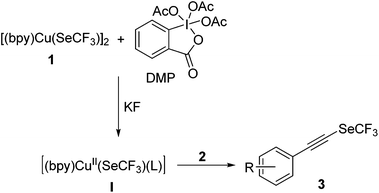
Alkynyl trifluoromethyl selenide synthesis via oxidative trifluoromethylselenolation of terminal alkynes†
Yuguang
Wang
,
Yi
You
* and
Zhiqiang
Weng
*
Department of Chemistry, Fuzhou University, Fuzhou 350108, China. E-mail: zweng@fzu.edu.cn; Fax: +86 591 22866121; Tel: +86 591 22866121
First published on 30th March 2015
Abstract
The synthesis of alkynyl trifluoromethyl selenides by Cu-mediated oxidative trifluoromethylselenolation of terminal alkynes is reported. Treatment of [(bpy)Cu(SeCF3)]2 with various terminal alkynes in the presence of an oxidant, Dess–Martin periodinane, led to the formation of trifluoromethylselenolated acetylenes bearing sensitive moieties such as alkoxy, halogens, trifluoromethyl, and heterocyclic groups.
The importance of organofluorine compounds in the fields of agricultural, medicinal, and materials chemistry has inspired chemists to continue developing mild and efficient methods for their synthesis.1–8 Among such compounds trifluoromethyl thioethers have attracted a great deal of attention in the past few years owing to their strong electron-withdrawing effects and high lipophilicity.9–12 The development of new synthetic routes to trifluoromethyl thioethers thus represents an important area of research in organic chemistry.13–18 Recently, the development of new trifluoromethylthiolating reagents19–30 and transition-metal-catalyzed or -mediated trifluoromethylthiolation has found many applications for assembling aryl and alkyl trifluoromethyl thioethers.24,28,31–41 Various methodologies have also been developed for introducing the trifluoromethanesulfenyl group into acetylenes. These include oxidative trifluoromethylthiolation of terminal alkynes with CF3SiMe3, and elemental sulphur42 or later with AgSCF3,43 copper-catalyzed trifluoromethylthiolation of terminal alkynes with a novel thioperoxy reagent,25 base-catalyzed trifluoromethylthiolation of terminal alkynes with a trifluoromethanesulfenamide reagent,44 and bismuth(III)-promoted trifluoromethylthiolation of trimethyl(alkynyl)silane with a trifluoromethanesulfanylamide reagent.45
Despite these seminal contributions made in trifluoromethylthiolation, to our knowledge, there are no examples in the literature for the construction of Csp–SeCF3 bonds. This is probably because the starting materials and reagents are either highly toxic, unstable, commercially unavailable, or require elaborate preparative methods.46–52
The organoselenium compounds also display significant potential as antioxidant, antitumor, antimicrobial, and antiviral agents.53 Therefore, development of efficient procedures for the synthesis of alkynyl trifluoromethyl selenides is of much importance.
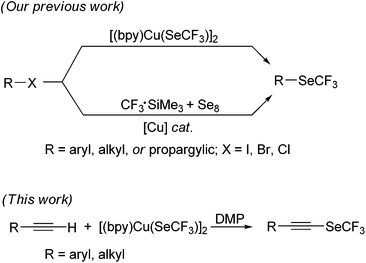
In our continuing efforts aimed at developing simple and efficient synthesis of trifluoromethylselenolated compounds by using the copper reagent [(bpy)Cu(SeCF3)]2 (1),54 we recently described the nucleophilic trifluoromethylselenolation of aryl and alkyl halides,54 α- or β-halo-α,β-unsaturated carbonyl compounds,55,56 propargylic chlorides, and allylic bromides.57 These organic halides were reacted smoothly with 1 to furnish the corresponding trifluoromethylselenolated products in good yield (Scheme 1). We further developed a method for the trifluoromethylselenolation of aryl, heteroaryl and alkyl halides by the Cu(I) catalyst.58 Since no method is available in the literature for the synthesis of trifluoromethylselenolated acetylenes, we developed a novel method to access them. Herein, we report the first example of copper-mediated oxidative trifluoromethylselenolation of terminal alkynes.
