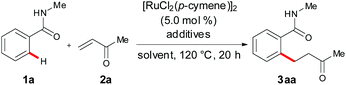Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carboxylate-assisted ruthenium(II)-catalyzed C–H activations of monodentate amides with conjugated alkenes†
Jie
Li
and
Lutz
Ackermann
*
Institut für Organische und Biomolekulare Chemie, Georg-August-Universität Göttingen, Tammannstrasse 2, 37077 Göttingen, Germany. E-mail: Lutz.Ackermann@chemie.uni-goettingen.de
First published on 18th June 2015
Abstract
Carboxylate assistance enabled efficient and chemoselective ruthenium(II)-catalyzed hydroarylations of α,β-unsaturated ketones via C–H activation on monodentate benzamides. Furthermore, the versatile ruthenium(II) catalyst set the stage for oxidative C–H functionalization on acetanilides, furnishing diversely decorated quinolines in a step-economical fashion.
Transition metal-catalyzed C–H functionalizations have been recognized as increasingly viable tools for the step-economical formation of C–C bonds.1 Particularly, metal-catalyzed hydroarylation reactions2via C–H activation are attractive because of their excellent atom-economy.3 Early findings by Lewis and Smith4 as well as Murai and co-workers5,6 indicated the considerable power of ruthenium(0) complexes as effective catalysts for hydroarylations through chelation-assisted C–H activation, which were proposed to proceed by oxidative addition of the C–H bond. Practical advances were achieved by Darses and Genet and co-workers through the in situ formation of [RuH2(PPh3)4] from [RuCl2(p-cymene)]2, NaO2CH and PPh3,7 thus avoiding sensitive and expensive ruthenium(0) complexes, such as [Ru3(CO)12], [RuH2(PPh3)4], [Ru(CO)2(PPh3)3], or [RuH2(CO)(PPh3)3]. As a part of our ongoing program on transition-metal-catalyzed C–H functionalizations,8 we recently developed ruthenium(II)-catalyzed hydroarylations via carboxylate-assisted C–H cleavages.9 Despite of these remarkable advances, the synthetically useful family of electron-deficient olefins,10 such as α,β-unsaturated ketones were thus far not viable substrates. While such transformations were accomplished with among others relatively expensive rhodium11 or rhenium12 catalysts, notable progress with ruthenium(II) complexes was very recently made by Chatani and co-workers highlighting that hydroarylations of α,β-unsaturated ketones could be realized, given that substrates displaying bidentate directing groups were employed.13,14 Herein, we report on an expedient access to β-aryl ketones and quinolines through ruthenium(II)-catalyzed hydroarylations and oxidative cascade annulations with α,β-unsaturated ketones, respectively. It is noteworthy that the ruthenium(II)-catalyzed C–H activation strategy was realized with synthetically useful amides as atom-economical mono-dentate directing groups.
We initiated our studies by testing the feasibility of the envisioned ruthenium(II)-catalyzed C–H alkylation of benzamide 1a with methyl vinyl ketone (2a) (Table 1). Interestingly, RuCl2(PPh3)3, which was previously used for hydroarylations with bidentate directing groups,13 unfortunately, failed to deliver the desired product 3aa with the assistance of the simple amide 1a (entries 1 and 2). Similar trends were observed when employing [RuCl2(p-cymene)]2 in combination with various additives (entries 3–5).
| Entry | Additive A [mol%] | Additive B [equiv.] | Solvent | Yieldb [%] |
|---|---|---|---|---|
| a General reaction conditions: 1a (0.50 mmol), 2a (1.00 mmol), [RuCl2(p-cymene)]2 (5.0 mol%), KO2CMes (30 mol%), MesCO2H (1.00 equiv.), solvent (2.0 mL), under N2, 120 °C, 20 h. b Isolated yield. c RuCl2(PPh3)3 (10 mol%). d Without [Ru]. | ||||
| 1 | NaOAc (30) | — | PhMe | —c |
| 2 | NaOAc (30) | — | H2O | —c |
| 3 | KPF6 (20) | — | H2O | — |
| 4 | KPF6 (20) | NaOAc (2.00) | H2O | — |
| 5 | PPh3 (15) | NaO2CH (0.30) | PhMe | — |
| 6 | KOAc (30) | HOAc (1.00) | H2O | 64 |
| 7 | KO2CMes (30) | MesCO2H (0.30) | H2O | 69 |
| 8 | KO 2 CMes (30) | MesCO 2 H (1.00) | H 2 O | 80 |
| 9 | KO2CMes (30) | — | H2O | 51 |
| 10 | KO2CMes (30) | MesCO2H (1.00) | H2O | —d |
| 11 | — | MesCO2H (1.00) | H2O | 29 |
A significant improvement was realized using cocatalytic amounts of KOAc and stoichiometric amounts of HOAc as the additives with H2O as inexpensive and nontoxic reaction medium15,16 (entry 6). Improved yields of the target compound 3aa were obtained when employing the bulky MesCO2K and MesCO2H as the cocatalysts (entry 7). Here, the use of stoichiometric MesCO2H provided the optimal results (entry 8). Furthermore, it is worth noting that the omission of either of the two additives resulted in significantly reduced yields of the alkylated benzamide 3aa (entries 9–11).
