Y. Li, F. Li, H. Zhang, Z. Xie, W. Xie, H. Xu, B. Li, F. Shen, L. Ye, M. Hanif, D. Ma and Y. Ma, Chem. Commun., 2007, 231–233 RSC.
- (a) Y. Lu, Y. Tan, Y. Gong, H. Li, W. Z. Yuan, Y. Zhang and B. Z. Tang, Chin. Sci. Bull., 2013, 58, 2719–2722 CrossRef CAS; (b) W. Wu, S. Ye, L. Huang, G. Yu, Y. Liu, J. Qin and Z. Li, Chin. J. Polym. Sci., 2013, 31, 1432–1442 CrossRef CAS PubMed; (c) W. Wu, S. Ye, L. Huang, L. Xiao, Y. Fu, Q. Huang, G. Yu, Y. Liu, J. Qin, Q. Li and Z. Li, J. Mater. Chem., 2012, 22, 6374–6382 RSC; (d) W. Wu, S. Ye, G. Yu, Y. Liu, J. Qin and Z. Li, Macromol. Rapid. Commun., 2012, 33, 164–171 CrossRef CAS PubMed.
- A. Qin, J. W. Y. Lam, L. Tang, C. K. W. Jim, H. Zhao, J. Sun and B. Z. Tang, Macromolecules, 2009, 42, 1421–1424 CrossRef CAS.
- W. Z. Yuan, H. Zhao, X. Y. Shen, F. Mahtab, J. W. Y. Lam, J. Z. Sun and B. Z. Tang, Macromolecules, 2009, 42, 9400–9411 CrossRef CAS.
- Z. Zhao, J. Liu, J. W. Yip Lam, C. Y. K. Chan, H. Qiu and B. Z. Tang, Dyes Pigm., 2011, 91, 258–263 CrossRef CAS PubMed.
- Y. N. Hong, Y. Q. Dong, H. Tong, M. Haussler, J. W. Y. Lam and B. Z. Tang, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 2007, 48, 367–368 CAS.
- C. K. Jim, A. Qin, J. W. Lam, F. Mahtab, Y. Yu and B. Z. Tang, Adv. Funct. Mater., 2010, 20, 1319–1328 CrossRef CAS.
- J. Liu, Y. Zhong, P. Lu, Y. Hong, J. W. Y. Lam, M. Faisal, Y. Yu, K. S. Wong and B. Z. Tang, Polym. Chem., 2010, 1, 426–429 RSC.
| This journal is © The Royal Society of Chemistry 2015 |