Construction of a series of zero-dimensional/one-dimensional crystalline Zn–S clusters – effect of the character of bridging organic ligands on structural diversity†
Xianghua
Zeng
a,
Xiaojing
Yao
b,
Junyong
Zhang
*a,
Qi
Zhang
a,
Wenqian
Wu
a,
Aihua
Chai
c,
Jinlan
Wang
*b,
Qingdao
Zeng
*d and
Jingli
Xie
*a
aCollege of Biological, Chemical Science and Engineering, Jiaxing University, Jiaxing 314001, P. R. China. E-mail: zhangjy@mail.zjxu.edu.cn; jlxie@mail.zjxu.edu.cn
bDepartment of Physics & School of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, P. R. China. E-mail: jlwang@seu.edu.cn
cCollege of Mathematics, Physics and Information Engineering, Jiaxing University, Jiaxing 314001, P. R. China
dCAS Key Laboratory of Standardization and Measurement for Nanotechnology, National Center for Nanoscience and Technology (NCNST), 11 Zhongguancun Beiyitiao, Beijing 100190, P. R. China. E-mail: zengqd@nanoctr.cn
First published on 19th December 2014
Abstract
A series of chalcogenide compounds with various compositions, i.e., octanuclear or tetranuclear Zn–S clusters, have been synthesised in a straighforward manner. Different fused-ring aromatic ligands were used as capping ligands and the corresponding zero-dimensional (0D) products were obtained. On the other hand, use of bridging ligands led to a family of one-dimensional (1D) coordination polymers, and an in situ ligand reaction has been observed in [Zn8S(SC6H5)13L1(H2O)]·2H2O (L = 3-carboxypryidyl) due to the hydrolysis of the cyano group of 3-pyridinecarbonitrile. A very rare 1D helical-chain structure was observed in [Zn4(SC6H5)8L1] (L = 4,4′-bipyridyl), providing evidence of the character of bridging organic ligands in the corresponding crystalline materials. First-principles calculations on [Zn4(SC6H5)8L1] (L = 4,4′-bipyridyl) further revealed that the two cluster units could rotate freely about the C–C single bond over a broad range, eventually leading to the formation of a one-dimensional helical structure.
Hybrid inorganic–organic framework materials have potential applications in many areas, such as chiral separations, asymmetric catalysis,1 ion exchange,2 adsorption–desorption,3 gas storage,4 non-linear optics,5 molecular magnetism6 and ferroelectrics.7 Hence, there are new development opportunities for these multifunctional materials. In comparison with metal–organic frameworks (MOFs) and porous coordination polymers (PCPs), which are constructed from metal atoms and organic ligands, the synergistic interactions between metal clusters and bridging organic ligands make the assembly and topological control of the corresponding crystalline materials a challenging task. The aim is to construct crystalline frameworks with intriguing topologies, and it is important to systematically investigate the relationship between the intrinsic character of bridging ligands and the specific framework materials to fully understand their supramolecular assembly.8 By using identical metal clusters as building blocks, structural diversity could be observed by virtue of different bridging ligands through modification of the length and the shape of the linkers.9 In this respect, chalcogenide clusters with well-defined molecular structures and interesting electrical/optical properties are promising building blocks. Vaqueiro has summarized a number of hybrid materials featuring chalcogenide clusters as inorganic units which are linked by organic ligands.10 Recently, Feng and co-workers reported that the precise doping of Mn2+ ions into coreless supertetrahedral chalcogenide nanoclusters could induce an unusual red shift, demonstrating potential applications for photonic devices and bio-imaging.11 By using a “bottom-up” synthetic strategy, herein we report eight chalcogenide compounds showing versatile structural characteristics, including zero-dimensional (0D) clusters and one-dimensional (1D) coordination polymers. It is demonstrated in this communication that various compositions can be achieved in a straightforward manner by deliberate use of capping ligands or bridging ligands.
