Planar copper and nickel triangles with a guanidine-derived ligand†
Takuto
Matsumoto
,
Yamato
Sato
,
Takuya
Shiga
* and
Hiroki
Oshio
*
Department of Chemistry, Graduate School of Pure and Applied Sciences, University of Tsukuba, Tennnodai 1-1-1, Tsukuba 305-8571, Japan. E-mail: oshio@chem.tsukuba.ac.jp; Fax: +81 29 853 4238; Tel: +81 29 853 4238
First published on 23rd June 2015
Abstract
A novel guanidine-derived ligand with three tetradentate N4 coordination sites and its trinuclear copper and nickel complexes, [Cu3] and [Ni3], were synthesized. X-ray structural analyses of [Cu3] and [Ni3] reveal the complexes to have planar triangular structures with pseudo C3 symmetry. Magnetic measurements for [Cu3] and [Ni3] complexes indicate that antiferromagnetic interactions are operative in both complexes with intratriangle exchange coupling constants of g = 2.08(1), J = −130(1) cm−1 for [Cu3], and g = 2.18(1), J = −14.9(1) cm−1 for [Ni3]. [Cu3] has a doublet spin ground state at low temperature, while the magnetic susceptibility data and magnetization curve suggest that the ground state of [Ni3] is spin singlet.
Introduction
Odd-membered antiferromagnetic rings continue to attract great interest due to their potential to exhibit spin frustration, non-collinear spin states and spin chirality.1 There are, however, relatively few reports concerning three-, five-, and seven-membered antiferromagnetic ring systems, whereas there are many studies on even-membered rings.2 Of the reported odd-membered systems, magnetically isolated three membered rings are the most widely studied, while examples of five- and seven-nuclear systems are rare. We reported the synthesis of a seven-membered antiferromagnetic vanadyl ring based on a cyclodextrin template that exhibited unusual stepped magnetization,3 but the scarcity of suitable templates renders the rational synthesis of five- and seven-membered rings challenging.4 On the other hand three-membered ring complexes can self-assemble from simple building blocks to yield clusters whose magnetic interactions can be studied to shed light on the fundamentals of quantum spins.5 In particular, the magnetic properties of odd-membered antiferromagnetic rings with S = 1/2 spins have been extensively studied for three-membered rings, which is the simplest system and close to a real quantum spin model. In one such example, half step magnetization and hysteresis curves originating from spin chirality were observed in the antiferromagnetically coupled [CuII3] polyoxometalate sandwich complex Na9[Cu3Na3(H2O)9(α-AsW9O33)2].6 The magnetization curve collected by the application of a rapidly pulsed field showed asymmetry between the positive and negative field regions. Motivated by interest in quantum spin systems, several [Cu3]-type molecules have been synthesized by molecular design, and their magnetic behaviour has been studied.7One-dimensional frustrated spin models have unique and diverse properties. In contrast to higher dimensional spin systems, quantum spin chains have no long-range order. In the absence of frustration, such as in an antiferromagnetic ladder with an even number of legs, S = 1/2 systems are expected to have an excitation gap.8 When frustration is present, the Lieb–Schultz–Mattis theorem suggests that the spin gap must be accompanied with at least doubly degenerate ground states.9 Three-leg spin tubes have a triangular column type structure, and antiferromagnetic interactions are operative between the spins. Although the theory of spin tubes is well studied and the expected physical properties are interesting from a fundamental viewpoint, there are only a few examples of compounds with triangular column type structures.10 The first example of a three-leg spin tube with S = 3/2, the inorganic chromium (CsCrF4, Cr3+; S = 3/2) was reported in 2009,11 with a space group of P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2m, and a columnar superstructure where identical Cr3 equilateral triangles are stacked without rotation along the c-axis. The distance between Cr ions in the equilateral triangle is 3.741 Å, while that along the c-axis is 3.857 Å. The magnetic susceptibility data show that antiferromagnetic interactions are operative between the chromium ions, and the Weiss temperature is −143 K. The specific heat capacity shows that there is no magnetic phase transition below 4 K. The heat capacity curve tends to a non-zero value at 0 K, and suggests that this compound has a gapless spin-liquid state, a so-called Tomonaga–Luttinger liquid.
