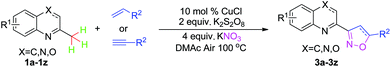Cu-catalyzed sp3 C–H bond oxidative functionalization of alkylazaarenes and substituted ethanones: an efficient approach to isoxazoline derivatives†
Gang-Wei
Wang‡
a,
Shi-Xia
Li‡
a,
Quan-Xiang
Wu
a and
Shang-Dong
Yang
*ab
aState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: yangshd@lzu.edu.cn; Fax: +86-931-8912859; Tel: +86-931-8912859
bState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Lanzhou 730000, P. R. China
First published on 27th March 2015
Abstract
Cu-catalyzed sp3 C–H bond oxidative functionalization of alkylazaarenes and substituted ethanones to different kinds of isoxazoline derivatives by 1,3-dipolar cycloaddition is reported. Cheap sources of nitro, commercially available substrates, as well as a variety of alkenes (alkynes) are applied in this transformation.
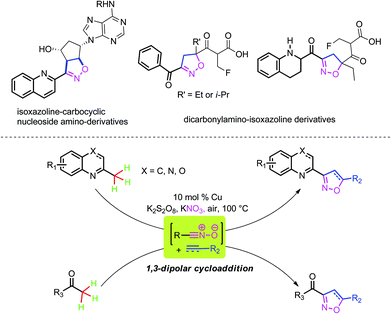
Isoxazoline derivatives display an array of significant biological properties including antiviral, antidiabetic, antitubulin, as well as antineoplastic activity.1 Currently, drugs containing the isoxazoline moiety have already been used in common clinical treatment, and some representative molecules are shown in Scheme 1.2 Meanwhile, isoxazoline derivatives are useful ligands3 and valuable synthetic intermediates for the construction of 1,3-diketones, β-hydroxyketones, and γ-amino alcohols.4 As a result, great efforts have been devoted in order to develop synthetic methods for isoxazoline derivatives during the past century.5 The classic, well-established approach of 1,3-dipolar cycloaddition of dipolarophiles with nitrile oxides has shown great advances in the synthesis of isoxazolines. However, as the nitrile oxides need to be prepared in situ from specific substrates, such as hydroximinoyl chlorides, aldoximes, and primary nitro compounds with certain reagents,4b,5 the development of alternative methods for the formation of nitrile oxides from stable, cheap, and readily accessible precursors as well as identifying convenient catalytic systems is still particularly challenging and highly attractive.
 | ||
| Scheme 1 Cu-catalyzed different types of sp3 C–H bond oxidative functionalization to synthesise isoxazolines. | ||
Expanding the applicability and exploring simple catalytic systems for metal-catalyzed sp3 C–H bond functionalizations have been ambitious goals for chemists during the past decade.6 Among them, the metal-catalyzed sp3 C–H bond nitration reaction is still an unresolved task.7 Herein, we used Cu-catalyzed sp3 C–H bond nitration to form the nitrile oxides and subsequently to go through 1,3-dipolar cycloadditions with alkenes or alkynes in order to obtain isoxazoline derivatives. The sp3 C–H bonds of the methylazaarenes and substituted ethanones were successfully converted into the oxidative products combined with various alkenes. Generally, by using a cheap nitro source (KNO3) and commercially available substrates, this method provided an efficient and concise approach to isoxazoline derivatives, which might possess great potential applications in the design of ligands as well as in tests of biological activity (Scheme 1).
Metal-catalyzed oxidation of the benzylic sp3 C–H bond of the methylazaarenes has stimulated great research efforts during the last decade,8 so we chose 2-methylquinoline 1a as the standard substrate to react with allylbenzene 2a in order to perform this sp3 C–H oxidative functionalization reaction. After a series of screens on different catalysts, oxidants, solvents, and nitro sources, we settled on the following reaction conditions: 10 mol% of CuCl as catalysts, 2 equiv. of K2S2O8 as the oxidant, 4 equiv. of KNO3 as the nitro source, and DMAc as a solvent at 100 °C for 12 h under an air atmosphere. The desired 1,3-dipolar cycloaddition product 3a was obtained in 82% yield combined with a trace amount of quinoline-2-carbaldehyde as the byproduct (see ESI†).