It is well known that zinc sulfide clusters exhibit different isomers based upon the sphalerite (cubic ZnS) and wurtzite (hexagonal ZnS) phases.12 The sphalerite (or zinc blende) structure has an expanded face-centered cubic (fcc) anion lattice, whereas the wurtzite structure is derived from an expanded hexagonally closed-packed (hcp) anion array. We have recently described a synthetic entry to a class of individual zinc sulfide clusters based on wurtzite-like Zn8S cores.13 This simple and efficient synthetic method provided clusters containing [Zn8S(SC6H5)14L2] (L = substituted organic ligands), and a recent example has shown that this class of Zn–S cluster could help to inhibit the photobleaching of fluorescent dyes to a certain degree,14 establishing such clusters as a promising new platform for future applications.
Complexes 1–8 were obtained by hydrothermal synthesis and their structures were determined by single-crystal X-ray diffraction (Table 1).15,16 Unlike the previous research using substituted pyridine ligands, fused-ring aromatic ligands 3-methylquinoline, 5-aminoquinoline, 3-(2-thienyl)pyridine and 4,7-phenanthroline were used as capping ligands, and the corresponding zero-dimensional (0D) products were Zn8S(SC6H5)14[3-H3CC9H6N]2 (1), Zn8S(SC6H5)14[5-H2NC9H6N]2 (2), Zn8S(SC6H5)14[C4H3SC5H4N]2 (3) and Zn8S(SC6H5)14[NC12H8N]2 (4) (Fig. 1), respectively. The Zn8S cores of neutral clusters display significant thermodynamic stability when different capping ligands are present, and therefore a large number of individual clusters could be generated upon using various capping ligands.
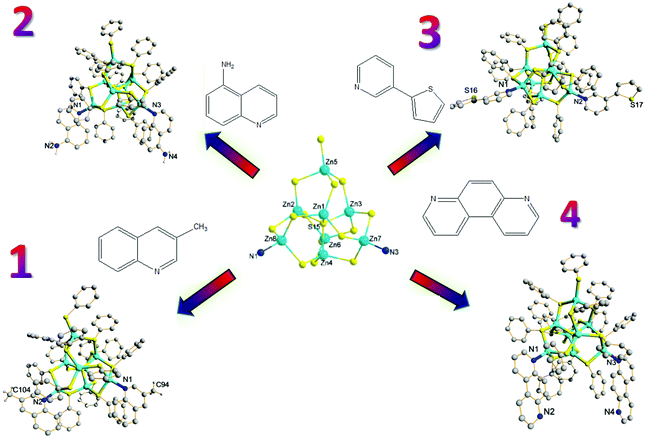 | ||
| Fig. 1 Structures of zero-dimensional (0D) products: Zn8S(SC6H5)14[3-H3CC9H6N]2 (1), Zn8S(SC6H5)14[5-H2NC9H6N]2 (2), Zn8S(SC6H5)14[C4H3SC5H4N]2 (3) and Zn8S(SC6H5)14[NC12H8N]2 (4). | ||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| Formula | C104H88N2S15Zn8 | C102H86N4S15Zn8 | C102H84N2S17Zn8 | C108H86N4S15Zn8 | C84H75NO5S14Zn8 | C96H78N4S16Zn8 | C60H50N2S8Zn4 | C116H96N4S16Zn8 |
| Fw | 2370.00 | 2371.98 | 2406.08 | 2444.05 | 2150.25 | 4477.831 | 1316.98 | 2581.89 |
| Cryst syst | Monoclinic | Monoclinic | Triclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | Cc |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n | Cc | C2c | P21/n | P21/c |
| a/Å | 25.9462(6) | 25.4629(19) | 13.3365(4) | 13.0752(5) | 16.951(2) | 53.269(3) | 13.3661(2) | 19.3609(10) |
| b/Å | 13.3330(2) | 13.3084(10) | 13.4427(4) | 25.2259(9) | 20.589(3) | 13.3807(3) | 24.0675(3) | 31.6252(18) |
| c/Å | 28.9328(5) | 29.022(2) | 29.4227(9) | 31.8774(13) | 25.375(4) | 38.045(2) | 19.0210(4) | 18.4565(9) |
| α/° | 90 | 90 | 90.982(2) | 90 | 90 | 90 | 90 | 90 |
| β/° | 98.500(2) | 98.821(4) | 91.336(2) | 96.401(2) | 96.243(2) | 133.037(9) | 105.480(2) | 99.136(2) |