2m, and a columnar superstructure where identical Cr3 equilateral triangles are stacked without rotation along the c-axis. The distance between Cr ions in the equilateral triangle is 3.741 Å, while that along the c-axis is 3.857 Å. The magnetic susceptibility data show that antiferromagnetic interactions are operative between the chromium ions, and the Weiss temperature is −143 K. The specific heat capacity shows that there is no magnetic phase transition below 4 K. The heat capacity curve tends to a non-zero value at 0 K, and suggests that this compound has a gapless spin-liquid state, a so-called Tomonaga–Luttinger liquid.
A spin tube with divalent copper ions, [Cl(CuCl2tachH)3]2+ (tach = cis,trans-1,3,5-triamino-cyclohexane), was reported in 2004 by Cronin et al. and the physical properties have been well studied.12 This Cu3 spin tube shows weak antiferromagnetic interactions between Cu(II) ions in the intra-triangular units, and has moderate antiferromagnetic interactions between Cu(II) ions of neighbouring Cu3 triangular units. Therefore, the spin system can be regarded as weakly antiferromagnetic-coupled three-leg chains. Magnetization measurements at low temperature suggest that this system has a gapless Tomonaga–Luttinger liquid ground state, although the theoretical prediction did indicate the existence of a spin gap. In order to access a novel quantum phase, fine-tuning of the magnetic interactions and spin topology is important.
In another example, a DABCO (= 1,4-diazabicyclo[2.2.2]octane) bridged-type copper spin tube structure catena-[Cu3(L1)3(dabco)3]·3Et2O (H2L1 = 1,1-(1,4-phenylene)-bis(4,4-dimethylpentane-1,3-dione)) was reported in 2006 by Lindoy et al.13 The planar triangular moiety consists of three copper(II) ions and three bis-β-diketone type bridging ligands, and the trinuclear metallocycles are bridged by DABCO moieties. Although this three-leg spin tube is not a twisted structure, the space group is P21/m, i.e. it has no crystallographic C3 axis. In this system, the physical properties appear likely to be very interesting but to date only the structure has been reported. From the viewpoint of molecular design of a magnetic system, the planar complex may be a useful building unit for the spin tube.
Towards the development of experimental spin tube models, it is important to construct triangular trinuclear complexes as building units through the use of appropriate planar bridging ligands whose coordination sites describe an equilateral triangle.
In this paper, the rational synthesis and magnetic properties of triangular metal complexes with highly planar structures were studied. A new guanidine-derived ligand, H2L·HCl (= 1,2,3-tris[(6-(1H-pyrazol-1-yl)pyrid-2-ylmethylene)amino]guanidinium chloride), was prepared and its copper and nickel complexes were synthesized. The ligand has three planar tetradentate N4 coordination sites and can coordinate the equatorial positions of transition metal ions, suggesting that it may be a useful building block for planar triangular complexes in line with findings with a similar guanidine-type ligand.14
Experimental section
Materials and general characterization
All chemicals were used without further purification except when noted. Diethyl ether was distilled over calcium hydride and then sodium/benzophenone. The precursors 1–3 of the guanidine-derivative ligand were prepared by modified versions of the published methods according to Scheme 1.9–11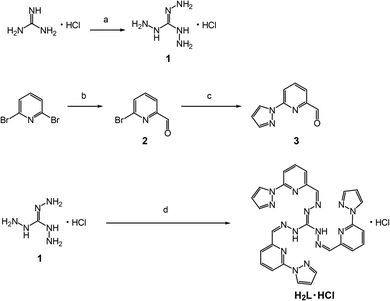 | ||
| Scheme 1 Synthesis of H2L·HCl. (a) Hydrazine monohydrate, 1,4-dioxane. (b) n-BuLi, DMF, Et2O. (c) Pyrazole, 1,10-phenanthroline monohydrate, CuI, K2CO3, toluene. (d) 3, EtOH. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6-(1H-pyrazol-1-yl)-2-pyridinecarboxaldehyde (3) (4.08 g, 23.6 mmol, 79% yield) as a white solid: 1H-NMR (CDCl3) δ 10.05 (s, 1H), 8.68 (d, 1H, J = 6.6 Hz), 8.23 (d, 1H, J = 8.4 Hz), 7.99 (dd, 1H, J = 8.0 Hz), 7.84 (d, 1H, J = 7.4 Hz), 7.78 (s, 1H), 6.52 (dd, 1H, J = 2.6 Hz, 2.2 Hz). Anal. (calc.) for C9H7N3O (3): C, 62.68 (62.42); H, 4.15 (4.07); N, 24.26 (24.26) %.
