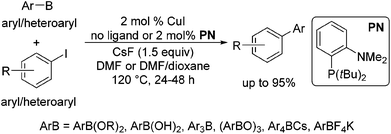
Copper-catalysed cross-couplings of arylboronate esters with aryl and heteroaryl iodides and bromides†‡
Santosh K.
Gurung
,
Surendra
Thapa
,
Bijay
Shrestha
and
Ramesh
Giri
*
Department of Chemistry & Chemical Biology, The University of New Mexico, Albuquerque, New Mexico 87131, USA. E-mail: rgiri@unm.edu; Tel: (+1) 505-277-1070, (+1) 505-277-2609
First published on 16th February 2015
Abstract
CuI-catalysed coupling of arylboronate esters with aryl and heteroaryl iodides and bromides is described. The transformation affords products in good yields using 5–10 mol% catalyst loadings. The described reaction requires a P,N-based bidentate ligand in combination with CuI for aryl–aryl coupling, but it proceeds without external ligands for aryl–heteroaryl coupling to afford the products. The reaction protocol can also be applied to achieve biarylation of diiodoarenes in reasonable yields.
Over the last three decades, Pd-catalysed Suzuki–Miyaura coupling has developed into a key synthetic tool that is capable of combining a wide variety of organoboron reagents with organohalides and pseudohalides to construct carbon–carbon (C–C) bonds.1 As a result, the cross-coupling process has been applied to the synthesis of a wide array of molecular targets, from materials and pharmaceuticals to organic building blocks and natural products.2 Despite the maturity of the transformation with regard to its substrate scope, catalytic turnover, and varied applications, the current desire remains high to achieve the reaction using inexpensive, less toxic, and sustainable transition metal (TM) catalysts. Towards this end, catalysts based on earth-abundant, 1st row late TMs are emerging as viable alternatives to palladium.3 In particular, Cu has demonstrated tremendous potential4 in catalysing such couplings for carbon–carbon (C–C) bond formation.5
In 2002, Rothenberg et al. revealed that a copper nanocluster enabled the coupling of phenylboronic acid with iodobenzene.6 Despite this seminal work more than a decade ago and subsequent reports,7 Cu-catalysed coupling was typically limited to the reaction of arylboronic acids with aryl iodides8 and in most cases required 10–20 mol% catalyst7b–f and stoichiometric amounts in others.7b In 2011, Liu et al. demonstrated that the reaction could be extended to the coupling of organoboronate esters, ArB(OR)2.9 However, the reaction was only suitable for coupling with primary alkyl halides and pseudohalides, proceeding via the SN2 mechanism. Recently, we have shown that a variety of organoboron reagents, such as ArB(OR)2, ArB(OH)2, Ar3B, (ArBO)3, Ar4BCs and ArBF4K, can undergo cross-coupling with aryl and heteroaryl iodides when CuI is utilized as a catalyst with or without the addition of the ligand o-(di-tert-butylphosphino)-N,N-dimethylaniline) (PN) (Scheme 1).10 Brown et al. also demonstrated that a similar reaction of ArB(OR)2 with aryl iodides could be achieved by the application of a bidentate xantphos ligand with CuCl, further attesting to the generality of Cu-based catalytic systems for Suzuki–Miyaura type cross-couplings.11
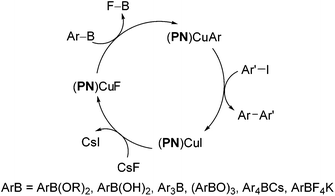
We further conducted detailed mechanistic studies and proposed a catalytic cycle based on the synthesis and characterisation of reaction intermediates, as well as followed the progress of the reaction in situ by 1H, 11B, 19F and 31P NMR spectroscopy (Scheme 2).10 Based on our studies, the cross-coupling proceeds via three elementary steps – exchange of a fluoride with the iodide in (PN)CuI, transmetallation of arylboron reagents12 with (PN)CuF,13 and the reaction of ArI with (PN)CuAr.14 In this article, we demonstrate that the reaction protocol can be extended to a variety of other mono- and di-iodoarenes and iodoheteroarenes to afford mono- and di-arylated products. In addition, we also show for the first time that these reaction conditions can be easily extended to the couplings of arylboronate esters with electron-deficient and heteroaryl bromides.
