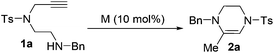Synthesis of morpholine or piperazine derivatives through gold-catalyzed cyclization reactions of alkynylamines or alkynylalcohols†
Liang-Feng
Yao
,
Yuan
Wang
and
Kuo-Wei
Huang
*
KAUST Catalysis Center and Division of Physical Sciences and Engineering, King Abdullah University of Science and Technology, Thuwal 23955-6900, Kingdom of Saudi Arabia. E-mail: hkw@kaust.edu.sa
First published on 20th April 2015
Abstract
A convenient and efficient synthetic method for the construction of morpholine and piperazine derivatives in moderate to good yields was established. The reaction proceeds smoothly with 1.0 mol% gold catalyst loading. A plausible mechanism involving the cascade cyclization and isomerizations to produce the six-membered ring was proposed and supported by deuterium labeling experiments.
Introduction
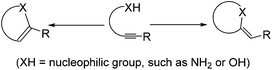
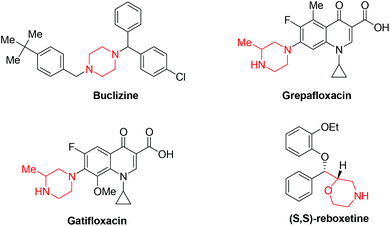
Morpholine and piperazine derivatives have attracted considerable attention owing to their occurrence in biologically active natural products1 and pharmaceuticals, such as antivertigo/antiemetic agent Buclizine,2 antibacterial agent Grepafloxacin hydrochloride,3 gyrase and topoisomerase IV inhibitor Gatifloxacin,4 and antidepressant drug Reboxetine (Fig. 1).5 They also serve as versatile building blocks in organic synthesis. While various methodologies have been established on the synthesis of morpholine6 and piperazine7 derivatives, there are still several challenges to overcome, including the limitation of starting materials, harsh reaction conditions, low efficiency, poor selectivity, etc. Only very few literature reports exist to provide a mild and highly regioselective access to functionalized-morpholine and piperazine derivatives.8 Alkynylamines and alkynylalcohols can be used to assemble various heterocyclic compounds and can serve as precursors for morpholine and piperazine derivatives. They typically undergo two different pathways to afford endo- or exo-cyclization products (Scheme 1).9 Recent reports have demonstrated that endo-cyclization of alkynylamines can be catalyzed by transition metal complexes, such as Pd,10 Ru,11 and W,12 as the catalysts. On the other hand, exo-cyclization of alkynylamines has been achieved in the presence of I2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 or tBuOK.14 Analogous cyclization reactions of alkynylalcohols have also been developed with a range of catalysts, such as Pd(OAc)2,15 Bu4NF,16 Ag2CO3,17 AuCl,18 AuCl(PPh3)/AgSbF6,19 Cu(OTf)2,20 NaH,21 PtCl2,22 and AgOTf.23 To the best of our knowledge, there is, however, no existing methodology to allow the preparation of morpholine and piperazine derivatives under the same reaction conditions. With this in mind, it is highly desired to develop a simple and facile method to prepare these molecules. Herein, we demonstrate a simple gold(I)-catalyzed reaction to afford morpholine and piperazine derivatives in moderate to good yields. These reactions can be conducted with only 1 mol% of the gold catalyst.
13 or tBuOK.14 Analogous cyclization reactions of alkynylalcohols have also been developed with a range of catalysts, such as Pd(OAc)2,15 Bu4NF,16 Ag2CO3,17 AuCl,18 AuCl(PPh3)/AgSbF6,19 Cu(OTf)2,20 NaH,21 PtCl2,22 and AgOTf.23 To the best of our knowledge, there is, however, no existing methodology to allow the preparation of morpholine and piperazine derivatives under the same reaction conditions. With this in mind, it is highly desired to develop a simple and facile method to prepare these molecules. Herein, we demonstrate a simple gold(I)-catalyzed reaction to afford morpholine and piperazine derivatives in moderate to good yields. These reactions can be conducted with only 1 mol% of the gold catalyst.