With optimized conditions in hand, we explored the substrate scope. First, a variety of alkenes were tested. In general, all these terminal alkenes with either electron-donating or electron-withdrawing substituents afforded the corresponding isoxazoline products in moderate to excellent yields (Table 1). For example, alkenes bearing useful functional groups, such as free alcohol, cyano, ester, phenylsulfinyl, and nitro led to the desired product in moderate to good yields (3e, 3g, 3h, 3i, 3l). Diphenyl(vinyl)phosphine oxide produced the product in an excellent yield of 92% (3j). Aliphatic alkenes were shown to be compatible with the optimized conditions (3a, 3c, 3d, 3o, 3p). When styrene was applied in the reaction, only a trace amount of the product was achieved (3k). Besides terminal alkenes, a cycloolefin was also a suitable substrate, although a low yield was achieved (3n). Next, substituted 2-methylquinoline was tested, wherein electronically disparate substituents on the aromatic ring had limited influence on the reaction process, and the corresponding products were obtained in moderate to good yields (3q–3u). We also used 3-methylquinoline and 4-methylquinoline as substrates to perform this reaction, however, 3-methylquinoline could only provide a trace amount of the desired product and 4-methylquinoline failed to provide any corresponding product. Different 2-methyl azaarenes were also applied into the catalytic system, and 2-methylbenzo[f]quinoxaline, 2-methylquinoxaline, 1-methylisoquinoline, and substituted benzoxazinones all gave the desired product in moderate to good yields (3v–3z), but 2-methylpyridine failed to give the desired product. An alkyne also served as a good dipolarophile under this catalytic system and 1-heptyne provided the desired product in 71% yield (3aa). But phenylacetylene was not a suitable substrate for this transformation. When 2-ethylquinoline was used as a substrate under the standard conditions, a cascade sp3 C–H bond oxidative functionalization was achieved to access the different isoxazoline derivatives.9
| Entry | Product | Yieldb,c [%] | Entry | Product | Yieldb,c [%] |
|---|---|---|---|---|---|
| a Reaction was carried out with CuCl (10 mol%), K2S2O8 (2.0 equiv.), KNO3 (4.0 equiv.), 2-methyl azaarenes (0.30 mmol), alkenes or alkyne (0.9 mmol) in DMAc (3.0 mL) at 100 °C for 12 h under air. b Isolated yield. c All the reaction systems produced trace amounts of corresponding quinoline-2-carbaldehyde as byproduct. d Mixed with trace amount of impurities. | |||||
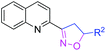
|
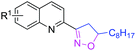
|
||||
| 3a | R2 = CH2Ph | 82 | 3q | R1 = 6-Br | 79 |
| 3b | R2 = (CH2)2Ph | 80 | 3r | R1 = 6-Me | 74 |
| 3c | R2 = C6H13 | 76 | 3s | R1 = 6-OMe | 63 |
| 3d | R2 = C8H17 | 81 | 3t | R1 = 6-OPh | 81 |
| 3e | R2 = CH2OH | 52 | 3u | R1 = 8-OMe | 85 |
| 3f | R2 = CH2Cl | 52 | |||
| 3g | R2 = CN | 74 | 3v |
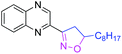
|
66 |
| 3h | R2 = CO2Bun | 77 | |||
| 3i | R2 = SO2Ph | 86 | |||
| 3j | R2 = P(O)Ph | 92 | |||
| 3k | R2 = Ph | Trace | |||
| 3l |
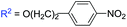
|
74 | 3w |
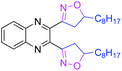
|
46 |
| 3m |

|
75 | 3x |
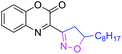
|
72 |
| 3n |
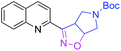
|
31 | 3y |
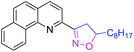
|
46d |
| 3o |
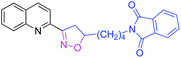
|
80 | 3z |
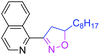
|
44 |
| 3p |
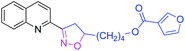
|
80 | 3aa |
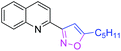
|
71 |
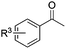
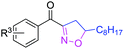