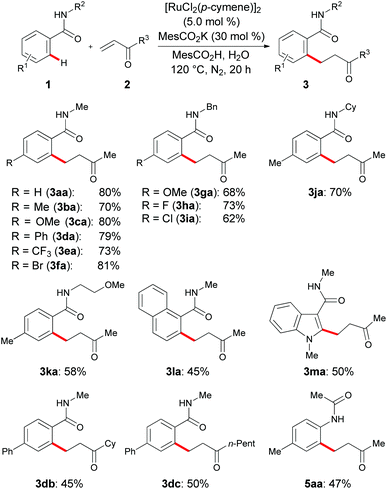
With the optimized reaction conditions in hand, we tested its versatility in the C–H alkylation with weakly coordinating17,18 amides 1 (Scheme 1). Notably, in these chelation-assisted direct C–H alkylations, both electron-rich as well as electron-poor para-substituted benzamides 1a–1f were identified as viable substrates. Moreover, a variation of the substitution pattern on the amide nitrogen with benzyl (1g–i), cyclohexyl (1j) or methoxyethyl (1k) groups, did not significantly alter the catalytic efficacy, while primary amides proved to be unsuitable substrates. More sterically hindered ortho-substituted benzamide 1l was successfully alkylated as well, albeit the desired product 3la was obtained in a slightly reduced yield. The widely applicable ruthenium(II) catalyst was not limited to aromatic benzamides 1, but the reaction of heteroaromatic indole derivative 1m also led to the site-selective C–H alkylation. In addition, among a representative set of α,β-unsaturated ketones, vinyl alkyl ketones 2b and 2c gave the alkylated products 3db and 3dc, respectively, in high yields. Interestingly, acetanilide 4a was identified as a suitable substrate for hydroarylations likewise.
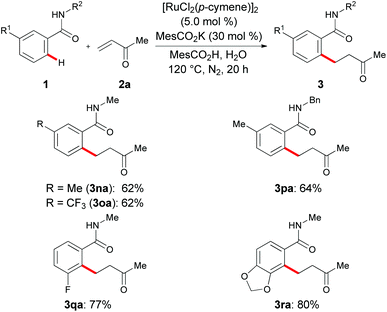
Intramolecular competition experiments with meta-methyl- or meta-trifluoromethyl-substituted arenes 1n–1p were largely governed by steric interactions to site-selectively deliver the alkylated products 3na–3pa at the sterically less hindered position (Scheme 2). In contrast, hydroarylations of the meta-substituted benzamides 1q and 1r featured a considerable ortho-orienting effect19 of the heteroatom substituent, thus leading to the site-selective formation of the sterically more hindered compounds 3qa and 3ra, respectively, as the sole products.
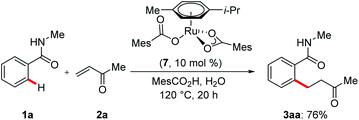
Remarkably, the well-defined, single-component [Ru(MesCO2)2(p-cymene)]20 catalyst 7 furnished the desired product, which illustrated the importance of carboxylate assistance (Scheme 3).21
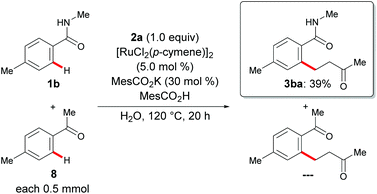
An intermolecular competition experiment between arenes with different directing groups clearly highlighted that amides 1 are more powerful than ketone 8 in the chelation-assisted C–H alkylation (Scheme 4).