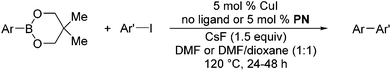
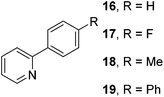
The results of our ongoing studies on the substrate scope for aryl–aryl and aryl–heteroaryl couplings in the presence of 5 mol% each of CuI and PN are summarized in Table 1. While the reactions of arylboronate esters with non-heteroaryl iodides required the PN ligand, the couplings of heteroaryl iodides were conducted with 5 mol% CuI in the absence of PN ligands (entries 1, 4).15 Without PN, the heteroaryl substrates and products could function as ligands for Cu. Cross-couplings involving heteroaryl coupling partners are generally challenging for Pd-catalysts because the heteroarenes bind to Pd competitively over the ligands, thus resulting in reaction inhibition and catalyst deactivation. Pd-based cross-couplings with heteroaryl coupling partners typically require highly sterically hindered phosphine- and N-heterocyclic carbene (NHC)-based ligands.16 Therefore, our reaction protocol provides an excellent complementary approach to Pd-catalysis for the synthesis of heterobiaryl molecules. In addition, the reaction tolerates halide substituents, such as chloride on the heteroaryl iodides (entry 1) and fluoride on arylboronate esters (entries 1, 5–8), affording cross-coupled products in good to excellent yields. We further demonstrated that the current reaction protocol can also be applied to the synthesis of terphenyl derivatives in reasonable yields either by mono-arylation of iodobiaryls (entries 2–4) or diarylation of di-iodoarenes (entries 5–8) that were not reported previously. Terphenyl derivatives are industrially important molecules that are widely utilized as preservatives, sunscreens, liquid crystals and proteomimics.17 As shown in entries 6–8, arylboronate esters containing multiple fluorinated and 3,5-bis-trifluoromethylated aryl groups were coupled with 1,4-diiodobenzene to afford the corresponding diarylated products in 47–73% yields.
| Entry | ArB(OR)2 | Ar′l | Ar–Ar′ | Yieldb (%) |
|---|---|---|---|---|
a 1.0 mmol scale. 5 mL DMF–dioxane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 48 h for aryl–aryl coupling, and 5 mL DMF, 24 h for aryl–heteroaryl coupling. PN was used for aryl–aryl coupling, and no ligand was used for aryl–heteroaryl coupling. CuI (99.999%) and CsF (99.9%) were used.
b Isolated yields.
c 20 mol% PN and CuI were used. 1), 48 h for aryl–aryl coupling, and 5 mL DMF, 24 h for aryl–heteroaryl coupling. PN was used for aryl–aryl coupling, and no ligand was used for aryl–heteroaryl coupling. CuI (99.999%) and CsF (99.9%) were used.
b Isolated yields.
c 20 mol% PN and CuI were used.