The functionalization of the α-C–H bond of carbonyl compounds has long been valued as a fundamental transformation in organic chemistry.10 Based on this transformation, Horiuchi and Roy developed approaches to isoxazolines11 by using acetone (or acetophenone) as the substrate and solvent combined with iron salts or cerium ammonium nitrate (CAN). These achievements, however elegant, could only provide a limited substrate scope. Different acetophenones were therefore tested in order to test the applicability of our transformation. We found that with a slightly changed catalytic system, acetophenones with electron-withdrawing groups substituted on the aromatic ring could provide the corresponding products in low to moderate yields, and a higher yield was achieved for more electron-deficient substrates (Table 2, 5a–5f). When 1-(quinolin-2-yl)ethanone was used as a substrate, 5g and 5h were formed in 67% and 62% yield, respectively. Other ketones, propiophenone and acetone for example, failed to give the desired product.
| Entry | Ketone | Alkene or alkyne | Product | Yieldb [%] |
|---|---|---|---|---|
| a The reaction was carried out with Cu(acac)2 (10 mol%), K2S2O8 (6.0 equiv.), KNO3 (4.0 equiv.), acetophenone (0.30 mmol), alkenes or alkynes (0.9 mmol) in DMF (3.0 mL) at 100 °C for 12 h under air. b Isolated yield. c Mixed with trace amount of impurities. | ||||
|
|
|||
| 4a | R3 = 4-Cl | 5a | 23%c | |
| 4b | R3 = 4-CN | 5b | 53% | |
| 4c | R3 = 4-CF3 | 5c | 50% | |
| 4d | R3 = NO2 |
|
5d | 63% |
| 4e | R3 = 4-OMe | 5e | Trace | |
| 4f | R3 = 2-F | 5f | 27% | |
| 4g |
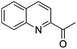
|
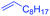
|
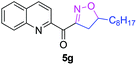
|
67% |
| 4h |
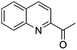
|

|
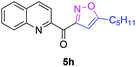
|
62% |
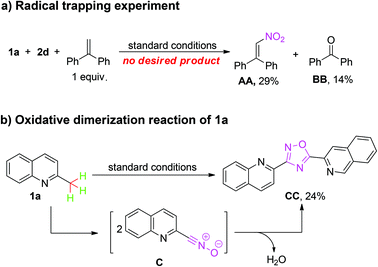
To gain insight into the mechanism, several control experiments were carried out by using 2-methylquinoline 1a as the substrate (Scheme 2). When a radical scavenger 1,1-diphenylethylene (1 equiv.) was added into the reaction, the desired product was not observed at all, but AA and BB were produced in yields of 29% and 14%, respectively. This result implied that the reaction might involve the generation of a nitro radical and oxygen.12 When 1a was applied to the reaction system in the absence of alkenes or alkynes, oxadiazole CC was achieved in a 24% yield. This result indicates that nitrile oxide could be the key intermediate for this reaction.5o
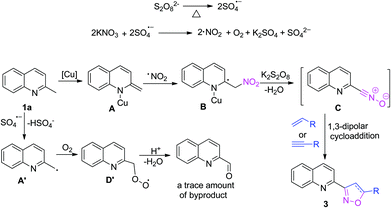
On the basis of the above results and previous literature,13,14 we proposed a tentative pathway for this transformation (Scheme 3). Decomposition of the potassium peroxydisulphate may initially occur in order to generate the sulfate radical anion, which subsequently reacted with KNO3 in order to form a nitrogen dioxide radical as well as oxygen.13a–d Meanwhile, 1a reacted with CuCl to produce the active metal enamide species A,14 and A was attacked by the NO2 radical in order to obtain the intermediate B, which provided the nitrile oxide C through the processes of oxidation and dehydration.7d,8,13e–f The nitrile oxide C then reacted with alkenes or alkynes through 1,3-dipolar cycloaddition reaction, and the final isoxazolines 3 were achieved.5o,8 At the same time, 1a could also be converted into a radical intermediate A′ through a single electron transfer (SET) process,7d then A′ reacted with oxygen to provide the byproduct quinoline-2-carbaldehyde in a trace amount (see ESI†).12 When substituted acetophenone 4 was used to provide the corresponding isoxazoline derivatives 5, a similar mechanism could be applied (active metal enol species was formed instead of enamide species A10) in the reaction course.