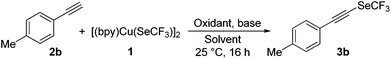
Our efforts to develop Cu-mediated methods for oxidative trifluoromethylselenolation of terminal alkynes were initiated with 4-ethynyltoluene (2b) as the substrate. Treatment of 2b with [(bpy)Cu(SeCF3)]2 (1) in CH3CN at 25 °C for 16 h gave (p-tolylethynyl)(trifluoromethyl)selane (3b) in 5% yield (Table 1, entry 1). These results encouraged us to optimize the other reaction parameters. After considerable efforts, we found that the yield of 3b (69%) could be enhanced in the presence of 2.0 equiv. of DMP as the oxidant and 3.0 equiv. of KF as the base (Table 1, entry 2). Of the solvents investigated, DMF was determined to be the optimal solvent for the trifluoromethylselenolation and the yield of 3b could be improved to 87% (Table 1, entry 8). Other solvents such as CH3CN, toluene, THF, dioxane, CH2Cl2, and DMSO gave inferior results (Table 1, entries 2–7). Among a set of oxidants, DMP gave the highest yield for this oxidative trifluoromethylselenolation (Table 1, entry 8). Other oxidants such as PhI(OAc)2, PIFA, DDQ, K2S2O8, air, DTBP and CuSO4 showed less or no efficiency (Table 1, entries 9–15). Further screening revealed that the amount of DMP affected the reaction, thus 1.0 equiv. of DMP offered the desired product 3b in a lower yield (80%; Table 1, entry 16). We also explored various bases in this reaction, and observed that KF was the most efficient base (Table 1, entries 17–20).
| Entry | Base | Oxidant (equiv.) | Solvent | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.060 mmol), 2b (0.10 mmol), base (0.30 mmol), solvent (1.0 mL), under N2 atmosphere, DMP = Dess–Martin periodinane, DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone. PIFA = [bis(trifluoroacetoxy)iodo]benzene, DTBP = di-tert-butyl peroxide, DMF = N,N-dimethylformamide, DMSO = dimethyl sulfoxide; the equiv. of oxidant is based on 2b. b Yields were determined by 19F NMR spectroscopy with PhOCF3 as internal standard. | ||||
| 1 | — | — | CH3CN | 5 |
| 2 | KF | DMP (2.0) | CH3CN | 69 |
| 3 | KF | DMP (2.0) | Toluene | 28 |
| 4 | KF | DMP (2.0) | THF | 52 |
| 5 | KF | DMP (2.0) | Dioxane | 28 |
| 6 | KF | DMP (2.0) | CH2Cl2 | 43 |
| 7 | KF | DMP (2.0) | DMSO | 16 |
| 8 | KF | DMP (2.0) | DMF | 87 |
| 9 | KF | PhI(OAc)2 (2.0) | DMF | 40 |
| 10 | KF | PIFA (2.0) | DMF | 62 |
| 11 | KF | DDQ (2.0) | DMF | 65 |
| 12 | KF | K2S2O8 (2.0) | DMF | 51 |
| 13 | KF | Air | DMF | 27 |
| 14 | KF | DTBP (2.0) | DMF | 5 |
| 15 | KF | CuSO4 (2.0) | DMF | 5 |
| 16 | KF | DMP (1.0) | DMF | 80 |
| 17 | NaF | DMP (2.0) | DMF | 80 |
| 18 | K2CO3 | DMP (2.0) | DMF | 26 |
| 19 | NaOH | DMP (2.0) | DMF | 28 |
| 20 | KOH | DMP (2.0) | DMF | 17 |
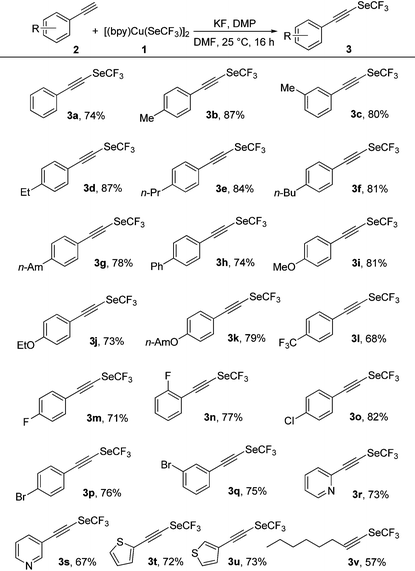
To explore the scope of the oxidative trifluoromethylselenolation, various substituted terminal alkynes were examined under the optimized reaction conditions (Table 2). Variation of the 3-alkyl- or 4-alkyl-substituted phenylacetylenes underwent the reaction with 1 to afford trifluoromethylselenolated acetylenes 3a–g in good yields (74–87%). (4-Phenylphenyl)ethyne also efficiently participated in the trifluoromethylselenolation to furnish the desired product 3h in 74% yield. Notably, the electronic effects of the substituents on the aromatic units had no significant influence on the outcome of the reaction. Indeed, both 4-alkoxy-substituted phenylacetylenes and 4-trifluoromethyl-substituted phenylacetylene produced the desired products 3i–l in good yields (68–81%). Interestingly, the reaction was also tolerated in the presence of a halogen atom on the aromatic rings such as fluorine, chlorine and bromine, affording the corresponding products 3m–q in good yields (71–82%). In addition, even more challenging and highly valuable heteroaromatic-substituted alkynes such as pyridines and thiophenes were readily transformed into the corresponding trifluoromethylselenolated products 3r–u in synthetically useful yields (67–73%). Additionally, the scope of the reaction was not limited to aromatic substrates and was successfully expanded to aliphatic alkyne (3v), thus showcasing the effectiveness of the reaction.