Results and discussion
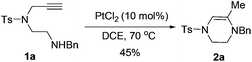
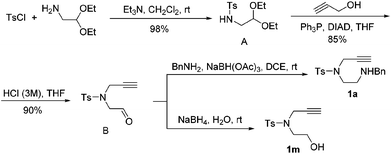
The Takemoto group has recently reported that the Lewis acid-catalyzed intramolecular hydroamination of N-Ns alkynylamide (Ns = o-nitrobenzenesulfonyl) in 1,2-dichloroethane proceeds smoothly to furnish the cyclization products.24 This work was done exclusively on phenyl alkynes and no mechanistic discussion was provided. On the basis of these results, we started to examine the reaction of the alkynylamines in the presence of carbophilic Lewis acids (Scheme 2).25 Suitable substrates were conveniently synthesized through the four steps shown in Scheme 3. Fragment A was prepared from toluenesulfonyl chloride and aminoacetaldehyde diethyl acetal in the presence of Et3N in 98% yield. Subsequent treatment of A under the Mitsunobu reaction conditions followed by hydrolysis with 3 M HCl aqueous solution gave alkynylaldehyde B in 77% yield over two steps. Reductive amination of B and reduction of B with NaBH4 afforded alkynylamine 1a and alkynylalcohol 1m in 75% and 95% yields, respectively.The reactivity of these substrates was examined in the presence of PtCl2. To our delight, the reaction proceeded smoothly and generated a 6-exo-cyclized product (2a) as a single product in 45% yield. In contrast to the earlier work, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was formed inside the ring. The reaction conditions were then optimized (Table 1). The temperature effect screening revealed that different temperatures gave similar results when PtCl2 (10 mol%) was used as the catalyst (entries 1–3). When different combinations of AuCl(PPh3)/AgX were examined, 2a was obtained in improved yields (entries 4–6). Among the silver salts screened, AgNTf2 was identified to offer the best result (entry 5). Using Lewis acid Bi(OTf)3 as a catalyst only 2a was delivered in 35% yield (entry 7). This reaction did not occur without the catalyst in DCE even at 80 °C overnight (entry 8). When the loading of the gold catalyst was reduced from 10 mol% to 1 mol%, the outcome of this reaction was not affected significantly and gave the corresponding product 2a in 82% yield (entry 9). When slightly elevating the temperature to 40 °C, 2a can be produced in 92% yield (entry 10).
C bond was formed inside the ring. The reaction conditions were then optimized (Table 1). The temperature effect screening revealed that different temperatures gave similar results when PtCl2 (10 mol%) was used as the catalyst (entries 1–3). When different combinations of AuCl(PPh3)/AgX were examined, 2a was obtained in improved yields (entries 4–6). Among the silver salts screened, AgNTf2 was identified to offer the best result (entry 5). Using Lewis acid Bi(OTf)3 as a catalyst only 2a was delivered in 35% yield (entry 7). This reaction did not occur without the catalyst in DCE even at 80 °C overnight (entry 8). When the loading of the gold catalyst was reduced from 10 mol% to 1 mol%, the outcome of this reaction was not affected significantly and gave the corresponding product 2a in 82% yield (entry 9). When slightly elevating the temperature to 40 °C, 2a can be produced in 92% yield (entry 10).
| Entry | Catalysts | Conditions | Yield (%) |
|---|---|---|---|
| a 1 mol% catalyst was used. | |||
| 1 | PtCl2 | DCE, 70 °C | 45 |
| 2 | PtCl2 | DCE, 40 °C | 65 |
| 3 | PtCl2 | DCE, rt | 63 |
| 4 | AuCl(PPh3)/AgOTf | DCE, rt | 75 |
| 5 | AuCl(PPh3)/AgNTf2 | DCE, rt | 85 |
| 6 | AuCl(PPh3)/AgBF4 | DCE, rt | 78 |
| 7 | Bi(OTf)3 | DCE, 70 °C | 35 |
| 8 | — | DCE, 80 °C | — |
| 9 | AuCl(PPh3)/AgNTf2 | DCE, rt | 82a |
| 10 | AuCl(PPh 3 )/AgNTf 2 | DCE, 40 °C | 92 |
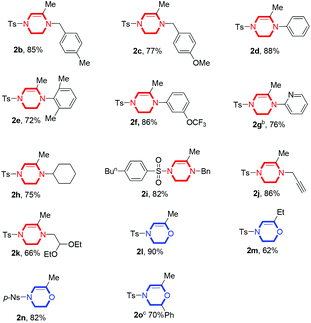
With the optimized reaction conditions, our attention was directed toward the exploration of the substrate scope. The screening results are shown in Table 2. We first tested various substituents at the terminal amino group. The benzyl substituted substrates 1b and 1c can be smoothly converted into the corresponding products 2b and 2c in high yields of 85% and 77%, respectively. When using phenyl groups in place of benzyl groups, 2d can be formed in 88% yield. In addition, the steric bulkiness of the substituent was also examined (1e), and to our delight, the reaction was not affected significantly and product 2e could also be furnished in 72% yield. Treatment with substrates containing a strong electron-withdrawing group at the meta position on the phenyl ring under the optimized conditions gave product 2f in 86% yield. Heteroatom containing substrate 1g, however, did not give the desired product 2g under the standard conditions, but 76% yield was achieved when the reaction temperature was elevated to 80 °C for 24 h. Various aliphatic substituents were also explored, including cyclohexyl (1h) and propargyl (1j), and the desired products 2h and 2j could be produced in 75% and 86% yield, respectively. It should be noted that the acid sensitive substrate 1k was also tolerated, giving the expected product 2k in 66% yield. Other alkynyl alcohol substrates 1l–1o were investigated as well, revealing that substrate 1l produced the corresponding morpholine derivative 2l in 90% yield under the otherwise identical conditions. A methyl group could be incorporated at the alkyne end, but furnishing product 2m in a lower yield of 62%. When a different sulfonyl group (1n) was involved, a similar result was obtained compared with that of 1l. Interestingly, a secondary alcohol substrate (1o) was also found suitable for this process, and the desired product 2o was obtained in 70% yield at a slightly higher temperature.