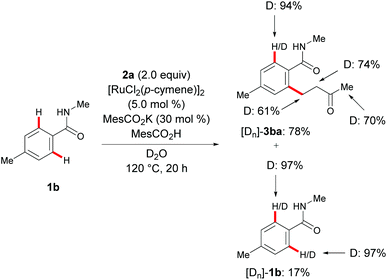
Given the unique reactivity of our carboxylate-assisted ruthenium(II) catalysis, we performed mechanistic studies to unravel its mode of action. To this end, strong evidence for a H/D exchange was gathered from C–H functionalization with starting material 1b in the presence of the deuterated solvent D2O (Scheme 5).9c This observation can be rationalized in terms of a reversible C–H metalation step in the ruthenium(II)-catalyzed direct hydroarylation.
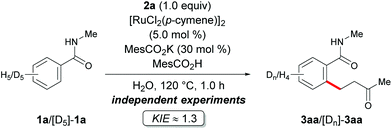
Moreover, the ruthenium-catalyzed C–H alkylation with isotopically labeled substrate [D5]-1a showed a negligible kinetic isotope effect (KIE) of kH/kD ≈ 1.3 for the intermolecular KIE experiment (Scheme 6). This data again suggests the C–H bond metalation not to be the rate-determining step.
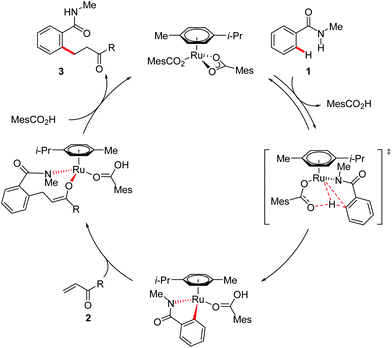
Based on these experimental findings and previous mechanistic insight, we propose a plausible catalytic cycle to involve an initial reversible C–H bond activation by carboxylate assistance, subsequent migratory insertion, and rate-determining reductive elimination (Scheme 7).
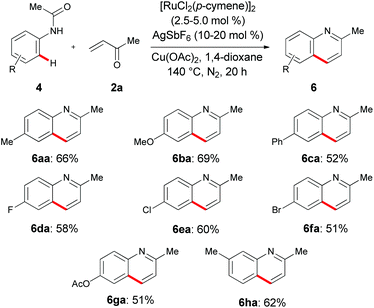
Inspired by our previous work on oxidative alkenylations,22 we subsequently probed the oxidative annulation of differently decorated acetanilides 4 with α,β-unsaturated ketone 2a (Scheme 8). Importantly, the catalytic system was not limited to the use of electron-rich N-phenylacetamides 4a–4c, but also allowed for the transformation of electron-poor substrates 4. Valuable electrophilic functional groups, such as fluoro, chloro, bromo and ester substituents, were well tolerated by the versatile ruthenium(II) catalyst. An intramolecular competition experiment with substrate 4h bearing a meta-methyl substituent showed that the cyclization was governed by steric interactions to deliver the product 6ha in high yield.
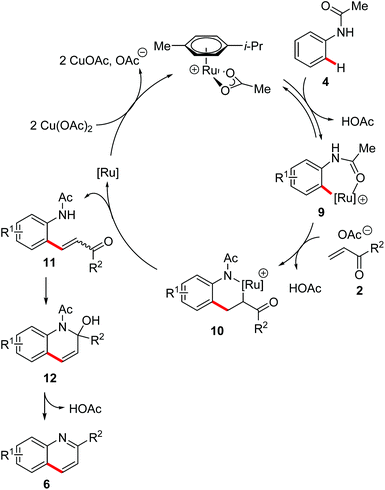
Based on our previous studies,22 we propose an initial C–H ruthenation to yield cycloruthenated complex 9 (Scheme 9). Thereafter, a migratory insertion of alkene 2 occurs to generate the intermediate 10. Then, β-hydride-elimination furnishes the product of oxidative alkenylation 11, while the catalytically active ruthenium(II) complex is regenerated by a sequence of reductive elimination and reoxidation. The desired quinoline 6 is obtained through an intramolecular nucleophilic attack of the anilide in intermediate 11, followed by β-elimination of acetic acid to deliver the desired product 6.