Conclusions
In conclusion, by using Cu-catalyzed sp3 C–H bond nitration to generate the nitrile oxides in situ and subsequently go through 1,3-dipolar cycloaddition with alkenes or alkynes, we developed an efficient and concise approach to isoxazoline derivatives. When an inexpensive nitro source (KNO3) and commercially available substrates are the starting points, the sp3 C–H bonds of methylazaarenes and substituted ethanones can be successfully functionalized with the assistance of different alkenes and alkynes. Current work is ongoing toward the application of these isoxazoline derivatives to biological activity tests.Acknowledgements
We are grateful for the NSFC (no. 21272100 and 21202075) and Program for New Century Excellent Talents in University (NCET-11-0215 and lzujbky-2013-k07) financial support. S.F. Reichard contributed editing.Notes and references
- (a) Y.-S. Lee and B. H. Kim, Bioorg. Med. Chem. Lett., 2002, 12, 1395 CrossRef CAS; (b) M. D. Mosher, L. G. Emmerich, K. S. Frost and B. Anderson, J. Heterocycl. Chem., 2006, 43, 535 CrossRef CAS; (c) J. Kaffy, R. Pontikis, D. Carrez, A. Croisy, C. Monneret and J.-C. Florent, Bioorg. Med. Chem., 2006, 14, 4067 CrossRef CAS PubMed; (d) A. Kamal, J. S. Reddy, M. J. Ramaiah, D. Dastagiri, E. V. Bharathi, M. A. Azhar, F. Sultana, S. N. C. V. L. Pushpavalli, M. Pal-Bhadra, A. Juvekar, S. Sen and S. Zingde, Eur. J. Med. Chem., 2010, 3924 CrossRef CAS PubMed; (e) A. Kamal, E. V. Bharathi, J. S. Reddy, M. J. Ramaiah, D. Dastagiri, M. K. Reddy, A. Viswanath, T. L. Reddy, T. B. Shaik, S. N. C. V. L. Pushpavalli and M. P. Bhadra, Eur. J. Med. Chem., 2011, 46, 691 CrossRef CAS PubMed.
- (a) T. M. Sielecki, J. Liu, S. A. Mousa, A. L. Racanelli, E. A. Hausner, R. R. Wexler and R. E. Olson, Bioorg. Med. Chem. Lett., 2001, 11, 2201 CrossRef CAS; (b) P. Quadrelli, M. Mella, L. Legnani and D. Al-Saad, Eur. J. Org. Chem., 2013, 4655 CrossRef CAS; (c) H.-K. Chang, Y.-S. Park, C.-W. Park, Y.-T. Jang, S.-S. Kim, M.-J. Kim, M.-J. Park, J.-G. Park, T.-K. Park, K.-S. Min, T.-S. Lee and S.-H. Lee, Caspase inhibitors containing dicarbonylamino-isoxazoline, WO, 2006/033551 A1, March 30, 2006 Search PubMed.