1) to give 6-(1H-pyrazol-1-yl)-2-pyridinecarboxaldehyde (3) (4.08 g, 23.6 mmol, 79% yield) as a white solid: 1H-NMR (CDCl3) δ 10.05 (s, 1H), 8.68 (d, 1H, J = 6.6 Hz), 8.23 (d, 1H, J = 8.4 Hz), 7.99 (dd, 1H, J = 8.0 Hz), 7.84 (d, 1H, J = 7.4 Hz), 7.78 (s, 1H), 6.52 (dd, 1H, J = 2.6 Hz, 2.2 Hz). Anal. (calc.) for C9H7N3O (3): C, 62.68 (62.42); H, 4.15 (4.07); N, 24.26 (24.26) %.
X-ray crystallography
A single crystal was removed from the mother liquor, mounted on a glass rod and intensity data were collected using a Bruker SMART or SMART APEX II CCD system with Mo-Kα radiation (λ = 0.71073 Å). The structure was solved by direct methods and refined by full-matrix least-squares techniques on F2 using SHELXTL.Magnetic measurements
Magnetic susceptibility data with an applied magnetic field of 500 Oe were collected using a Quantum Design MPMS-5S SQUID magnetometer. Magnetic data were corrected for the diamagnetism of the sample holder and for the diamagnetism of the sample using Pascal's constants.NMR measurements
1H-NMR spectra were measured on a Bruker AVANCE400 spectrometer at room temperature. Chemical shifts in NMR were reported in ppm (δ), relative to the internal standard of tetramethylsilane (TMS). The signals observed were described as s (singlet), d (doublet), t (triplet), and m (multiplets). The number of protons (n) for a given resonance is indicated as nH. Coupling constants are reported as J in hertz.Elemental analysis
Elemental analyses were performed using a Perkin Elmer 2400 elemental analyser.Results and discussion
Structures
[Cu3] crystallized in the P21/n space group and the structural data were collected at 100 K.18 [Cu3] has a planar trinuclear triangular core, consisting of three copper ions (Fig. 1). The asymmetric unit includes one ligand, three copper ions, three coordinated chloride ions, one uncoordinated chloride ion and five water molecules. All copper ions have a square pyramidal geometry with N4Cl donor sets and show elongated-type Jahn–Teller distortion with the elongated axis lying perpendicular to the ligand plane. The coordination bond lengths, charge balance and coordination geometries of the copper ions suggest that all are divalent. Three chloride ions coordinate the copper ions as axial ligands, and the remaining chloride ion is free in the crystal lattice. The ligand, L2−, coordinates the equatorial positions of all copper ions, acting as a bridge between all three centres. The distances between the copper ions are Cu1–Cu2 = 4.8360(16) Å, Cu2–Cu3 = 4.8026(15) Å and Cu3–Cu1 = 4.8353(16) Å, respectively. The structure suggests that strong antiferromagnetic interactions are operative between the copper ions, through Cu–N–N–Cu magnetic pathways and the magnetic orbitals on equatorial planes. The Cu–N–N–Cu torsion angles are close to linear, ranging from 173.10° to 177.71°. Hydrogen bonds exist between the trinuclear complex cations, forming a columnar structure.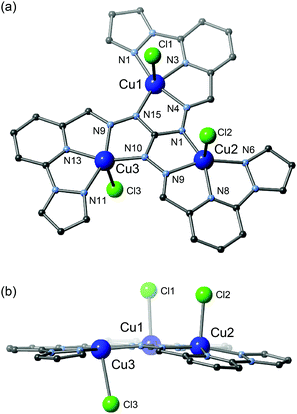 | ||