|
||||
| 1 |
|
|
|
70 |
| 2 |
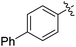
|
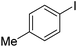
|
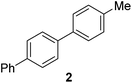
|
64 |
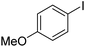
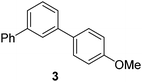
| 3 |
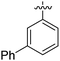
|
|
|
66 |
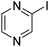
| 4 |
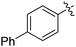
|
|
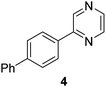
|
69 |
| 5 |
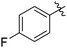
|
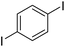
|
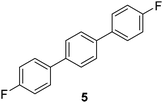
|
69c |
| 6 |
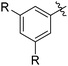
|
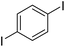
|
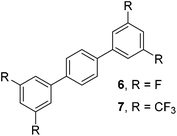
|
71 |
| 7 | 47 | |||
| 8 |
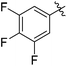
|
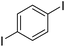
|
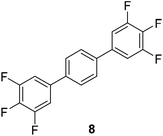
|
73c |
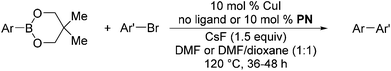
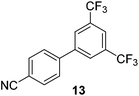
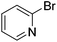
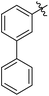
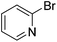
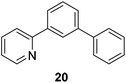
We also have found that the current combination of CuI with the sterically hindered and electron-rich PN ligand as a catalyst has enabled us to conduct cross-couplings of activated aryl and heteroaryl bromides to afford the coupled products in reasonable yields (Table 2).18 Since the Cu-catalysed cross-coupling of aryl bromides, which are less expensive and more readily available than aryl iodides, are rare5j,7e–f and generally require stoichiometric quantities of Cu-catalysts,5l the current reaction protocol provides an excellent opportunity to synthesize biaryl molecules using catalytic amounts of Cu-salts. The reaction proceeds well with both the electron deficient and electron rich arylboronate esters, affording the products in good yields. The reaction tolerates very sensitive and synthetically useful functional groups, including nitriles (entries 1–7).19 In addition, the coupling with heteroaryl bromides does not require the addition of any ancillary ligands (entries 8–12), an observation that is analogous to the coupling with heteroaryl iodides. This Cu-catalysed process provides a very cost-effective alternative to Pd-based systems for the synthesis of heterobiaryl molecules.
| Entry | ArB(OR)2 | Ar′Br | Ar–Ar′ | Yieldb (%) |
|---|---|---|---|---|
a 1.0 mmol scale. 5 mL DMF–dioxane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 48 h for aryl–aryl coupling, and 5 mL DMF, 36 h for aryl–heteroaryl coupling. PN was used for aryl–aryl coupling, and no ligand was used for aryl–heteroaryl coupling. CuI (99.999%) and CsF (99.9%) were used.
b Isolated yields.
c 2-Phenyl-1,3,2-dioxaborolane was used. 1), 48 h for aryl–aryl coupling, and 5 mL DMF, 36 h for aryl–heteroaryl coupling. PN was used for aryl–aryl coupling, and no ligand was used for aryl–heteroaryl coupling. CuI (99.999%) and CsF (99.9%) were used.
b Isolated yields.
c 2-Phenyl-1,3,2-dioxaborolane was used.
|
||||
| 1 | 69 | |||
| 2 |
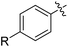
|
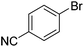
|
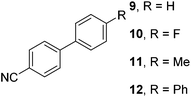
|
48 |
| 3 | 45 | |||
| 4 | 48 | |||
| 5 |
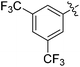
|
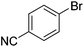
|
|
34 |
| 6 |
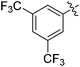
|
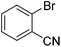
|
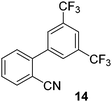
|
53 |
| 7 |
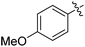
|
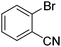
|
|
42 |
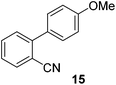
| 8 | 65c | |||
| 9 |
|
|
|
74 |
| 10 | 47 | |||
| 11 | 61 | |||
| 12 |
|
|
|
84 |
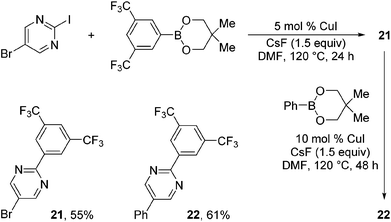
Taking advantage of the differential reaction rates of aryl iodides and bromides, we have further demonstrated that the current protocol can be extended to sequential arylations of haloarenes containing both iodo- and bromo-substituents. As outlined in Scheme 3, 5-bromo-2-iodopyrimidine was successively arylated with 3,5-bis(trifluoromethyl)phenyl- and phenylboronic acid neopentyl glycol esters under the standard reaction conditions to afford the mono- and diarylated products 21 and 22 in 55% and 61% yields, respectively.