| a 1 (0.18 mmol), 2 (0.30 mmol), DMP (0.60 mmol), KF (0.90 mmol), DMF (2.5 mL), 25 °C, 16 h, N2. All reported yields are isolated yields. |
|---|
|
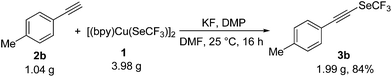
To demonstrate the practical utility, the reaction of 1 and 2b was conducted on a 9.0 mmol scale. As shown in Scheme 2, 2b underwent the oxidative trifluoromethylselenolation without any loss in effectiveness and the corresponding product 3b was obtained in 84% yield (1.99 g).
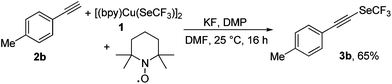
In an effort to understand the mechanistic details of the transformation, a series of experiments were undertaken. First, the trifluoromethylselenolation of 2b was conducted in the presence of 1.0 equiv. of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) as a radical scavenger, and the desired product 3b was obtained in 65% yield (Scheme 3). This observation suggests against the fact that the reaction proceeds via a radical pathway.
To examine whether any reactive species is involved in the oxidative trifluoromethylselenolation, the progress of the reaction was monitored by 19F NMR (see ESI†). The results revealed that a copper intermediate (I) is formed immediately in 75% yield during the reaction of 1 with DMP in the presence of KF. The amount of copper in I was determined by ICP, which gave Cu 8.18 wt%. In addition, the oxidative state of copper in I was verified by using X-ray photoelectron spectroscopy (XPS). The binding energy for Cu 2p3/2 is 934.2 eV, which is characteristic of a Cu(II) state59 (see ESI†). The 19F NMR spectrum of intermediate I showed a peak at −37.1 ppm. When 2b was added to this mixture, the resonances for intermediate I decreased and, instead, a new peak at −36.8 ppm was increased corresponding to the product (p-tolylethynyl)(trifluoromethyl)selane (3b).
Based on aforementioned experimental evidence, a plausible preparation mechanism for the alkynyl trifluoromethyl selenides (3) is proposed: the copper reagent 1 undergoes oxidation by DMP in the presence of KF to form an intermediate I. The subsequent electrophilic trifluoromethylselenolation of terminal alkynes 2 with I under copper-mediated conditions would produce the corresponding products 3 (Scheme 4).
Conclusions
In conclusion, we have discovered a new copper-mediated oxidative trifluoromethylselenolation of terminal alkynes. This reaction readily converts a variety of terminal alkynes to the corresponding alkynyl trifluoromethyl selenides in good yields. Importantly, several synthetically valuable functional groups, such as alkoxy, halides, trifluoromethyl, and heterocyclic groups, were tolerated under the developed reaction conditions. Further applications of this method to the synthesis of other related compounds are in progress.Acknowledgements
Financial support from the National Natural Science Foundation of China (NSFC) (grant number 21372044), the Research Fund for the Doctoral Program of Higher Education of China (grant number 20123514110003), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, P. R. China (grant number 2012-1707), the Science Foundation of the Fujian Province, China (grant number 2013J01040), and Fuzhou University (grant numbers 022318, 022494) is gratefully acknowledged.Notes and references
- P. Kirsch, Modern fluoroorganic chemistry: synthesis, reactivity, applications, Wiley-VCH, Weinheim, 2004 Search PubMed.