| Fig. 1 Molecular structure of [Cu3]. (a) Top view. (b) Side view. Counter anions, hydrogen atoms and solvent molecules have been omitted for clarity. | ||
The three copper ions and all atoms of the ligand L2− lie on the same plane with a deviation of 0.422(2) Å from the least-squares planes defined by all atoms. The Cu1, Cu2 and Cu3 ions deviate from the equatorial plane defined by four donor atoms (N1, N3, N4, N15), (N5, N6, N8, N9) and (N10, N11, N13, N14) by −0.081(4), −0.243(4), and 0.052(4) Å, respectively. There are some hydrogen bonding interactions between the chloride ions and water molecules, forming a one-dimensional network along the b-axis. π–π interactions are observed between neighbouring triangles, expanding the one-dimensional ribbon along the a-axis. The shortest intermolecular metal–metal distance is 6.7814(15) Å, through a translational operation along the b-axis.
[Ni3] crystallized in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and the structural data were collected at 100 K.19 [Ni3] has a similar trinuclear triangular core, consisting of one ligand, three nickel ions, five coordinated methanol moieties and one water molecule, with four tetrafluoroborate ions included in the crystal lattice as counter anions (Fig. 2). The distances between the nickel ions are Ni1–Ni2 = 4.9599(12) Å, Ni2–Ni3 = 4.9877(12) Å and Ni3–Ni1 = 4.9957(12) Å, respectively. All nickel ions have six-coordinate octahedral coordination geometries suggesting that all nickel ions are divalent and in their HS state (S = 1). All nickel centres are coordinated in a meridional fashion by the N-donor atoms of the ligand and by two oxygen atoms from solvent molecules. The Ni1 ion is coordinated by one water molecule and one methanol molecule at the apical positions, while Ni2 and Ni3 ions are coordinated by two methanol molecules.
space group and the structural data were collected at 100 K.19 [Ni3] has a similar trinuclear triangular core, consisting of one ligand, three nickel ions, five coordinated methanol moieties and one water molecule, with four tetrafluoroborate ions included in the crystal lattice as counter anions (Fig. 2). The distances between the nickel ions are Ni1–Ni2 = 4.9599(12) Å, Ni2–Ni3 = 4.9877(12) Å and Ni3–Ni1 = 4.9957(12) Å, respectively. All nickel ions have six-coordinate octahedral coordination geometries suggesting that all nickel ions are divalent and in their HS state (S = 1). All nickel centres are coordinated in a meridional fashion by the N-donor atoms of the ligand and by two oxygen atoms from solvent molecules. The Ni1 ion is coordinated by one water molecule and one methanol molecule at the apical positions, while Ni2 and Ni3 ions are coordinated by two methanol molecules.
