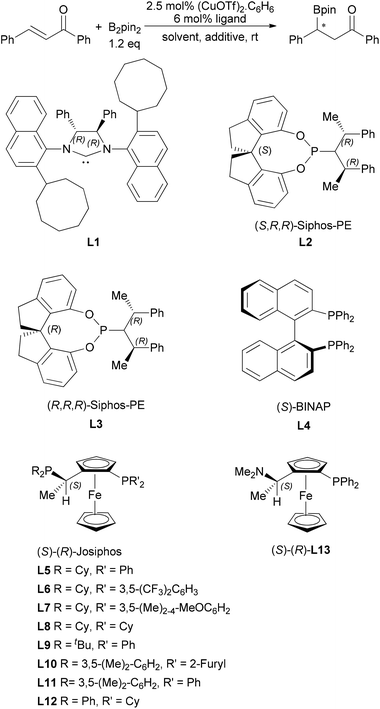
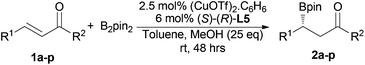
Asymmetric boron conjugate addition to α,β-unsaturated carbonyl compounds catalyzed by CuOTf/Josiphos under non-alkaline conditions†
Jian-Bo
Xie
a,
Siqi
Lin
a,
Jian
Luo
a,
Jianbin
Wu
a,
Timothy R.
Winn
a and
Guigen
Li
*ab
aDepartment of Chemistry and Biochemistry, Texas Tech University, Lubbock, Texas 79409-1061, USA. E-mail: guigen.li@ttu.edu
bInstitute of Chemistry & BioMedical Sciences (ICBMS), Nanjing University, Nanjing 210093, P. R. China
First published on 1st December 2014
Abstract
The asymmetric boron conjugate addition onto α,β-unsaturated ketones and esters has been developed by using the CuOTf/Josiphos complex as the catalyst under non-alkaline conditions. It was found that the addition of MeOH into the reaction system is crucial to the catalytic reactivity. Good to excellent enantioselectivity (up to 96% ee) and yields (up to 98%) have been achieved for 15 examples.
In the past few decades, the synthesis of α-chiral boron compounds has attracted much attention in chemical synthesis since they serve as precursors to many chiral products containing C–O, C–N and C–C bonds;1 they are also important bioactive building blocks for drug design and synthesis.2 Among the efforts to obtain α-chiral boron compounds, the copper catalyzed asymmetric boron conjugate addition to α,β-unsaturated carbonyl compounds has undoubtedly achieved great success.3 However, to the best of our knowledge, all the existing asymmetric catalytic systems with copper salts require the use of strong inorganic bases, such as NaOtBu, Cs2CO3, etc., as additives, or copper precursors (e.g. Cu(OH)2,4a CuCO3,4betc.4c,d) should be used with water as the solvent. The strong base additives were believed to play important roles in achieving high catalytic reactivity,5 and they can also act as catalysts in the boron additions at high temperatures.6 It is necessary to develop a catalytic system in the absence of base additives to avoid potential problems like elimination or racemization in complex compounds.3q,7
Several examples of non-alkaline catalytic systems for the boron conjugate addition reaction have been reported, which include the use of platinum catalysts,8 CuOTf/monophosphine catalysts9 and organo catalysts (phosphines,10a NHC,10betc.). Meanwhile, a mixed sp2–sp3 hybridized diboron reagent11 can enable this reaction to be performed under non-alkaline conditions. However, there is no successful enantioselective boron addition example based on these catalytic systems reported so far. Herein, we would like to report a new Cu(I)-catalyzed asymmetric boron conjugate addition reaction under non-alkaline conditions.