- (a) M. A. Arai, T. Arai and H. Sasai, Org. Lett., 1999, 1, 1795 CrossRef CAS; (b) M. A. Arai, M. Kuraishi, T. Arai and H. Sasai, J. Am. Chem. Soc., 2001, 123, 2907 CrossRef CAS; (c) T. Tsujihara, T. Shinohara, K. Takenaka, S. Takizawa, K. Onitsuka, M. Hatanaka and H. Sasai, J. Org. Chem., 2009, 74, 9274 CrossRef CAS PubMed.
- (a) D. P. Curran, J. Am. Chem. Soc., 1983, 105, 5826 CrossRef CAS; (b) J. W. Bode, N. Fraefel, D. Muri and E. M. Carreira, Angew. Chem., Int. Ed., 2001, 40, 2082 CrossRef CAS; (c) A. R. Minter, A. A. Fuller and A. K. Mapp, J. Am. Chem. Soc., 2003, 125, 6846 CrossRef CAS PubMed; (d) N. Sewald, Angew. Chem., Int. Ed., 2003, 42, 5794 CrossRef CAS PubMed; (e) A. A. Fuller, B. Chen, A. R. Minter and A. K. Mapp, J. Am. Chem. Soc., 2005, 127, 5376 CrossRef CAS PubMed; (f) F. Kleinbeck and E. M. Carreira, Angew. Chem., Int. Ed., 2009, 48, 578 CrossRef CAS PubMed; (g) H. Jiang, P. Elsner, K. L. Jensen, A. Falcicchio, V. Marcos and K. A. Jørgensen, Angew. Chem., Int. Ed., 2009, 48, 6844 CrossRef CAS PubMed.
- (a) A. Werner and H. Buss, Chem. Ber., 1894, 27, 2193 CrossRef; (b) T. Mukaiyama and T. Hoshino, J. Am. Chem. Soc., 1960, 82, 5339 CrossRef CAS; (c) R. Huisgen, Angew. Chem., Int. Ed. Engl., 1963, 2, 565 CrossRef; (d) S. Kanemasa and K. Onimura, Tetrahedron, 1992, 48, 8645 CrossRef CAS; (e) Y. Basel and A. Hassner, Synthesis, 1997, 309 CrossRef CAS PubMed; (f) S. Béha, D. Giguère and R. Patnam, Synlett, 2006, 11, 1739 Search PubMed; (g) N. Chatterjee, P. Pandit, S. Halder, A. Patra and D. K. Maiti, J. Org. Chem., 2008, 73, 7775 CrossRef CAS PubMed; (h) R. G. Chary, G. R. Reddy, Y. S. S. Ganesh, K. V. Prasad, A. Raghunadh, T. L. Cecchi, F. D. Sarlo and F. Machetti, Chem. – Eur. J., 2008, 14, 7903 CrossRef PubMed; (i) S. Grecian and V. V. Fokin, Angew. Chem., Int. Ed., 2008, 47, 8285 CrossRef CAS PubMed; (j) J. L. Frie, C. S. Jeffrey and E. J. Sorensen, Org. Lett., 2009, 11, 5394 CrossRef CAS PubMed; (k) N. Lohse-Fraefel and E. M. Carreira, Chem. – Eur. J., 2009, 15, 12065 CrossRef CAS PubMed; (l) M. J. Raihan, V. Kavala, C.-W. Kuo, R. Raju and C.-F. Yao, Green Chem., 2010, 12, 1090 RSC; (m) T. Jen, B. A. Mendelsohn and M. A. Ciufolini, J. Org. Chem., 2011, 76, 728 CrossRef CAS PubMed; (n) S. Minakata, S. Okumura, T. Nagamachi and Y. Takeda, Org. Lett., 2011, 13, 2966 CrossRef CAS PubMed; (o) K.-I. Itoh, T. Aoyama, H. Satoh, Y. Fujii, H. Sakamaki, T. Takido and M. Kodomari, Tetrahedron Lett., 2011, 52, 6892 CrossRef CAS PubMed; (p) T. F. Niu, M. F. Lv, L. Wang, W. B. Yi and C. Cai, Org. Biomol. Chem., 2013, 11, 1040 RSC; (q) A. Yoshimura, K. R. Middleton, A. D. Todora, B. J. Kastern, S. R. Koski, A. V. Maskaev and V. V. Zhdankin, Org. Lett., 2013, 15, 4010 CrossRef CAS PubMed; (r) R. G. Chary, G. R. Reddy, Y. S. S. Ganesh, K. V. Prasad, A. Raghunadh, T. Krishna, S. Mukherjee and M. Pal, Adv. Synth. Catal., 2014, 356, 160 CrossRef; (s) J. S. Oakdale, R. K. Sit and V. V. Fokin, Chem. – Eur. J., 2014, 20, 11101 CrossRef CAS PubMed; (t) Y. Liu, J.-L. Zhang, R.-J. Song, P.-C. Qian and J.-H. Li, Angew. Chem., Int. Ed., 2014, 53, 9017 CrossRef CAS PubMed.