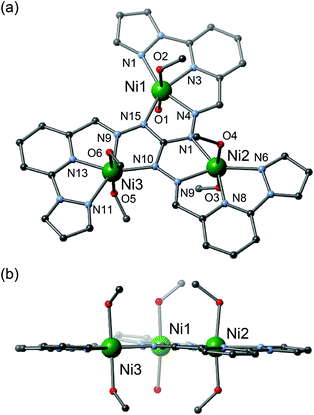 | ||
| Fig. 2 Molecular structure of [Ni3]. (a) Top view. (b) Side view. Counter anions, hydrogen atoms and solvent molecules have been omitted for clarity. | ||
[Ni3] has a planar structure similar to [Cu3]. All atoms of the ligand and the three nickel ions lie on the same plane with a deviation of less than 0.35 Å. The Ni1, Ni2 and Ni3 ions deviate from the equatorial plane defined by their four donor atoms (N1, N3, N4, N15), (N5, N6, N8, N9) and (N10, N11, N13, N14) by −0.027(3), −0.006(3), and −0.023(3) Å, respectively. The shortest intermetallic separation of Ni3–Ni3#1 ions between neighboring triangular units is 9.5053(17) Å (symmetry operation #1: −x + 1, −y, −z + 1).
Overall, the guanidine derivative ligand, H2L·HCl, was shown to support [M3L]n+ (M; metal ion) type structure, acting as a −2 anionic ligand. Guanidine has two single bonds and one double bond between the nitrogen and carbon atoms. In this system, the bond lengths between the central carbon atom (C28) and nitrogen atoms are C28–N5 = 1.345(10) Å, C28–N10 = 1.360(10) Å, and C28–N15 = 1.373(10) Å for [Cu3] and C28–N5 = 1.359(8) Å, C28–N10 = 1.376(8) Å, and C28–N15 = 1.368(8) for [Ni3], respectively. These bond lengths are intermediates between single and double bonds, and show that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond in both complexes is delocalized between the four atoms. Therefore, all carbon and nitrogen atoms form sp2-like hybrid orbitals, and complexes with highly planar structures were produced.
N double bond in both complexes is delocalized between the four atoms. Therefore, all carbon and nitrogen atoms form sp2-like hybrid orbitals, and complexes with highly planar structures were produced.
Magnetic properties
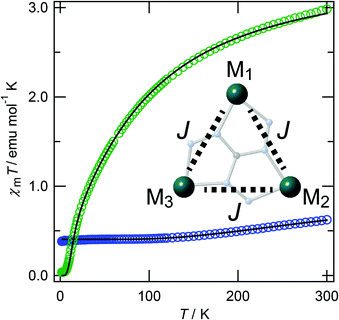
DC magnetic susceptibility data of [Cu3] and [Ni3] were measured in the temperature range of 1.8–300 K under an applied magnetic field of 500 Oe and the χmT versus T plots are shown in Fig. 3.The χmT value of [Cu3] at 300 K was 0.622 emu mol−1 K, which is smaller than the expected value of three magnetically isolated S = 1/2 spins (1.125 emu mol−1 K (g = 2.00)), and decreased with cooling to reach a plateau below 130 K. The χmT value of [Cu3] at 100 K was 0.411 emu mol−1 K, close to the spin only value of 0.375 emu mol−1 K (g = 2.00), expected for the isolated spin of one CuII ion (S = 1/2). The χmT value of [Ni3] at 300 K was 3.20 emu mol−1 K, slightly larger than the spin only value of 3.00 emu mol−1 K (g = 2.00), calculated from the sum of the uncorrelated spins of three NiII ions (S = 1). The χmT value also showed a decrease with lowered temperature. The χmT value at 1.8 K was 0.039 emu mol−1 K, suggesting that the spin ground state of [Ni3] at low temperature is spin singlet. The triangular spin Hamiltonian model of H = −2J(S1·S2 + S2·S3 + S3·S1), where J represents the exchange of the coupling constant between metal ions, was used to analyse the magnetic susceptibility data of [Cu3] and [Ni3] (the schematic spin model is shown as an inset in Fig. 3). The solid lines are the least squares fits and the parameters for [Cu3] are g = 2.08(1), J = −130(1) cm−1, and for [Ni3] are g = 2.18(1), J = −14.9(1) cm−1, showing that strong antiferromagnetic interactions are operative between the metal ions, respectively. In the case of [Cu3], the magnetic exchange interactions between copper ions are operative through N–N bonds and involve the overlapping of the magnetic equatorial orbitals of the copper ions, thus promoting strong interactions. We recently reported the magnetic behaviour of a linear trinuclear copper complex with a planar structure, which shows similar strong magnetic interactions J = −194 cm−1 between neighbouring copper ions when bridged by pyrazole groups.20 In both cases the magnetic pathways are mediated by similar sp2-type N–N bridges, indicating that the strength of the magnetic interaction in [Cu3] is reasonable from a structural viewpoint.