| γ /° | 90 | 90 | 106.792(3) | 90 | 90 | 90 | 90 | 90 |
| V/Å3 | 9899.1(3) | 9718.3(13) | 5047.0(3) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 448.7(7) 448.7(7) |
8804(2) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820(3) 820(3) |
5896.87(17) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 157.4(10) 157.4(10) |
| Z | 4 | 4 | 2 | 4 | 4 | 4 | 4 | 4 |
| d calc/g cm−3 | 1.590 | 1.621 | 1.583 | 1.498 | 1.622 | 1.501 | 1.483 | 1.537 |
| GOF | 1.041 | 1.007 | 1.125 | 0.889 | 1.026 | 1.010 | 1.032 | 1.029 |
| R 1 [I > 2σ(I)] | 0.0740 | 0.1046 | 0.1373 | 0.0663 | 0.0694 | 0.0939 | 0.0334 | 0.0569 |
| wR2[I > 2σ(I)] | 0.1789 | 0.3357 | 0.3721 | 0.1610 | 0.1661 | 0.2469 | 0.0641 | 0.1284 |
The results of the photoluminescence properties of 2 (c = 2.5 × 10−6 M in DMSO) at room temperature are shown in Fig. S1a.† The fluorescence emission of 2 occurs at 476 nm due to excitation at λex 370 nm, compared to the typical absorption maximum at about 260 nm of Zn8S(SC6H5)14[phenanthridyl]2 as previously reported,13a suggesting that there is a relationship between the terminal capping ligands and their corresponding spectral properties. Excitation of 3 at λex 296 nm leads to a prominent emission peak at 350 nm (fwhm ∼ 50 nm) with a blue shift compared to that of compound 2, presumably due to the perturbation of the ion electronic state induced by the terminal 3-(2-thienyl)pyridyl ligands (Fig. S1b, ESI†). Surprisingly, there is no observable fluorescence emission for compounds 1 and 4, indicating that the photoluminescence of this class of clusters is very hard to tune simply by changing the surface-capping ligands.
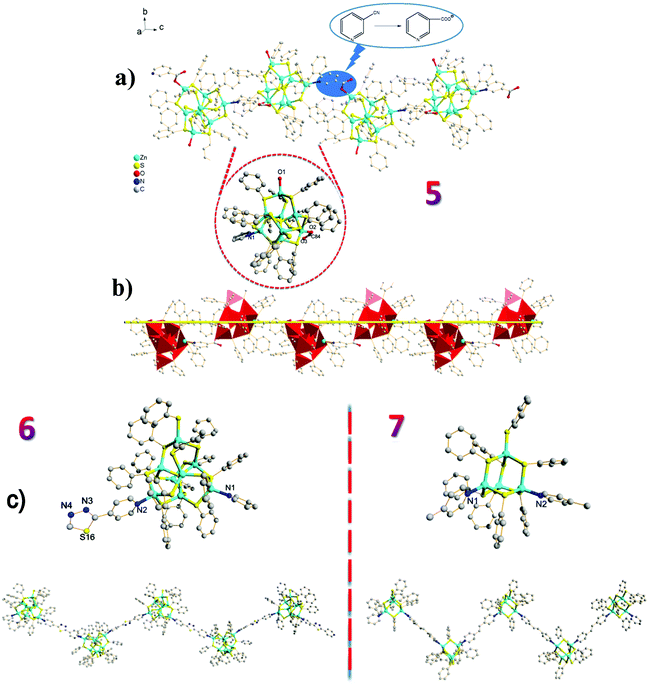
Apart from using capping ligands to obtain the zero-dimensional nanoclusters 1–4, it is highly desirable to use bridging ligands to link chalcogenide clusters which behave as large “artificial atoms”. The aim is to organize uniform molecular-level integration of crystalline frameworks using chalcogenide clusters as building blocks.17 To the best of our knowledge, however, only a few hybrid materials featuring similar compositions as building blocks linked by organic ligands have been reported.17b,18 Extending our methodology to the targeted synthesis of 1D coordination polymers led successfully to 5–8, in which bridging ligands link clusters together. Using 3-pyridinecarbonitrile, 2,5-bis(4-pyridyl)-1,3,4-thiadiazole, trans-1,2-bis(4-pyridyl)ethylene and 4,4′-bipyridyl as bridging ligands led to [Zn8S(SC6H5)13L1(H2O)]·2H2O (L = 3-carboxypyridyl) (5), [Zn8S(SC6H5)14L1] (L = 2,5-bis(4-pyridyl)-1,3,4-thiadiazole) (6), [Zn4(SC6H5)8L1] (L = trans-1,2-bis(4-pyridyl)ethylene) (7) and [Zn4(SC6H5)8L1] (L = 4,4′-bipyridyl) (8), respectively. Compounds 5–7 displayed a 1D zig-zag chain (Fig. 2), whereas a very rare 1D helical-chain structure was observed for compound 8 (Fig. 3).