- For reviews on metal-catalyzed sp3 C–H bond functionalization, see: (a) K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS PubMed; (b) M. Tobisu and N. Chatani, Angew. Chem., Int. Ed., 2006, 45, 1683 CrossRef CAS PubMed; (c) K. R. Campos, Chem. Soc. Rev., 2007, 36, 1069 RSC; (d) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (e) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (f) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (g) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem. – Eur. J., 2010, 16, 2654 CrossRef CAS PubMed; (h) O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902 RSC; (i) B.-J. Li and Z.-J. Shi, Chem. Soc. Rev., 2012, 41, 5588 RSC; (j) T. A. Ramirez, B. Zhao and Y. Shi, Chem. Soc. Rev., 2012, 41, 931 RSC; (k) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed.
- For selected examples on nitration of aliphatic hydrocarbons see: (a) G. W. Smithand and H. D. Williams, J. Org. Chem., 1961, 26, 2207 CrossRef; (b) G. A. Olah and H. C. Lin, J. Am. Chem. Soc., 1971, 93, 1259 CrossRef CAS; (c) G. A. Olah, P. Ramaiah, C. B. Rao, G. Sandfold, R. Golam, N. J. Trivediand and J. A. Olah, J. Am. Chem. Soc., 1993, 115, 7246 CrossRef CAS; (d) D. Wu, J. Zhang, J. H. Cui, W. Zhang and Y. K. Liu, Chem. Commun., 2014, 50, 10857 RSC.
- Examples on metal-catalyzed benzylic C–H oxidation see: (a) A. J. Catino, J. M. Nichols, H. Choi, S. Gottipamula and M. P. Doyle, Org. Lett., 2005, 7, 5167 CrossRef CAS PubMed; (b) Y. Bonvin, E. Callens, I. Larrosa, D. A. Henderson, J. Oldham, A. J. Burton and A. G. M. Barrett, Org. Lett., 2005, 7, 4549 CrossRef CAS PubMed; (c) M. Nakanishi and C. Bolm, Adv. Synth. Catal., 2007, 349, 861 CrossRef CAS; (d) C. S. Yi, K.-H. Kwon and D. W. Lee, Org. Lett., 2009, 11, 1567 CrossRef CAS PubMed; (e) B. Qian, P. Xie, Y. Xie and H. Huang, Org. Lett., 2011, 13, 2580 CrossRef CAS PubMed; (f) Y. Yan, K. Xu, Y. Fang and Z. Wang, J. Org. Chem., 2011, 76, 6849 CrossRef CAS PubMed; (g) Z.-Q. Wang, W.-W. Zhang, L.-B. Gong, R.-Y. Tang, X.-H. Yang, Y. Liu and J.-H. Li, Angew. Chem., Int. Ed., 2011, 50, 8968 CrossRef CAS PubMed; (h) J. D. Houwer, K. A. Tehrani and B. U. W. Maes, Angew. Chem., Int. Ed., 2012, 51, 2745 CrossRef PubMed; (i) S.-J. Lou, D.-Q. Xu, D.-F. Shen, Y.-F. Wang, Y.-K. Liu and Z.-Y. Xu, Chem. Commun., 2012, 48, 11993 RSC; (j) Y. Li, F. Guo, Z. Zha and Z. Yang, Chem. – Asian. J., 2013, 8, 534 CrossRef CAS PubMed; (k) Y.-G. Zhang, J.-K. Xu, X.-M. Li and S.-K. Tian, Eur. J. Org. Chem., 2013, 3468 Search PubMed; (l) X. Gao, F. Zhang, G. Den and L. Yang, Org. Lett., 2014, 16, 3664 CrossRef CAS PubMed; (m) M. Itoh, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2014, 16, 2050 CrossRef CAS PubMed.