The field-dependence of magnetization data for [Cu3] and [Ni3] at 1.8 K is shown in Fig. S1.† The magnetic moment of [Cu3] at 5 T reached 0.974Nβ, suggesting that the ground state of [Cu3] is ST = 1/2. The magnetization curve was analysed using an S = 1/2 Brillouin function and the estimated curve is shown as a solid line. The observed data are slightly lower than the estimated curve across the majority of the applied magnetic fields, suggesting that weak antiferromagnetic interactions are likely to be operative between neighbouring triangular molecules at low temperature. Calculated magnetization curves of [Cu3] at 1.8 K, estimated from a regular triangular model with the above-mentioned g and J values are shown in Fig. S2.† At low magnetic fields, the ground state ST = 1/2 is strongly stabilized and the next level crossing point (to ST = 3/2) is estimated to occur at 280 T. The magnetic moment of [Ni3] at 5 T was 0.068Nβ, showing that the ground state of [Ni3] is ST = 0 (Fig. S1†). The calculated magnetization curves of [Ni3] at 1.8 K showed that level crossing is estimated to occur at 19.6 T (ST = 0 → 1), 41.2 T (ST = 1 → 2), and 61.8 T (ST = 2 → 3) (Fig. S3†). In order to observe spin flipping under a pulsed magnetic field, the strength of the magnetic interactions should be weak. Further study on spin frustration and spin chirality will require detailed EPR experiments. We are currently applying our learning from the presented systems to aid in the construction of spin tubes.
Conclusions
A novel guanidine derivative and its tri-nuclear metal complexes were synthesized. The ligand can coordinate three metal ions in its N4 binding positions, forming planar triangular structures. The exchange coupling constants were g = 2.08(1), J = −130(1) cm−1 for [Cu3], and g = 2.18(1), J = −14.9(1) cm−1 for [Ni3], suggesting that antiferromagnetic interactions are operative between the metal ions in both complexes. The ground spin state of [Cu3] at low temperature is spin doublet, while in the case of [Ni3], the magnetic susceptibility data and magnetization curve suggest that its ground state is spin singlet. The synthesized guanidine derivative ligand has proven itself useful in the construction of antiferromagnetic triangular units with planar molecular structures; however, in this example, the counterions and solvent molecules precluded the formation of supramolecular spin-tube type arrangements. Further work will attempt to connect the units to form extended structures. The presented complexes are 4+ cations, a fact that may be used to their advantage by employing anionic bridging units to link them into extended systems. These results will shed light on the molecular design of planar triangular building blocks and extended spin-tubes.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (A) and (C) (no. 25248014 and no. 26410065) from the Japan Society for the Promotion of Science (JSPS).Notes and references
- (a) M. L. Baker, G. A. Timco, S. Piligkos, J. S. Mathieson, H. Mutka, F. Tuna, P. Kozlowski, M. Antkowiak, T. Guidi, T. Gupta, H. Rath, R. J. Woolfson, G. Kamieniarz, R. G. Pritchard, H. Weihe, L. Cronin, G. Rajaraman, D. Collison, E. J. L. McInnes and R. E. P. Winpenny, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 19113–19118 CrossRef CAS PubMed; (b) J. Schnack, Dalton Trans., 2010, 39, 4677–4686 RSC.