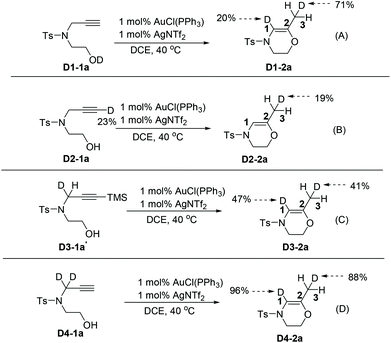
To shed light on the mechanism of the above-mentioned reaction, deuterium labeling studies have been performed. The results are summarized in Scheme 4. Accordingly, incorporation of the D-label at C1 and C3 positions in product D1-2a were achieved at 20% and 71%, respectively. The scrambling of the D label suggests the deprotonation and protonation events at C1 and the protonation event at C3 (Scheme 4A). When substrate 1a (with a D-label at the C3 position) was involved, almost no loss of the D-label at the C3 position in product D2-2a was observed, indicating that the formation of a gold acetylide intermediate was unlikely (Scheme 4B). In both cases of substrates D3-1a′ (mono-D-labeled at the C1 position) and D4-1a (di-D-labeled at the C1 position) under the optimized reaction conditions, the D labels were distributed at C1 and C3 almost equally, suggesting a reversible deprotonation/protonation process at the C1 position before the irreversible formation of the final product (Scheme 4C and D).
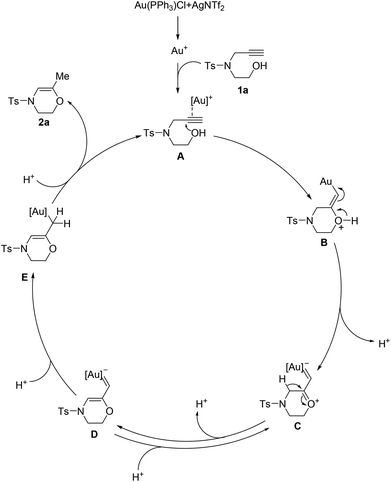
Scheme 5 depicts a plausible mechanism for the present cyclization reaction. Initially, the π-activation of the triple bond by using gold(I) takes place to furnish intermediate A, which triggers the 6-exo cyclization through a nucleophilic addition to the internal carbon–carbon triple bond to afford intermediate B.26 Subsequent loss of a proton forms the gold carbenoid intermediate C.27 Deprotonation of intermediate C results in the formation of intermediate D, a process which is rationalized to be reversible based on the D-labeling results. The protonation of the gold carbenoid intermediate D then commences, leading to intermediate E. Finally, protonation of intermediate E takes place to release the product (2a) and regenerate the active gold(I) species to complete the catalytic cycle.
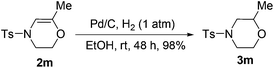
Lastly, it is important to note that the hydrogenation of the 6-exo cyclization product 2m could be conveniently achieved according to literature procedures by using Pd/C as a catalyst to give morpholine derivative 3m in high yield (Scheme 6).