Conclusions
We have demonstrated a broad substrate scope of the CuI-catalyzed Suzuki–Miyaura-type couplings. The reactions of arylboronate esters proceed well with aryl- and heteroaryl iodides and activated aryl and heteroaryl bromides. In general, the reaction requires a bidentate PN ligand for aryl–aryl couplings; however, no ligand is required for aryl–heteroaryl couplings. The current reaction protocol can also be applied to biarylation of diiodoarenes, affording doubly cross-coupled products in reasonable yields.Acknowledgements
We thank the University of New Mexico (UNM) for financial support, and upgrades to the NMR (NSF grants CHE08-40523 and CHE09-46690) and mass spectrometry facilities.Notes and references
- (a) N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef CAS; (b) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef CAS PubMed; (c) G. C. Fu, Acc. Chem. Res., 2008, 41, 1555 CrossRef CAS PubMed; (d) A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed; (e) C. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314 CrossRef CAS PubMed.
- (a) A. M. Rouhi, Chem. Eng. News, 2004, 82, 49 Search PubMed; (b) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS PubMed.
- For selected examples of Ni-catalysed Suzuki–Miyaura coupling, see: (a) J. Zhou and G. C. Fu, J. Am. Chem. Soc., 2004, 126, 1340 CrossRef CAS PubMed; (b) F. González-Bobes and G. C. Fu, J. Am. Chem. Soc., 2006, 128, 5360 CrossRef PubMed; (c) B. Saito and G. C. Fu, J. Am. Chem. Soc., 2007, 129, 9602 CrossRef CAS PubMed; (d) M. Tobisu, T. Shimasaki and N. Chatani, Angew. Chem., Int. Ed., 2008, 47, 4866 CrossRef CAS PubMed; (e) K. W. Quasdorf, X. Tian and N. K. Garg, J. Am. Chem. Soc., 2008, 130, 14422 CrossRef CAS PubMed; (f) B.-T. Guan, Y. Wang, B.-J. Li, D.-G. Yu and Z.-J. Shi, J. Am. Chem. Soc., 2008, 130, 14468 CrossRef CAS PubMed; (g) A. Antoft-Finch, T. Blackburn and V. Snieckus, J. Am. Chem. Soc., 2009, 131, 17750 CrossRef CAS PubMed; (h) K. W. Quasdorf, M. Riener, K. V. Petrova and N. K. Garg, J. Am. Chem. Soc., 2009, 131, 17748 CrossRef CAS PubMed; (i) K. W. Quasdorf, A. Antoft-Finch, P. Liu, A. L. Silberstein, A. Komaromi, T. Blackburn, S. D. Ramgren, K. N. Houk, V. Snieckus and N. K. Garg, J. Am. Chem. Soc., 2011, 133, 6352 CrossRef CAS PubMed; (j) Z. Lu, A. Wilsily and G. C. Fu, J. Am. Chem. Soc., 2011, 133, 8154 CrossRef CAS PubMed; (k) M. Tobisu, T. Xu, T. Shimasaki and N. Chatani, J. Am. Chem. Soc., 2011, 133, 19505 CrossRef CAS PubMed; (l) S. Ge and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 12837 CrossRef CAS PubMed. For Fe-catalysed Suzuki–Miyaura couplings, see: (m) Y. Guo, D. J. Young and T. S. Andy Hor, Tetrahedron Lett., 2008, 49, 5620 CrossRef CAS PubMed; (n) T. Hatakeyama, T. Hashimoto, Y. Kondo, Y. Fujiwara, H. Seike, H. Takaya, Y. Tamada, T. Ono and M. Nakamura, J. Am. Chem. Soc., 2010, 132, 10674 CrossRef CAS PubMed.