- Ring compound review: (a) G. A. Timco, T. B. Faust, F. Tuna and R. E. P. Winpenny, Chem. Soc. Rev., 2011, 40, 3067–3075 RSC; (b) M. Affronte, S. Carretta, G. A. Timco and R. E. P. Winpenny, Chem. Commun., 2007, 1789–1797 RSC; (c) R. W. Saalfrank, H. Maid and A. Scheurer, Angew. Chem., Int. Ed., 2008, 47, 8794–8824 CrossRef CAS PubMed; J. J. Bodwin, A. D. Cutland, R. G. Malkani and V. L. Pecoraro, Coord. Chem. Rev., 2001, 216–217, 489–512 Search PubMed.
- N. Hoshino, M. Nakano, H. Nojiri, W. Wernsdorfer and H. Oshio, J. Am. Chem. Soc., 2009, 131, 15100–15101 CrossRef CAS PubMed.
- Odd-membered rings: (a) A. J. Stemmler, J. W. Kampf, M. L. Kirk, B. H. Atasi and V. L. Pecoraro, Inorg. Chem., 1999, 38, 2807–2817 CrossRef CAS PubMed; (b) C. S. Campos-Fernández, R. Clérac and K. R. Dunbar, Angew. Chem., Int. Ed., 1999, 38, 3477–3479 CrossRef; (c) C. S. Campos-Fernández, R. Clérac, J. M. Koomen, D. H. Russell and K. R. Dunbar, J. Am. Chem. Soc., 2001, 123, 773–774 CrossRef.
- Three-membered rings: (a) N. F. Curtis and K. R. Morgan, J. Mol. Struct., 2011, 1006, 375–378 CrossRef CAS; (b) A. Escuer, G. Vlahopoulou, F. Lloret and F. A. Mautner, Eur. J. Inorg. Chem., 2014, 83–92 CrossRef CAS; (c) M. Wenzel, R. S. Forgan, A. Faure, K. Mason, P. A. Tasker, S. Piligkos, E. K. Brechin and P. G. Plieger, Eur. J. Inorg. Chem., 2009, 4613–4617 CrossRef CAS; (d) E. Y. Tsui, M. W. Day and T. Agapie, Angew. Chem., Int. Ed., 2011, 50, 1668–1672 CrossRef CAS PubMed; (e) D. Cage, F. A. Cotton, N. S. Dalal, E. A. Hillard, B. Rakvin and C. M. Ramsey, J. Am. Chem. Soc., 2003, 125, 5270–5271 CrossRef PubMed.
- (a) K.-Y. Choi, Y. Matsuda, H. Nojiri, U. Kortz, F. Hussain, A. C. Stowe, C. Ramsey and D. S. Dalal, Phys. Rev. Lett., 2006, 96, 107202 CrossRef PubMed; (b) K.-Y. Choi, Z. Wang, H. Nojiri, J. van Tol, P. Kumar, P. Lemmens, B. S. Bassil, U. Kortz and N. S. Dalal, Phys. Rev. Lett., 2012, 108, 067206 CrossRef PubMed.
- (a) M. Casarin, C. Corvaja, C. di Nicola, D. Falcomer, L. Franco, M. Monari, L. Pandolfo, F. Pettinari and P. Tagliatesta, Inorg. Chem., 2004, 43, 5865–5876 CrossRef CAS PubMed; (b) J.-P. Costes, F. Dahan and J.-P. Laurent, Inorg. Chem., 1986, 25, 413–416 CrossRef CAS; (c) A. Escuer, R. Vicente, E. Peñalba, X. Solans and M. Font-Bardía, Inorg. Chem., 1996, 35, 248–251 CrossRef CAS PubMed.