We initiated this work with 5 mol% CuOTf and 6 mol% monodentate ligands as it was described that no desired product was formed with the bidentate phosphine ligand at room temperature in Hosomi's work.9 The commercially available bis(pinacolato)diboron (B2Pin2) and chalcone were chosen as the boron reagent and the standard substrate, respectively. We first employed the C2 symmetric chiral NHC ligand (L1).12 However, only poor or moderate enantioselectivity was obtained (Table 1, entries 1–3). When methanol was added as an additive, the enantioselectivity and reactivity were significantly enhanced (Table 1, entry 2).13 The reactivity can be further increased albeit it caused slight harm to the enantioselectivity when methanol was used as the solvent (entry 3). The spiro monophosphoramidite ligand [(S,R,R)-L2] was then applied for this boron addition reaction. The enantioselectivity was close to the result obtained by the NHC ligand L1 while the reactivity was much higher (entry 4). When its diastereoisomer, (R,R,R)-L3, was applied, we observed the inversed configuration in the product which means that the chirality on the spiro backbone is more important than the chirality on the amine part of the ligand (entry 5). We also increased the loading of the ligand to 12 mol% for comparison and almost the same result was obtained (entry 6) which could indicate that only one molecule of phosphoramidite was coordinated to the Cu(I) catalyst species.
| Entry | Ligand | Solvent | Additive (equiv.) | Time (h) | Conv.b (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Reactions were run under argon protection and mostly repeated twice. Reaction conditions: 0.1 mmol scale, [substrate] = 0.04 M, 2.5 mol% (CuOTf)2. Benzene, 6 mol% ligand, solvent volume = 2.5 mL, room temperature (23–26 °C). b Determined by crude 1H NMR analysis. c Determined by chiral HPLC analysis. d The (NHC)CuOTf catalyst was prepared by anion exchange with (NHC)CuCl and AgOTf. e 12 mol% ligand. | ||||||
| 1d | L1 | Toluene | — | 16 | 7 | 8(S) |
| 2d | L1 | Toluene | MeOH (1) | 16 | 17 | 47 |
| 3d | L1 | MeOH | — | 16 | 46 | 41 |
| 4 | L2 | Toluene | MeOH (1.5) | 30 | 97 | 42 |
| 5 | L3 | Toluene | MeOH (1.5) | 46 | 35 | −50(R) |
| 6e | L2 | Toluene | MeOH (1.5) | 30 | 95 | 43 |
| 7 | L4 | Toluene | MeOH (1.5) | 30 | 2 | ND |
| 8 | L5 | Toluene | MeOH (1.5) | 30 | 74 | −92 |
| 9 | L13 | Toluene | MeOH (1.5) | 30 | 100 | −45 |
| 10 | L5 | Toluene | — | 30 | 0 | — |
| 11 | L5 | Toluene | MeOH (12) | 30 | 80 | −93 |
| 12 | L5 | Toluene | MeOH (25) | 18 | 93 | −91 |
| 13 | L5 | MeOH | — | 30 | 100 | −33 |
| 14 | L5 | DCM | MeOH (25) | 30 | 0 | — |
| 15 | L5 | THF | MeOH (25) | 30 | 62 | −6 |
| 16 | L5 | CH3CN | MeOH (25) | 30 | 5 | ND |
| 17 | L5 | DMF | MeOH (25) | 30 | 94 | −51 |
| 18 | L5 | Toluene | EtOH (25) | 46 | 64 | −92 |
| 19 | L5 | Toluene | iPrOH (25) | 46 | 24 | −93 |
| 20 | L5 | Toluene | t BuOH (25) | 46 | <2 | ND |
| 21 | L5 | Toluene | AcOH (25) | 46 | 14 | 0 |
| 22 | L5 | Toluene | PhOH (2.5) | 46 | 23 | −4 |
| 23 | L6 | Toluene | MeOH (25) | 46 | 44 | −70 |
| 24 | L7 | Toluene | MeOH (25) | 20 | 95 | −13 |
| 25 | L8 | Toluene | MeOH (25) | 20 | 90 | 7 |
| 26 | L9 | Toluene | MeOH (25) | 46 | <2 | ND |
| 27 | L10 | Toluene | MeOH (25) | 20 | 100 | −4 |
| 28 | L11 | Toluene | MeOH (25) | 20 | 94 | −13 |
| 29 | L12 | Toluene | MeOH (25) | 20 | 51 | −39 |