Conclusions
In summary, we have developed unprecedented ruthenium(II)-catalyzed hydroarylations and oxidative annulations on benzamides 1 and acetanilides 4 with α,β-unsaturated ketones 2 through C–H activation. The use of benzamides with monodentate directing groups renders our approach highly atom-economical, and the aqueous reaction conditions makes the process environmentally-benign. Detailed experimental mechanistic studies indicated a facile H/D-exchange. In addition, a cascade oxidative annulation of α,β-unsaturated ketones 2a with acetanilides 4 was developed to deliver decorated quinolines 6 in a highly step-economic fashion.Acknowledgements
Support by the European Research Council under the European Community's Seventh Framework Program (FP7 2007–2013)/ERC Grant agreement no. 307535, and the Chinese Scholarship Council (fellowship to J.L.) is gratefully acknowledged.Notes and references
- (a) X. Cui, J. Mo, L. Wang and Y. Liu, Synthesis, 2015, 439–459 CrossRef PubMed; (b) L. Ackermann, Org. Process Res. Dev., 2015, 19, 260–269 CrossRef CAS; (c) A. F. Noisier and M. A. Brimble, Chem. Rev., 2014, 114, 8775–8806 CrossRef CAS PubMed; (d) X.-S. Zhang, K. Chen and Z.-J. Shi, Chem. Sci., 2014, 5, 2146–2159 RSC; (e) V. S. Thirunavukkarasu, S. I. Kozhushkov and L. Ackermann, Chem. Commun., 2014, 50, 29–39 RSC; (f) N. Kuhl, N. Schröder and F. Glorius, Adv. Synth. Catal., 2014, 356, 1443–1460 CrossRef CAS PubMed; (g) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed; (h) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215–1292 CrossRef CAS PubMed; (i) L. Ackermann, R. Vicente and A. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed; (j) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed ; and references cited therein.
- (a) K. Gao and N. Yoshikai, Acc. Chem. Res., 2014, 47, 1208–1219 CrossRef CAS PubMed; (b) N. A. Foley, J. P. Lee, Z. Ke, T. B. Gunnoe and T. R. Cundari, Acc. Chem. Res., 2009, 42, 585–597 CrossRef CAS PubMed; (c) A. M. Echavarren and C. Nevado, Synthesis, 2005, 167–182 CrossRef PubMed; F. Kakiuchi and N. Chatani, Adv. Synth. Catal., 2003, 345, 1077–1101 Search PubMed.
- B. M. Trost, Science, 1991, 254, 1471–1477 CAS.
- L. N. Lewis and J. F. Smith, J. Am. Chem. Soc., 1986, 108, 2728–2735 CrossRef CAS.
- S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Nature, 1993, 366, 529–531 CrossRef CAS PubMed.
- N. Chatani, T. Asaumi, S. Yorimitsu, T. Ikeda, F. Kakiuchi and S. Murai, J. Am. Chem. Soc., 2001, 123, 10935–10941 CrossRef CAS PubMed.
- (a) M.-O. Simon, R. Martinez, J.-P. Genet and S. Darses, J. Org. Chem., 2010, 75, 208–210 CrossRef CAS; (b) R. Martinez, M. O. Simon, R. Chevalier, C. Pautigny, J. P. Genet and S. Darses, J. Am. Chem. Soc., 2009, 131, 7887–7895 CrossRef CAS PubMed; (c) R. Martinez, J. P. Genet and S. Darses, Chem. Commun., 2008, 3855–3857 RSC; (d) R. Martinez, R. Chevalier, S. Darses and J. P. Genet, Angew. Chem., Int. Ed., 2006, 45, 8232–8235 CrossRef CAS PubMed.
- (a) L. Ackermann, Acc. Chem. Res., 2014, 47, 281–295 CrossRef CAS PubMed; (b) L. Ackermann, Isr. J. Chem., 2010, 50, 652–663 CrossRef CAS PubMed; (c) L. Ackermann, Synlett, 2007, 507–526 CrossRef CAS PubMed.
- (a) M. Schinkel, L. Wang, K. Bielefeld and L. Ackermann, Org. Lett., 2014, 16, 1876–1879 CrossRef CAS PubMed; (b) M. Schinkel, J. Wallbaum, S. I. Kozhushkov, I. Marek and L. Ackermann, Org. Lett., 2013, 15, 4482–4484 CrossRef CAS PubMed; (c) M. Schinkel, I. Marek and L. Ackermann, Angew. Chem., Int. Ed., 2013, 52, 3977–3980 CrossRef CAS PubMed.
- F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani and S. Murai, Bull. Chem. Soc. Jpn., 1995, 68, 62–83 CrossRef CAS.