- For a few examples of the effects of Cu-salts on Pd- and Ni-catalysed cross-couplings, see: (a) X. X. Liu and M. Z. Deng, Chem. Commun., 2002, 622 RSC; (b) J. Z. Deng, D. V. Paone, A. T. Ginnetti, H. Kurihara, S. D. Dreher, S. A. Weissman, S. R. Stauffer and C. S. Burgey, Org. Lett., 2009, 11, 345 CrossRef CAS PubMed; (c) B. H. Lipshutz, D. M. Nihan, E. Vinogradova, B. R. Taft and Z. V. Bogkovic, Org. Lett., 2008, 10, 4279 CrossRef CAS PubMed.
- For Ni-catalysed Hiyama coupling with RX, see: (a) D. A. Powell and G. C. Fu, J. Am. Chem. Soc., 2004, 126, 7788 CrossRef CAS PubMed; (b) N. A. Strotman, S. Sommer and G. C. Fu, Angew. Chem., Int. Ed., 2007, 46, 3556 CrossRef CAS PubMed; (c) X. Dai, N. A. Strotman and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 3302 CrossRef CAS PubMed. For Cu-catalysed Stille coupling, see: (d) T. Takeda, K. I. Matsunaga, Y. Kabasawa and T. Fujiwara, Chem. Lett., 1995, 771 CrossRef CAS; (e) J. R. Falck, R. K. Bhatt and J. Ye, J. Am. Chem. Soc., 1995, 117, 5973 CrossRef CAS; (f) G. D. Allred and L. S. Liebeskind, J. Am. Chem. Soc., 1996, 118, 2748 CrossRef CAS; (g) S.-K. Kang, J.-S. Kim and S.-C. Choi, J. Org. Chem., 1997, 62, 4208 CrossRef CAS; (h) N. S. Nudelman and C. Carro, Synlett, 1999, 1942 CrossRef CAS PubMed. For a Cu-catalysed Heck-type coupling, see: (i) T. W. Liwosz and S. R. Chemler, Org. Lett., 2013, 15, 3034 CrossRef CAS PubMed. For a Cu-catalysed coupling with organoindium reagents, see: (j) S. Thapa, S. K. Gurung, D. A. Dickie and R. Giri, Angew. Chem., Int. Ed., 2014, 53, 11620 CrossRef CAS PubMed. For Cu-catalysed Hiyama-type couplings, see: (k) S. K. Gurung, S. Thapa, A. S. Vangala and R. Giri, Org. Lett., 2013, 15, 5378 CrossRef CAS PubMed; (l) S. K. Gurung, S. Thapa, B. Shrestha and R. Giri, Synthesis, 2014, 1933 Search PubMed; (m) A. Tsubouchi, D. Muramatsu and T. Takeda, Angew. Chem., Int. Ed., 2013, 52, 12719 CrossRef CAS PubMed; (n) L. Cornelissen, M. Lefrancq and O. Riant, Org. Lett., 2014, 16, 3024 CrossRef CAS PubMed; (o) L. Cornelissen, V. Cirriez, S. Vercruysse and O. Riant, Chem. Commun., 2014, 50, 8018 RSC.
- M. B. Thathagar, J. Beckers and G. Rothenberg, J. Am. Chem. Soc., 2002, 124, 11858 CrossRef CAS PubMed.
- For other examples of Cu-catalysed Suzuki–Miyaura coupling with ArB(OH)2, see: (a) M. B. Thathagar, J. Beckers and G. Rothenberg, Adv. Synth. Catal., 2003, 345, 979 CrossRef CAS; (b) J.-H. Li and D.-P. Wang, Eur. J. Org. Chem., 2006, 2063 CrossRef CAS; (c) J.-H. Li, J.-L. Li, D.-P. Wang, S.-F. Pi, Y.-X. Xie, M.-B. Zhang and X.-C. Hu, J. Org. Chem., 2007, 72, 2053 CrossRef CAS PubMed; (d) J.-H. Li, J.-L. Li and Y.-X. Xie, Synthesis, 2007, 984 CrossRef CAS PubMed; (e) J. Mao, J. Guo, F. Fang and S.-J. Ji, Tetrahedron, 2008, 64, 3905 CrossRef CAS PubMed; (f) Y.-M. Ye, B.-B. Wang, D. Ma, L.-X. Shao and J.-M. Lu, Catal. Lett., 2010, 139, 141 CrossRef CAS; (g) S. Wang, M. Wang, L. Wang, B. Wang, P. Li and J. Yang, Tetrahedron, 2011, 67, 4800 CrossRef CAS PubMed.