Under similar conditions to those used in the synthesis of zero-dimensional compounds, the cyano group in 3-pyridinecarbonitrile was hydrolysed to give the 3-carboxypyridyl in compound 5, and consequently, a 1D zig-zag chain was achieved by virtue of the bridging ligand 3-carboxypyridyl (Fig. 2a). The observation of this in situ ligand reaction further demonstrated the importance of the ligand in constructing functional crystalline clusters.13a
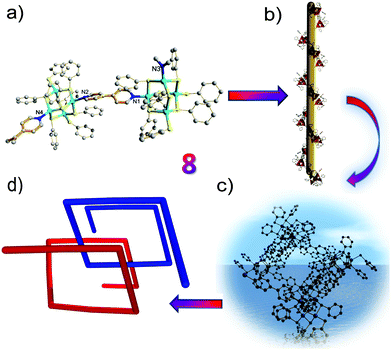
Interestingly, in the absence of thiourea, T2 clusters (supertetrahedral clusters are denoted by Tn, where n is the number of metal layers in each cluster) have evolved in 7/8 and are linked by ditopic ligands. Compared to the 1D zig-zag chain in 7, the intriguing characteristic of 8 is its helical-chain structure, which is very rare in the family of hybrid materials with chalcogenide clusters as fundamental building blocks.18,19 As demonstrated in Fig. 3, the structure of 8 shows a fragment of the sphalerite (cubic ZnS) phase, and two crystallographically independent adamantane-type Zn4S8N2 clusters are linked by two independent 4,4′-bipyridyl ligands in an asymmetric unit. The Zn–S bond lengths range from 2.2501(17) to 2.4236(14) Å and the Zn–N bond lengths vary from 2.041(4) and 2.060(4) Å. Of particular note is the twist character of the two pyridine rings in 4,4′-bipyridyl, i.e., the dihedral angle between the ring of N1, C51–C55 atoms and the ring of N2, C56–C60 atoms is 32.0°, whereas the angle between the ring of N3A, C49A, C50A, C114–C116 atoms and the ring of N4, C109–C113 atoms is 35.4°. This distorted character of the bridging ligands may be the basis of the observation of a 21 helix along the b axis. The pitch range of each chain is 31.625 Å, which is equal to the unit cell parameter along the b axis and increases to 31.993 Å, which is derived from its room temperature structure (8 RT).20 Although it is not uncommon to integrate a right-handed helix and left-handed helix into an overall achiral structure as in 8, the exploration of ligands with greater flexibility will facilitate the discovery of more helical structures.21
To further understand the twist character of the cluster units around the C–C single bond in the 4,4′-bipyridyl ligand, a first-principles computational study was performed. The first-principles calculations based on density functional theory were carried out within the Vienna ab initio simulation package.22,23 The electron-ion interaction was described by the projected augmented wave (PAW) potentials24 and the plane-wave basis set, with the local density approximation (LDA) exchange–correlation functional.25 The kinetic energy cutoff was set as 400 eV. The Hellmann–Feynman forces acting on each atom were less than 0.05 V Å−1. To simplify the periodic structure to a cluster model, we used a benzene ring (highlighted by green circles in Fig. 4a) to replace the three dangling C rings in [Zn4S(SC6H5)8L1] (L = 4,4′-bipyridyl) (see Fig. 3a). We employed a supercell of 25 × 26 × 41 Å3 for all calculations, so that the interactions generated by the periodic boundary condition could be neglected. The built cluster model was first fully optimized without any symmetry constraints. Then, we calculated the rotation energy of part A (Zn4S8N2 cluster) to part B (another Zn4S8N2 cluster) in the cluster model around the C–C single bond (schematic diagram shown in Fig. 4a) within 90 degrees, and the results suggested that the two parts can rotate freely, with an energy difference that is relatively small in comparison with the initial position (Fig. 4b). As clearly shown in Fig. 4b, when the dihedral angle is 32.0° or 35.4° (crystallographic results), the rotation energies of part A to part B around the C–C single bond are rather small (∼0.04 eV). On the other hand, the energy difference is relatively high when the rotation angle is around 80 degrees. This suggests that the two cluster units can freely rotate (from theoretical calculations). Thus, the results of the theoretical study match with the observed twist character, and we believe that the crystal packing effect and intermolecular interactions play an important role in the formation of the helical structure.