As the enantioselectivity has been proven to be low to moderate when using a monodentate ligand, we next attempted to utilize bidentate diphosphine ligands (L4 and L5) and aminophos ligand (L12). Although the bidentate ligands were proved to show no catalytic activity at all in the boron addition reaction (Table 1, entry 10) in Hosomi's work, the reactivity was dramatically changed by adding MeOH as an additive into the reaction system, and the enantioselectivity reached 92% ee by the use of Josiphos (L5) (entry 8). Nevertheless, another diphosphine ligand, Binap, showed very poor catalytic activity even by addition of MeOH (entry 7). The aminophos ligand (L12) achieved a higher reactivity though only 43% ee was obtained (entry 9). Then we continued catalytic condition optimization by using Josiphos L5. Increasing the loading of methanol could enhance the reactivity further while a slight decrease of enantioselectivity was also observed with 25 equiv. of MeOH (entry 12). If methanol was used as the solvent, the enantioselectivity dropped to 33% (entry 13). We screened several other common solvents, but only DMF could give moderate enantioselectivity and good reactivity (entries 14–17). We then compared other protonic additives in toluene. When various alcohols were used (Table 1), the reactivity decreased while the bulk of the alcohol increased. But the enantioselectivity can be maintained well with the two alcohol additives (entries 18–20). The acidic additives, such as acetic acid and phenol, would lead to dramatically decreases of the reactivity and enantioselectivity (entries 21 and 22). Other Josiphos type ligands were also checked for this reaction with results shown in Table 1. However, changing the substituent groups on the P atoms cannot lead to higher enantioselectivity (entries 23–29).
The substrate scope was examined by using CuOTf (5 mol%) and Josiphos ligand L5 (6 mol%) in toluene and MeOH (25.0 eq.) as the additive for 48 h (Table 2). The reactions were very clean with no side product observed by crude 1H-NMR analysis. For the chalcone substrates, the substituents on the phenyl rings had a small influence on the enantioselectivity (entries 2–11), but the substituents on the ortho position of the phenyl ring which is adjacent to the newly formed chiral center led to a significant decrease in the reactivity (entries 2 and 11). It should be noted that the NO2 substituent can tolerate the catalytic conditions and give the desired product with 95% yield and 95% ee (entry 3). As far as we know, it is the first example of using a substrate with the NO2 group in such a catalytic boron addition reaction. When the phenyl group on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in chalcone was replaced by the methyl group, the highest ee value (96%) was obtained (entry 13), but its isomer, (E)-4-phenylbut-3-en-2-one, only gave 72% ee and lower conversion (entry 12). The α,β-unsaturated esters were also analysed in this reaction. Good enantioselectivity was obtained for both of the tested ester substrates (entries 14 and 15). As we have developed a non-alkaline catalytic system, cinnamic acid was also studied as the substrate. Unfortunately, no reaction was observed in this case.
C bond in chalcone was replaced by the methyl group, the highest ee value (96%) was obtained (entry 13), but its isomer, (E)-4-phenylbut-3-en-2-one, only gave 72% ee and lower conversion (entry 12). The α,β-unsaturated esters were also analysed in this reaction. Good enantioselectivity was obtained for both of the tested ester substrates (entries 14 and 15). As we have developed a non-alkaline catalytic system, cinnamic acid was also studied as the substrate. Unfortunately, no reaction was observed in this case.