- G.-W. Wang, M.-X. Cheng, R.-S. Ma and S.-D. Yang, Chem. Commun. 10.1039/C5CC01004G.
- For recent reviews, see: (a) D. A. Culkin and J. F. Hartwig, Acc. Chem. Res., 2003, 36, 234 CrossRef CAS PubMed; (b) F. Bellina and R. Rossi, Chem. Rev., 2009, 110, 1082 CrossRef PubMed; (c) A. C. B. Burtoloso, Synlett, 2009, 320 CrossRef CAS PubMed; (d) C. C. C. Johansson and T. J. Colacot, Angew. Chem., Int. Ed., 2010, 49, 676 CrossRef CAS PubMed; (e) T. Ankner, C. C. Cosner and P. Helquist, Chem. – Eur. J., 2013, 19, 1858 CrossRef CAS PubMed.
- (a) K.-I. Itoh and C. A. Horiuchi, Tetrahedron, 2004, 60, 1671 CrossRef CAS PubMed; (b) K.-I. Itoh, H. Sakamak, N. Nakazato, A. Horiuchi, E. Horn and C. A. Horiuchi, Synthesis, 2005, 3541 CAS; (c) S. Béha, D. Giguére, R. Patnam and R. Roy, Synlett, 2006, 11, 1739 Search PubMed.
- (a) S. Hirashima, Y. Kudo, T. Nobuta, N. Tada and A. Itoh, Tetrahedron Lett., 2009, 50, 4328 CrossRef CAS PubMed; (b) S. Maity, S. Manna, S. Rana, T. Naveen, A. Mallick and D. Maiti, J. Am. Chem. Soc., 2013, 135, 3355 CrossRef CAS PubMed.
- (a) Z. Zhang, S. Fang, Q. Liu and G. Zhang, J. Org. Chem., 2012, 77, 7665 CrossRef CAS PubMed; (b) S. Manna, S. Maity, S. Rana, S. Agasti and D. Maiti, Org. Lett., 2012, 14, 1736 CrossRef CAS PubMed; (c) Y.-M. Li, X.-H. Wei, X.-A. Li and S.-D. Yang, Chem. Commun., 2013, 49, 11701 RSC; (d) Y. Liu, B. Jiang, W. Zhang and Z. Xu, J. Org. Chem., 2013, 78, 966 CrossRef CAS PubMed; (e) Y.-K. Liu, S.-J. Lou, D.-Q. Xu and Z.-Y. Xu, Chem. – Eur. J., 2010, 16, 13590 CrossRef CAS PubMed; (f) E. Begari, C. Singh, U. Nookaraja and P. Kumar, Synlett, 2014, 25, 1997 CrossRef CAS PubMed.
- B. Qian, S. Guo, J. Shao, Q. Zhu, L. Yang, C. Xia and H. Huang, J. Am. Chem. Soc., 2010, 132, 3650 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00053j |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2015 |