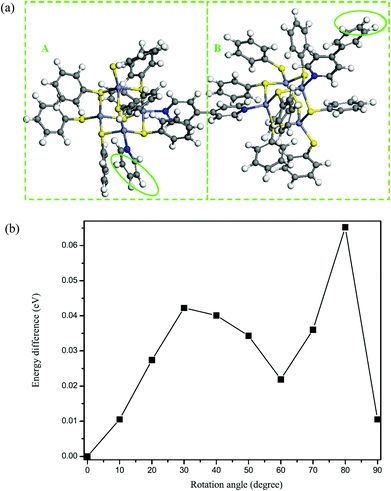 | ||
| Fig. 4 (a) The cluster model for the rotational calculations for 8. (b) The energy difference vs. rotation angle (from 0° to 90°). | ||
In summary, we have shown that a family of chalcogenide compounds with versatile characters (0D and 1D) can be constructed by the rational combination of Zn–S clusters and capping/bridging ligands. The solution-state properties of compounds 1–8 could be measured by NMR and MS and will be reported elsewhere.26 An in situ ligand reaction was observed for 5, namely, the hydrolysis of the cyano group of 3-pyridinecarbonitrile, giving the 3-carboxypyridyl bridging ligand, which helped to construct [Zn8S(SC6H5)13L1(H2O)]·2H2O (L = 3-carboxypyridyl). A rare 1D helical-chain structure in [Zn4(SC6H5)L1] (L = 4,4′-bipyridyl) (8) indicated that organic ligands play an important role in affecting the assembly of hybrid inorganic–organic materials. It is well-known that bridging ligands are designated di-, tri- or tetratopic depending on the number of donor atoms, and we believe that bidentate bridging ligands such as those shown in this work are the best choice for linking crystalline clusters to obtain one-dimensional coordination polymers, in which the rigidity/flexibility of ligands could be the basis for the variation of structural topologies. Furthermore, the use of tri- and tetratopic ligands may make it possible to obtain higher dimensional polymers.
Acknowledgements
Financial support from the NSFC (21401077 and 21371078) and the start-up fund of Jiaxing University is gratefully acknowledged.Notes and references
- (a) M. Zhao, S. Ou and C.-D. Wu, Acc. Chem. Res., 2014, 47, 1199 CrossRef CAS PubMed; (b) R. E. Morris and X. Bu, Nat. Chem., 2010, 2, 353 CrossRef CAS PubMed.
- D. F. Sava, V. C. Kravtsov, F. Nouar, L. Wojtas, J. F. Eubank and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 3768 CrossRef CAS PubMed.
- (a) Y. Jiang, J. Huang, B. Kasumaj, G. Jeschke, M. Hunger, T. Mallat and A. Baiker, J. Am. Chem. Soc., 2009, 131, 2058 CrossRef CAS PubMed; (b) Y. Kubota, M. Takata, T. C. Kobayashi and S. Kitagawa, Coord. Chem. Rev., 2007, 251, 2510 CrossRef CAS; (c) B. Panella, K. Hönes, U. Müller, N. Trukhan, M. Schubert, H. Pütter and M. Hirscher, Angew. Chem., Int. Ed., 2008, 47, 2138 CrossRef CAS PubMed.
- (a) D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058 CrossRef PubMed; (b) S. Ma and H.-C. Zhou, J. Am. Chem. Soc., 2006, 128, 11734 CrossRef CAS PubMed.
- C. Janiak, Dalton Trans., 2003, 2781 RSC.