| Entry | R1 | R2 | Prod. | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions are the same as those listed in Table 1, entry 12. b Yields of the isolated products. c % ee was determined by chiral HPLC analysis; the absolute configuration was determined by comparison of optical rotation with literature data. | |||||
| 1 | C6H5 | C6H5 | 2a | 90 | 91(R) |
| 2 | 2-ClC6H4 | C6H5 | 2b | 24 | 93(R) |
| 3 | 3-NO2C6H4 | C6H5 | 2c | 95 | 95(+) |
| 4 | 4-MeOC6H4 | C6H5 | 2d | 89 | 93(R) |
| 5 | 4-FC6H4 | C6H5 | 2e | 96 | 95(−) |
| 6 | 4-ClC6H4 | C6H5 | 2f | 98 | 95(R) |
| 7 | C6H5 | 4-MeOC6H4 | 2g | 98 | 90(R) |
| 8 | C6H5 | 4-FC6H4 | 2h | 96 | 95(R) |
| 9 | C6H5 | 4-ClC6H4 | 2i | 86 | 92(−) |
| 10 | 4-FC6H4 | 4-FC6H4 | 2j | 95 | 94(−) |
| 11 | 2,4-(MeO)2C6H3 | 4-FC6H4 | 2k | 50 | 82(−) |
| 12 | C6H5 | Me | 2l | 76 | 72(R) |
| 13 | Me | C6H5 | 2m | 95 | 96(S) |
| 14 | C6H5 | OBn | 2n | 62 | 84(R) |
| 15 | Me | OBn | 2o | 96 | 88(S) |
| 16 |
|
2p | NR | — | |
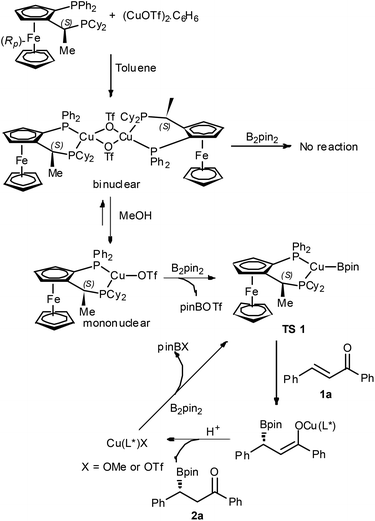
The catalytic mechanism and the role of MeOH in this reaction are proposed in Scheme 1 based on the previous catalytic boron conjugate addition work involving copper14 and the coordination chemistry between Cu(I)X and Josiphos in various solvents reported by Feringa and co-workers.15 The Cu(I)X and Josiphos form different complexes in different solvents, which could be a mononuclear or a binuclear complex. Furthermore, the rapid equilibration to form either a mononuclear or a binuclear complex depending on the solvent employed was also observed by Feringa's group and indeed a mononuclear complex would form in methanol. We assume that CuOTf and Josiphos firstly formed a binuclear complex in toluene, in which the copper has already four coordination numbers and cannot react with B2Pin2 as the copper was saturated with 18e. By adding MeOH, a mononuclear copper complex is formed and reacted with B2Pin2 to produce (L*)Cu–BPin species (TS 1). The (L*)Cu–BPin species has been known as an efficient catalyst in the boron addition reaction, and reacts with chalcone followed by protonation to release the chiral product and (L*)CuX species. The species of (L*)CuX then reacts with B2Pin2 to produce (L*)Cu–BPin species (TS 1) again and finally to complete the catalytic cycle.
In conclusion, we have developed a novel non-alkaline catalytic system for asymmetric boron conjugate addition reaction with α,β-unsaturated ketones and esters, which involved the Cu(I) triflate and Josiphos ligand as the catalyst and chiral ligand, respectively. Methanol was proved to be a crucial additive for high reactivity while there would be no reaction in the absence of the additive in toluene. Further studies on the mechanism and applications of the non-alkaline catalytic system to other base sensitive chiral products are ongoing in our laboratory.
Acknowledgements
We gratefully acknowledge the financial support from the Robert A. Welch Foundation (D-1361), NSFC (no. 21332005) and the Jiangsu Innovation Programs (P. R. China). We also thank NSF Grant CHE-1048553 and the CRIF program for supporting our NMR facility.Notes and references
- (a) T. Awano, T. Ohmura and M. Suginome, J. Am. Chem. Soc., 2011, 133, 20738 CrossRef CAS PubMed; (b) S. L. Poe and J. P. Morken, Angew. Chem., Int. Ed., 2011, 50, 4189 CrossRef CAS PubMed; (c) M. Tortosa, Angew. Chem., Int. Ed., 2011, 50, 3950 CrossRef CAS PubMed; (d) R. P. Sonawane, V. Jheengut, C. Rabalakos, R. Larouche-Gauthier, H. K. Scott and V. K. Aggarwal, Angew. Chem., Int. Ed., 2011, 50, 3760 CrossRef CAS PubMed; (e) J. K. Park, H. H. Lackey, B. A. Ondrusek and D. T. McQuade, J. Am. Chem. Soc., 2011, 133, 2410 CrossRef CAS PubMed; (f) S. Nave, R. P. Sonawane, T. G. Elford and V. K. Aggarwal, J. Am. Chem. Soc., 2010, 132, 17096 CrossRef CAS PubMed; (g) T. Ohmura, T. Awano and M. Suginome, J. Am. Chem. Soc., 2010, 132, 13191 CrossRef CAS PubMed; (h) J. Chang, H. Lee and D. G. Hall, J. Am. Chem. Soc., 2010, 132, 5544 CrossRef PubMed; (i) D. Imao, B. W. Glasspoole, V. S. Laberge and C. M. Crudden, J. Am. Chem. Soc., 2009, 131, 5024 CrossRef CAS PubMed; (j) E. Hupe, P. Marek and I. Knochel, Org. Lett., 2002, 4, 2861 CrossRef CAS PubMed; (k) Y. Yamamoto and N. Asao, Chem. Rev., 1993, 93, 2207 CrossRef CAS.