- (a) For few examples of reactions with aryl bromides, see: ref. 7e and f.
- C.-T. Yang, Z.-Q. Zhang, Y.-C. Liu and L. Liu, Angew. Chem., Int. Ed., 2011, 50, 3904 CrossRef CAS PubMed.
- S. K. Gurung, S. Thapa, A. Kafle, D. A. Dickie and R. Giri, Org. Lett., 2014, 16, 1264 CrossRef CAS PubMed.
- Y. Zhou, W. You, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2014, 53, 3475 CrossRef CAS PubMed.
- A. M. Whittaker, R. P. Rucker and G. Lalic, Org. Lett., 2010, 12, 3216 CrossRef CAS PubMed.
- For examples of other ligated CuF species, see: (a) D. J. Gulliver, W. Levason and M. Webster, Inorg. Chim. Acta, 1981, 52, 153 CrossRef CAS; (b) J. R. Herron and Z. T. Ball, J. Am. Chem. Soc., 2008, 130, 16486 CrossRef CAS PubMed; (c) J. R. Herron, V. Russo, E. J. Valente and Z. T. Ball, Chem. – Eur. J., 2009, 15, 8713 CrossRef CAS PubMed; (d) V. Russo, J. R. Herron and Z. T. Ball, Org. Lett., 2009, 12, 220 CrossRef PubMed.
- For examples of [CuAr] complexes, see: (a) H.-Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185 CrossRef CAS PubMed; (b) P. Leoni, M. Pesquali and C. A. Ghilardi, J. Chem. Soc., Chem. Commun., 1983, 240 RSC; (c) A. Doshi, A. Sundararaman, K. Venkatasubbaiah, L. N. Zakharov, A. L. Rheingold, M. Myahkostupov, P. Piotrowiak and F. Jäkle, Organometallics, 2011, 31, 1546 CrossRef; (d) M. Nilsson and O. Wennerstrom, Acta Chem. Scand., 1970, 24, 482 CrossRef CAS PubMed.
- For limited examples of Cu-catalzyed Suzuki–Miyaura reaction for heteroaryl couplings, see: ref. 5j–l, 7d and 10.
- (a) N. Kudo, M. Perseghini and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1282 CrossRef CAS PubMed; (b) K. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3358 CrossRef CAS PubMed; (c) K. L. Billingsley and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 4695 CrossRef CAS PubMed; (d) D. W. Robbins and J. F. Hartwig, Org. Lett., 2012, 14, 4266 CrossRef CAS PubMed; (e) M. A. Düfert, K. L. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 12877 CrossRef PubMed; (f) G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151 RSC.
- (a) B. P. Orner, J. T. Ernst and A. D. Hamilton, J. Am. Chem. Soc., 2001, 123, 5382 CrossRef CAS; (b) L. Oriol, M. Pinol, J. L. Serrano, C. Martinez, R. Alcala, R. Cases and C. Sanchez, Polymer, 2001, 42, 2737 CrossRef CAS; (c) C. Willemin and D. Candau, PatentFR2903008A1, 2008 Search PubMed.
- For optimization of reaction conditions and screening of various organoboron reagents with aryl and heteroaryl bromides, see the ESI.‡ In general, phenylboronic acid neopentyl glycol ester afforded the best product yields.
- Reactions of 2-, 3- or 4-bromobenzoic acid methyl esters afforded the corresponding coupled products in less than 10% yields.
Footnotes |
| † Dedicated to Professor Ei-ichi Negishi on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures and the characterization data of new compounds. See DOI: 10.1039/c4qo00331d |
| This journal is © the Partner Organisations 2015 |