Conclusion
In conclusion, we have established a simple and efficient synthetic route to morpholine and piperazine derivatives through a gold(I)-catalyzed 6-exo cyclization reaction with only 1 mol% catalyst loading. This methodology therefore has considerable potential toward the preparation of heterocyclic motifs. Further studies to extend their applications are currently underway and will be reported in due course.Acknowledgements
We would like to acknowledge the financial support from the King Abdullah University of Science and Technology and the assistance given by the Core Laboratories.Notes and references
- (a) V. Hahn, A. Mikolasch, K. Wende, H. Bartrow, U. Lindequist and F. Schauer, Biotechnol. Appl. Biochem., 2009, 54, 187 CrossRef CAS PubMed; (b) J. R. Hwu, M.-H. Hsu and R. C. C. Huang, Bioorg. Med. Chem., 2008, 18, 1884 CrossRef CAS PubMed; (c) M. Kimura, T. Masuda, K. Yamada, M. Mitani, N. Kubota, N. Kawakatsu, K. Kishii, M. Inazu, Y. Kiuchi, K. Oguchi and T. Namiki, Bioorg. Med. Chem., 2004, 12, 3069 CrossRef CAS PubMed; (d) G.-Y. Li, S.-G. Yan, S. Jiang, X.-H. Qian, Q.-C. Huang and R. Zhang, Chin. J. Org. Chem., 2008, 28, 2001 CAS; (e) L. d. M. Lima, P. Castro, A. L. Machado, C. A. M. Fraga, C. Lugnier, V. L. G. de Moraes and E. J. Barreiro, Bioorg. Med. Chem., 2002, 10, 3067 CrossRef CAS; (f) J. N. Narendra Sharath Chandra, C. T. Sadashiva, C. V. Kavitha and K. S. Rangappa, Bioorg. Med. Chem., 2006, 14, 6621 CrossRef CAS PubMed; (g) A. Ryckebusch, M.-A. Debreu-Fontaine, E. Mouray, P. Grellier, C. Sergheraert and P. Melnyk, Bioorg. Med. Chem., 2005, 15, 297 CrossRef CAS PubMed.
- B. Mahmut, Improved montelukast formulations, WO2012064302A2, 2012 Search PubMed.
- K. Bostian, T. Glinka, O. Lomovskaya, M. Surber, N. Berkley and D. Griffith, Bacterial efflux pump inhibitors for the treatment of ophthalmic and otic infections in co-administration with antimicrobial agents, US20080132457A1, 2008 Search PubMed.
- M. Kahn, J.-L. Teo, M. McMillan, Y. Zhao and Y. Wu, CBP/catenin antagonists for enhancing asymmetric division of somatic stem cells to treat age-related conditions or diseases, WO2012068299A2, 2012 Search PubMed.
- A. Weizman and M. Poyurovsky, Method for weight-gain management using combination of H3-receptor antagonists and norepinephrine reuptake inhibitors, WO2012070043A1, 2012 Search PubMed.
- (a) D. Albanese, M. Salsa, D. Landini, V. Lupi and M. Penso, Eur. J. Org. Chem., 2007, 2107 CrossRef CAS PubMed; (b) E. Bouron, G. Goussard, C. Marchand, M. Bonin, X. Panneconcke, J.-C. Quirion and H.-P. Husson, Tetrahedron Lett., 1999, 40, 7227 CrossRef CAS; (c) M. Breuning, M. Winnacker and M. Steiner, Eur. J. Org. Chem., 2007, 2100 CrossRef CAS PubMed; (d) B. A. Lanman and A. G. Myers, Org. Lett., 2004, 6, 1045 CrossRef CAS PubMed; (e) M. L. Leathen, B. R. Rosen and J. P. Wolfe, J. Org. Chem., 2009, 74, 5107 CrossRef CAS PubMed; (f) V. Lupi, D. Albanese, D. Landini, D. Scaletti and M. Penso, Tetrahedron, 2004, 60, 11709 CrossRef CAS PubMed; (g) R. Pedrosa, C. Andrés, P. Mendiguchía and J. Nieto, J. Org. Chem., 2006, 71, 8854 CrossRef CAS PubMed; (h) M. Penso, V. Lupi, D. Albanese, F. Foschi, D. Landini and A. Tagliabue, Synlett, 2008, 2451 CrossRef CAS PubMed; (i) B. Ritzen, S. Hoekman, E. D. Verdasco, F. L. van Delft and F. P. J. T. Rutjes, J. Org. Chem., 2010, 75, 3461 CrossRef CAS PubMed.