- (a) J.-B. Xie, J. Luo, T. R. Winn, D. B. Cordes and G. Li, Beilstein J. Org. Chem., 2014, 10, 746 CrossRef PubMed; (b) K. Hong and J. P. Morken, J. Am. Chem. Soc., 2013, 135, 9252 CrossRef CAS PubMed; (c) M. A. Beenen, C. An and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 6910 CrossRef CAS PubMed.
- (a) L. Zhao, Y. Ma, F. He, W. Duan, J. Chen and C. Song, J. Org. Chem., 2013, 78, 1677 CrossRef CAS PubMed; (b) T. Iwai, Y. Akiyama and M. Sawamura, Tetrahedron: Asymmetry, 2013, 24, 729 CrossRef CAS PubMed; (c) J.-L. Zhang, L.-A. Chen, R.-B. Xu, C.-F. Wang, Y.-P. Ruan, A.-E. Wang and P.-Q. Huang, Tetrahedron: Asymmetry, 2013, 24, 492 CrossRef CAS PubMed; (d) C. Sole, A. Bonet, A. H. M. de Vries, J. G. de Vries, L. Lefort, H. Gulyás and E. Fernández, Organometallics, 2012, 31, 7855 CrossRef CAS; (e) C. Solé, A. Tatla, J. A. Mata, A. Whiting, H. Gulyás and E. Fernández, Chem. – Eur. J., 2011, 17, 14248 CrossRef PubMed; (f) I. Ibrahem, P. Breistein and A. Córdova, Angew. Chem., Int. Ed., 2011, 50, 12036 CrossRef CAS PubMed; (g) A. Bonet, C. Sole, H. Gulyás and E. Fernández, Chem. – Asian J., 2011, 6, 1011 CrossRef CAS PubMed; (h) C. Solé, A. Whiting, H. Gulyás and E. Fernández, Adv. Synth. Catal., 2011, 353, 376 CrossRef; (i) B. Hong, Y. Ma, L. Zhao, W. Duan, F. He and C. Song, Tetrahedron: Asymmetry, 2011, 22, 1055 CrossRef CAS PubMed; (j) X. Feng and J. Yun, Chem. – Eur. J., 2010, 16, 13609 CrossRef CAS PubMed; (k) J. M. O'Brien, K.-S. Lee and A. H. Hoveyda, J. Am. Chem. Soc., 2010, 132, 10630 CrossRef PubMed; (l) J. K. Park, H. H. Lackey, M. D. Rexford, K. Kovnir, M. Shatruk and D. T. McQuade, Org. Lett., 2010, 12, 5008 CrossRef CAS PubMed; (m) W. J. Fleming, H. Müller-Bunz, V. Lillo, E. Fernández and P. J. Guiry, Org. Biomol. Chem., 2009, 7, 2520 RSC; (n) H.-S. Sim, X. Feng and J. Yun, Chem. – Eur. J., 2009, 15, 1939 CrossRef CAS PubMed; (o) C. Solé and E. Fernández, Chem. – Asian J., 2009, 4, 1790 Search PubMed; (p) V. Lillo, A. Prieto, A. Bonet, M. M. Díaz-Requejo, J. Ramírez, P. J. Pérez and E. Fernández, Organometallics, 2009, 28, 659 CrossRef CAS; (q) J.-E. Lee and J. Yun, Angew. Chem., Int. Ed., 2008, 47, 145 CrossRef CAS PubMed.