- J.-P. Begue and D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008 Search PubMed.
- I. Ojima, Fluorine In Medicinal Chemistry And Chemical Biology, John Wiley & Sons Ltd., 2009 Search PubMed.
- V. A. Petrov, Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, Wiley, Hoboken, 2009 Search PubMed.
- K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- Z. Jin, G. B. Hammond and B. Xu, Aldrichimica Acta, 2012, 45, 67–83 CAS.
- P. Chen and G. Liu, Synthesis, 2013, 2919–2939 CAS.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- A. Leo, C. Hansch and D. Elkins, Chem. Rev., 1971, 71, 525–616 CrossRef CAS.
- L. M. Yagupol'skii, A. Y. Ilchenko and N. V. Kondratenko, Russ. Chem. Rev., 1974, 43, 32–47 CrossRef PubMed.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- R. Filler, Biomedical Aspects of Fluorine Chemistry, Kodansha, Tokyo, 1982 Search PubMed.
- A. Tlili and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 6818–6819 CrossRef CAS PubMed.
- F. Toulgoat, S. Alazet and T. Billard, Eur. J. Org. Chem., 2014, 2415–2428 CrossRef CAS.
- L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513–1522 CrossRef CAS PubMed.
- H. Wang and D. A. Vicic, Synlett, 2013, 1887–1898 CAS.
- W. He and Z. Weng, Prog. Chem., 2013, 25, 1071–1078 CAS.
- X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731–764 CrossRef CAS PubMed.
- A. Ferry, T. Billard, B. R. Langlois and E. Bacqué, Angew. Chem., Int. Ed., 2009, 48, 8551–8555 CrossRef CAS PubMed.
- F. Baert, J. Colomb and T. Billard, Angew. Chem., Int. Ed., 2012, 51, 10382–10385 CrossRef CAS PubMed.
- J. Liu, L. Chu and F.-L. Qing, Org. Lett., 2013, 15, 894–897 CrossRef CAS PubMed.
- Y.-D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki, M. Shiro and N. Shibata, J. Am. Chem. Soc., 2013, 135, 8782–8785 CrossRef CAS PubMed.
- T. Bootwicha, X. Liu, R. Pluta, I. Atodiresei and M. Rueping, Angew. Chem., Int. Ed., 2013, 52, 12856–12859 CrossRef CAS PubMed.
- R. Pluta, P. Nikolaienko and M. Rueping, Angew. Chem., Int. Ed., 2014, 53, 1650–1653 CrossRef CAS PubMed.
- X. Shao, X. Wang, T. Yang, L. Lu and Q. Shen, Angew. Chem., Int. Ed., 2013, 52, 3457–3460 CrossRef CAS PubMed.
- X. Wang, T. Yang, X. Cheng and Q. Shen, Angew. Chem., Int. Ed., 2013, 52, 12860–12864 CrossRef CAS PubMed.
- E. V. Vinogradova, P. Müller and S. L. Buchwald, Angew. Chem., Int. Ed., 2014, 53, 3125–3128 CrossRef CAS PubMed.
- C. Xu, B. Ma and Q. Shen, Angew. Chem., Int. Ed., 2014, 53, 9316–9320 CrossRef CAS PubMed.
- S.-G. Li and S. Z. Zard, Org. Lett., 2013, 15, 5898–5901 CrossRef CAS PubMed.
- Z. Weng, W. He, C. Chen, R. Lee, D. Tan, Z. Lai, D. Kong, Y. Yuan and K.-W. Huang, Angew. Chem., Int. Ed., 2013, 52, 1548–1552 CrossRef CAS PubMed.
- C. Chen, Y. Xie, L. Chu, R.-W. Wang, X. Zhang and F.-L. Qing, Angew. Chem., Int. Ed., 2012, 51, 2492–2495 CrossRef CAS PubMed.