- Spin gap reference S = 1/2 chain (a) H. Tsunetsugu, Y. Hatsugai, K. Ueda and M. Sigrist, Phys. Rev. B: Condens. Matter, 1992, 46, 3175–3178 CrossRef; (b) C. C. Yu and S. R. White, Phys. Rev. Lett., 1993, 71, 3866–3869 CrossRef CAS PubMed.
- E. Lieb, T. Schultz and D. Mattis, Ann. Phys., 1961, 16, 407–466 Search PubMed.
- (a) K. Kawano and M. Takahashi, J. Phys. Soc. Jpn., 1997, 66, 4001–4008 CrossRef CAS; (b) T. Sakai and M. Sato, Phys. Rev. B: Condens. Matter, 2007, 75, 014411 CrossRef; (c) T. Sakai, M. Sato, K. Okunishi, Y. Otuka, K. Okamoto and C. Itoi, Phys, Rev. B: Condens. Matter, 2008, 78, 184415 CrossRef.
- H. Manaka, Y. Hirai, Y. Hachigo, M. Mitsunaga, M. Ito and N. Terada, J. Phys. Soc. Jpn., 2009, 78, 093701 CrossRef.
- (a) G. Seeber, P. Kögerler, B. M. Kariuki and L. Cronin, Chem. Commun., 2004, 1580–1581 RSC; (b) N. B. Ivanov, J. Schnack, R. Schnalle, J. Richter, P. Kögerler, G. N. Newton, L. Cronin, Y. Oshima and H. Nojiri, Phys. Rev. Lett., 2010, 105, 037206 CrossRef PubMed.
- J. K. Clegg, L. F. Lindoy, J. C. McMurtrie and D. Schilter, Dalton Trans., 2006, 3114–3121 RSC.
- I. M. Müller and D. A. Möller, Eur. J. Inorg. Chem., 2005, 257–267 CrossRef.
- (a) Y.-H. Gong, F. Miomandre, R. Méallet-Renault, S. Badré, L. Galmiche, J. Tang, P. Audebert and G. Clavier, Eur. J. Org. Chem., 2009, 6121–6128 CrossRef CAS; (b) J. Zhu, J. Hiltz, R. B. Lennox and R. Schirrmacher, Chem. Commun., 2013, 49, 10275–10277 RSC.
- Z. He, D. C. Craig and S. B. Colbran, Dalton Trans., 2002, 4224–4235 RSC.
- (a) F. Zeng and Z. Yu, Organometallics, 2009, 28, 1855–1862 CrossRef CAS; (b) S. Heider, H. Petzold, G. Chastanet, S. Schlamp, T. Rüffer, B. Weber and J.-F. Létard, Dalton Trans., 2013, 42, 8575–8584 RSC.
- Crystallographic data for [Cu3] (C28H31N15Cl4Cu3O5 = 990.10 g mol−1) monoclinic, P21/n, a = 11.665(3) Å, b = 6.7814(15) Å, c = 48.532(11) Å, β = 94.167(4)°, V = 3828.8(15) Å3, Z = 4, Dcalcd = 1.718 g cm−3, R1 = 0.0907 (I > 2σ(I)), wR2 = 0.2438 (all data) (CCDC 1403544).
- Crystallographic data for [Ni3] (C34H47N15B4F16Ni3O7 = 1301.23 g mol−1) triclinic, Pν, a = 10.0521(8) Å, b = 11.4299(9) Å, c = 22.6136(18) Å, α = 77.6660(10)°, β = 83.379(2)°, γ = 83.524(2)°, V = 2510.9(3) Å3, Z = 2, Dcalcd = 1.721 g cm−3, R1 = 0.0861 (I > 2σ(I)), wR2 = 0.2276 (all data) (CCDC 1403545).
- S. Terashima, G. N. Newton, T. Shiga and H. Oshio, Inorg. Chem. Front., 2015, 2, 125–128 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional structural and magnetic data. CCDC 1403544 and 1403545 for [Cu3] and [Ni3], respectively. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qi00085h |
| This journal is © the Partner Organisations 2015 |