- (a) S. Kobayashi, P. Xu, T. Endo, M. Ueno and T. Kitanosono, Angew. Chem., Int. Ed., 2012, 51, 12763 CrossRef CAS PubMed; (b) G. Stavbera and Z. Časar, Appl. Organomet. Chem., 2013, 27, 159 CrossRef; (c) T. Kitanosono, P. Xu and S. Kobayashi, Chem. – Asian J., 2014, 9, 179 CrossRef CAS PubMed; (d) T. Kitanosono and S. Kobayashi, Asian J. Org. Chem., 2013, 2, 961 CrossRef CAS.
- J. A. Schiffner, K. Müther and M. Oestreich, Angew. Chem., Int. Ed., 2010, 49, 1194 CrossRef CAS PubMed.
- A. Bonet, C. Pubill-Ulldemolins, C. Bo, H. Gulyás and E. Fernández, Angew. Chem., Int. Ed., 2011, 50, 7158 CrossRef CAS PubMed.
- Z.-T. He, Y.-S. Zhao, P. Tian, C.-C. Wang, H.-Q. Dong and G.-Q. Lin, Org. Lett., 2014, 16, 1426 CrossRef CAS PubMed.
- Y. G. Lawson, M. J. G. Lesley, T. B. Marder, N. C. Norman and C. R. Rice, Chem. Commun., 1997, 2051 RSC.
- H. Ito, H. Yamanaka, J.-I. Tateiwab and A. Hosomi, Tetrahedron Lett., 2000, 41, 6821 CrossRef CAS.
- (a) C. Pubill-Ulldemolins, A. Bonet, H. Gulyás, C. Bo and E. Fernández, Org. Biomol. Chem., 2012, 10, 9677 RSC; (b) K.-S. Lee, A. R. Zhugralin and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 7253 CrossRef CAS PubMed. Hoveyda and co-workers also reported highly stereoselective boron conjugate addition with the chiral NHC catalyst but with an excess of base: (c) H. Wu, S. Radomkit, J. M. O'Brien and A. H. Hoveyda, J. Am. Chem. Soc., 2012, 134, 8277 CrossRef CAS PubMed.
- M. Gao, S. B. Thorpe and W. L. Santos, Org. Lett., 2009, 11, 3478 CrossRef CAS PubMed.
- For more details about the NHC ligand L1, see: L. Wu, L. Falivene, E. Drinkel, S. Grant, A. Linden, L. Cavallo and R. Dorta, Angew. Chem., Int. Ed., 2012, 51, 2870 CrossRef CAS PubMed.
- For the alcohol acceleration effect, see: S. Mun, J.-E. Lee and J. Yun, Org. Lett., 2006, 8, 4887 CrossRef CAS PubMed.
- (a) L. Dang and Z. Lin, Organometallics, 2008, 27, 4443 CrossRef CAS; (b) D. S. Laitar, E. Y. Tsui and J. P. Sadighi, J. Am. Chem. Soc., 2006, 128, 11036 CrossRef CAS PubMed; (c) K. Takahashi, T. Ishiyama and N. Miyaura, Chem. Lett., 2000, 982 CrossRef CAS.
- (a) S. R. Harutyunyan, F. López, W. R. Browne, A. Correa, D. Peña, R. Badorrey, A. Meetsma, A. J. Minnaard and B. L. Feringa, J. Am. Chem. Soc., 2006, 128, 9103 CrossRef CAS PubMed; (b) F. López, S. R. Harutyunyan, A. Meetsma, A. J. Minnaard and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 2752 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-, 13C-NMR data and spectra, HRMS for the new compounds; 1H NMR data, HPLC spectra and optical rotation for all products; experimental procedures and details. See DOI: 10.1039/c4qo00271g |
| This journal is © the Partner Organisations 2015 |