- X.-Y. Wang, Z.-M. Wang and S. Gao, Chem. Commun., 2008, 281 RSC.
- W. Zhang and R.-G. Xiong, Chem. Rev., 2012, 112, 1163 CrossRef CAS PubMed.
- U. Schubert, Chem. Soc. Rev., 2011, 40, 575 RSC.
- R. Robson, Dalton Trans., 2008, 5113 RSC.
- P. Vaqueiro, Dalton Trans., 2010, 39, 5965 RSC.
- J. Lin, Q. Zhang, L. Wang, X. Liu, W. Yan, T. Wu, X. Bu and P. Feng, J. Am. Chem. Soc., 2014, 136, 4769 CrossRef CAS PubMed.
- (a) C. A. Blindauer and P. J. Sadler, Acc. Chem. Res., 2005, 38, 62 CrossRef CAS PubMed; (b) H. Vahrenkamp, Dalton Trans., 2007, 4751 RSC; (c) G. Henkel and B. Krebs, Chem. Rev., 2004, 104, 801 CrossRef CAS PubMed; (d) I. G. Dance and K. Fisher, Prog. Inorg. Chem., 1994, 41, 637 CrossRef CAS.
- (a) J. Xie, S. R. Batten, Y. Zou and X. Ren, Cryst. Growth Des., 2011, 11, 16 CrossRef CAS; (b) J. Xie, Inorg. Chem., 2008, 47, 5564 CrossRef CAS PubMed.
- J. Xie, S. Cao, D. Good, M. Wei and X. Ren, Inorg. Chem., 2010, 49, 1319 CrossRef CAS PubMed.
- (a) G. M. Sheldrick, SHELXTL V5.1 Software Reference Manual, Bruker AXS, Inc., Madison, USA, 1997 Search PubMed; (b) G. M. Sheldrick, SHELX97, Programs for Crystal Structure Analysis, Institüt für Anorganische Chemie der Universität Göttingen, 1998 Search PubMed.
- G. M. Sheldrick, SADABS, V2.01, Empirical Absorption CorrectionProgram, Institüt für Anorganische Chemie der Universität Göttingen, 1996 Search PubMed.
- (a) W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688 CrossRef CAS PubMed; (b) J. Xie, X. Bu, N. Zheng and P. Feng, Chem. Commun., 2005, 4916 RSC.
- Z. Chen, D. Luo, M. Kan and Z. Lin, Inorg. Chem., 2011, 50, 4674 CrossRef CAS PubMed.
- I. Dance, J. Am. Chem. Soc., 1980, 102, 3445 CrossRef CAS.
- Unit cell parameters of 8 at room temperature: a = 19.4573(4) Å, b = 31.9930(6) Å, c = 18.6800(4) Å, β = 99.1590(10)°, V = 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480.0(4) Å3, Z = 4, Dc = 1.438 g cm−3, GOF = 1.018, total of 88
480.0(4) Å3, Z = 4, Dc = 1.438 g cm−3, GOF = 1.018, total of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524 reflections (21
524 reflections (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 822 unique) with Rint = 0.0452, R1 = 0.0708, wR2 = 0.1841 (I > 2σ(I)). CCDC 1012594.
822 unique) with Rint = 0.0452, R1 = 0.0708, wR2 = 0.1841 (I > 2σ(I)). CCDC 1012594. - (a) X.-D. Zheng and T.-B. Lu, CrystEngComm, 2010, 12, 324 RSC; (b) J. Zhang and X. Bu, Chem. Commun., 2009, 206 RSC; (c) J. Zhang, S. Chen, A. Zingiryan and X. Bu, J. Am. Chem. Soc., 2008, 130, 17246 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter, 1993, 48, 13115 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15 CrossRef CAS.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- J. P. Perdew and A. Zunger, Phys. Rev. B, 1981, 23, 5048 CrossRef CAS.
- X. Zeng, J. Zhang and J. Xie, unpublished results.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and general procedures, synthesis of compounds 1–8, PL spectra of 2 and 3, X-ray crystallographic file in CIF format. CCDC 1012102 (1), 1012103 (2), 1012104 (3), 1012105 (4), 795210 (5), 1012106 (6), 1012107 (7), 1012108 (8). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00227j |
| This journal is © the Partner Organisations 2015 |