- (a) C. M. Filloux and T. Rovis, J. Am. Chem. Soc., 2015, 137, 508–517 CrossRef CAS PubMed; (b) L. Yang, B. Qian and H. Huang, Chem. – Eur. J., 2012, 18, 9511–9515 CrossRef CAS PubMed; (c) J. Ryu, S. Hwan Cho and S. Chang, Angew. Chem., Int. Ed., 2012, 51, 3677–3681 CrossRef CAS PubMed; (d) F. W. Patureau, T. Besset and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 1064–1067 CrossRef CAS PubMed; (e) L. Yang, C. A. Correia and C. J. Li, Org. Biomol. Chem., 2011, 9, 7176–7179 RSC; (f) F. W. Patureau and F. Glorius, J. Am. Chem. Soc., 2010, 132, 9982–9983 CrossRef CAS PubMed; (g) D. A. Colby, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2006, 128, 5604–5605 CrossRef CAS PubMed; (h) S.-G. Lim, J.-A. Ahn and C.-H. Jun, Org. Lett., 2004, 6, 4687–4690 CrossRef CAS PubMed. For further transition metal catalysts, see also: (i) T. Shibata and H. Takano, Org. Chem. Front., 2015, 2, 383–387 RSC; (j) S. Pan, N. Ryu and T. Shibata, Adv. Synth. Catal., 2014, 356, 929–933 CrossRef CAS PubMed; (k) Y. Kommagalla, K. Srinivas and C. V. Ramana, Chem. – Eur. J., 2014, 20, 7884–7889 CrossRef CAS PubMed; (l) B. Zhou, P. Ma, H. Chen and C. Wang, Chem. Commun., 2014, 50, 14558–14561 RSC; (m) T. Shibata and T. Shizuno, Angew. Chem., Int. Ed., 2014, 53, 5410–5413 CrossRef CAS PubMed ; and references cited therein.
- (a) H. Jin, Z. Zhu, N. Jin, J. Xie, Y. Chenga and C. Zhu, Org. Chem. Front., 2015, 2, 378–382 RSC; (b) Y. Kuninobu, K. Kikuchi, Y. Tokunaga, Y. Nishina and K. Takai, Tetrahedron, 2008, 64, 5974–5981 CrossRef CAS PubMed; (c) Y. Kuninobu, Y. Nishina, K. Okaguchi, M. Shouho and K. Takai, Bull. Chem. Soc. Jpn., 2008, 81, 1393–1401 CrossRef CAS.
- G. Rouquet and N. Chatani, Chem. Sci., 2013, 4, 2201–2208 RSC.
- For selected examples of using bidentate directing groups, see: (a) Q. Gu, H. H. Al Mamari, K. Graczyk, E. Diers and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 3868–3871 CrossRef CAS PubMed; (b) W. Song, S. Lackner and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 2477–2480 CrossRef CAS PubMed; (c) Y. Aihara and N. Chatani, Chem. Sci., 2013, 4, 664–670 RSC; (d) V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154–13155 CrossRef CAS PubMed.
- B. Li and P. H. Dixneuf, Chem. Soc. Rev., 2013, 42, 5744–5767 RSC.
- R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302–6337 CrossRef CAS PubMed.
- S. De Sarkar, W. Liu, S. I. Kozhushkov and L. Ackermann, Adv. Synth. Catal., 2014, 356, 1461–1479 CrossRef CAS PubMed.
- K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788–802 CrossRef CAS PubMed.
- E. Clot, O. Eisenstein, N. Jasim, S. A. Macgregor, J. E. McGrady and R. N. Perutz, Acc. Chem. Res., 2011, 44, 333–348 CrossRef CAS PubMed.
- (a) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed; (b) L. Ackermann, J. Pospech and H. K. Potukuchi, Org. Lett., 2012, 14, 2146–2149 CrossRef CAS PubMed; (c) S. Warratz, C. Kornhaaß, A. Cajaraville, B. Niepötter, D. Stalke and L. Ackermann, Angew. Chem., Int. Ed., 2015, 54, 5513–5517 CrossRef CAS PubMed.
- L. Ackermann, Chem. Rev., 2011, 111, 1315–1345 CrossRef CAS PubMed.
- Selected examples: (a) J. Li, M. John and L. Ackermann, Chem. – Eur. J., 2014, 20, 5403–5408 CrossRef CAS PubMed; (b) L. Ackermann, L. Wang, R. Wolfram and A. V. Lygin, Org. Lett., 2012, 14, 728–731 CrossRef CAS PubMed; a reiew: (c) S. I. Kozhushkov and L. Ackermann, Chem. Sci., 2013, 4, 886–896 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00167f |
| This journal is © the Partner Organisations 2015 |