- C.-P. Zhang and D. A. Vicic, Chem. – Asian J., 2012, 7, 1756–1758 CrossRef CAS PubMed.
- X. Shao, T. Liu, L. Lu and Q. Shen, Org. Lett., 2014, 16, 4738–4741 CrossRef CAS PubMed.
- J. Xu, X. Mu, P. Chen, J. Ye and G. Liu, Org. Lett., 2014, 16, 3942–3945 CrossRef CAS PubMed.
- G. Danoun, B. Bayarmagnai, M. F. Gruenberg and L. J. Goossen, Chem. Sci., 2014, 5, 1312–1316 RSC.
- L. D. Tran, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237–18240 CrossRef CAS PubMed.
- C. Chen, X.-H. Xu, B. Yang and F.-L. Qing, Org. Lett., 2014, 16, 3372–3375 CrossRef CAS PubMed.
- C. Xu and Q. Shen, Org. Lett., 2014, 16, 2046–2049 CrossRef CAS PubMed.
- F. Hu, X. Shao, D. Zhu, L. Lu and Q. Shen, Angew. Chem., Int. Ed., 2014, 53, 6105–6109 CrossRef CAS PubMed.
- G. Teverovskiy, D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 7312–7314 CrossRef CAS PubMed.
- C.-P. Zhang and D. A. Vicic, J. Am. Chem. Soc., 2012, 134, 183–185 CrossRef CAS PubMed.
- C. Chen, L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 12454–12457 CrossRef CAS PubMed.
- S.-Q. Zhu, X.-H. Xu and F.-L. Qing, Eur. J. Org. Chem., 2014, 4453–4456 CrossRef CAS.
- S. Alazet, L. Zimmer and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 10814–10817 CrossRef CAS PubMed.
- J. Sheng and J. Wu, Org. Biomol. Chem., 2014, 12, 7629–7633 CAS.
- T. Billard and B. R. Langlois, Tetrahedron Lett., 1996, 37, 6865–6868 CrossRef CAS.
- T. Billard, S. Large and B. R. Langlois, Tetrahedron Lett., 1997, 38, 65–68 CrossRef CAS.
- G. Blond, T. Billard and B. R. Langlois, Tetrahedron Lett., 2001, 42, 2473–2475 CrossRef CAS.
- S. Large, N. Roques and B. R. Langlois, J. Org. Chem., 2000, 65, 8848–8856 CrossRef CAS PubMed.
- C. Pooput, M. Medebielle and W. R. Dolbier, Org. Lett., 2004, 6, 301–303 CrossRef CAS PubMed.
- C. Pooput, W. R. Dolbier and M. Médebielle, J. Org. Chem., 2006, 71, 3564–3568 CrossRef CAS PubMed.
- N. V. Kondratenko, A. A. Kolomeytsev, V. I. Popov and L. M. Yagupolskii, Synthesis, 1985, 667–669 CrossRef CAS.
- C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255–6286 CrossRef CAS PubMed.
- C. Chen, L. Ouyang, Q. Lin, Y. Liu, C. Hou, Y. Yuan and Z. Weng, Chem. – Eur. J., 2014, 20, 657–661 CrossRef CAS PubMed.
- P. Zhu, X. He, X. Chen, Y. You, Y. Yuan and Z. Weng, Tetrahedron, 2014, 70, 672–677 CrossRef CAS PubMed.
- C. Hou, X. Lin, Y. Huang, Z. Chen and Z. Weng, Synthesis, 2015, 47, 969–975 CrossRef CAS PubMed.
- M. Rong, R. Huang, Y. You and Z. Weng, Tetrahedron, 2014, 70, 8872–8878 CrossRef CAS PubMed.
- C. Chen, C. Hou, Y. Wang, T. S. A. Hor and Z. Weng, Org. Lett., 2014, 16, 524–527 CrossRef CAS PubMed.
- H. Zhang, B. Yao, L. Zhao, D.-X. Wang, B.-Q. Xu and M.-X. Wang, J. Am. Chem. Soc., 2014, 136, 6326–6332 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and analytical data for all new compounds. See DOI: 10.1039/c5qo00045a |
| This journal is © the Partner Organisations 2015 |