- (a) M. E. Jung and J. C. Rohloff, J. Org. Chem., 1985, 50, 4909 CAS; (b) K. G. Liu and A. J. Robichaud, Tetrahedron Lett., 2005, 46, 7921 CrossRef CAS PubMed; (c) P. Maity and B. Konig, Org. Lett., 2008, 10, 1473 CrossRef CAS PubMed; (d) G. J. Mercer and M. S. Sigman, Org. Lett., 2003, 5, 1591 CrossRef CAS PubMed; (e) S. Soukara and B. Wünsch, Synthesis, 1999, 1739 CrossRef CAS PubMed; (f) P. Vairaprakash and M. Periasamy, J. Org. Chem., 2006, 71, 3636 CrossRef CAS PubMed.
- L. Revesz, E. Blum and R. Wicki, Tetrahedron Lett., 2005, 46, 5577 CrossRef CAS PubMed.
- J. E. Baldwin, J. Chem. Soc., Chem. Commun., 1976, 734–746 RSC.
- (a) S. Arimitsu and G. B. Hammond, J. Org. Chem., 2007, 72, 8559–8561 CrossRef CAS PubMed; (b) J. C. Jury, N. K. Swamy, A. Yazici, A. C. Willis and S. G. Pyne, J. Org. Chem., 2009, 74, 5523–5527 CrossRef CAS PubMed; (c) C. Martínez, R. Alvarez and M. Aurrecoechea, J. Org. Lett., 2009, 11, 1083–1086 CrossRef PubMed; (d) Y. Hu, Y. Zhang, Z. Yeng and R. Fathi, J. Org. Chem., 2002, 67, 2365–2368 CrossRef CAS PubMed.
- (a) B. M. Trost and Y. H. Rhee, J. Am. Chem. Soc., 2002, 124, 2528–2533 CrossRef CAS PubMed; (b) B. M. Trost, M. T. Rudd, M. G. Costa, P. I. Lee and A. E. Pomerantz, Org. Lett., 2004, 6, 4235–4238 CrossRef CAS PubMed.
- F. E. McDonald, Chem. – Eur. J., 1999, 5, 3103–3106 CrossRef CAS.
- S. Mehta, T. Yao and R. C. Larock, J. Org. Chem., 2012, 77, 10938–10944 CrossRef CAS PubMed.
- C. Koradin, W. Dohle, A. L. Rodriguez, B. Schmid and P. Knochel, Tetrahedron, 2003, 59, 1571–1578 CrossRef CAS.
- F.-T. Luo, I. Schreuder and R.-T. Wang, J. Org. Chem., 1992, 57, 2213–2215 CrossRef CAS.
- K. Hiroya, R. Jouka, M. Kameda, A. Yasuhara and T. Sakamoto, Tetrahedron, 2001, 57, 9697–9710 CrossRef CAS.
- P. Pale and J. Chuche, Eur. J. Org. Chem., 2000, 1019–1025 CrossRef CAS.
- Y. Liu, F. Song, Z. Song, M. Liu and B. Yan, Org. Lett., 2005, 7, 5409–5412 CrossRef CAS PubMed.
- L. Z. Dai, M. J. Qi, Y. L. Shi, X. G. Liu and S. Min, Org. Lett., 2007, 9, 3191–3194 CrossRef CAS PubMed.
- C. Praveen, C. Iyyappan and P. T. Perumal, Tetrahedron Lett., 2010, 51, 4767–4771 CrossRef CAS PubMed.
- J. K. Vandavas, W. P. Hu, H. Y. Chen, G. C. Senadi, C. Y. Chen and J. J. Wang, Org. Lett., 2012, 14, 3134–3137 CrossRef PubMed.
- A. L. Girard, T. Enomoto, S. Yokouchi, C. Tsukano and Y. Takemoto, Chem. – Asian. J., 2011, 6, 1321–1324 CrossRef CAS PubMed.
- M. Maheswara, Y. Y. Lee and E. J. Kang, Synlett, 2012, 2481–2484 CAS.
- S. Obika, Y. Yasui, R. Yanada and Y. Takemoto, J. Org. Chem., 2008, 73, 5206–5520 CrossRef CAS PubMed.
- (a) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef PubMed; (b) T. Shimada, I. Nakamura and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 10546 CrossRef CAS PubMed.
- T. Yang, L. Campbell and D. J. Dixon, J. Am. Chem. Soc., 2007, 129, 12070 CrossRef CAS PubMed.
- N. D. Shapiro and F. D. Toste, J. Am. Chem. Soc., 2007, 129, 4160 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, characterization data (1H, 2H and 13C spectroscopic data), and HRMS of compounds 1a–1o, 2a–2o and 3m. See DOI: 10.1039/c5qo00060b |
| This journal is © the Partner Organisations 2015 |