Visible-light photoredox catalysis: direct synthesis of fused β-carbolines through an oxidation/[3 + 2] cycloaddition/oxidative aromatization reaction cascade in batch and flow microreactors†
D.
Chandrasekhar
a,
Satheesh
Borra
a,
Jeevak Sopanrao
Kapure
b,
Ghule Shailendra
Shivaji
b,
Gannoju
Srinivasulu
b and
Ram Awatar
Maurya
*ac
aDivision of Medicinal Chemistry and Pharmacology, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, India. E-mail: ramaurya@iict.res.in
bNational Institute of Pharmaceutical Education and Research, Balanagar, Hyderabad-500035, India
cAcademy of Scientific and Innovative Research, New Delhi 110025, India
First published on 14th August 2015
Abstract
Fused β-carbolines were synthesized via a visible light photoredox catalyzed oxidation/[3 + 2] cycloaddition/oxidative aromatization reaction cascade in batch and flow microreactors. Several structurally diverse heterocyclic scaffolds were obtained in good yields by coupling of tetrahydro-β-carbolines with a variety of dipolarophiles under photoredox multiple C–C bond forming events. The photoredox coupling of tetrahydro-β-carboline with 1,4-benzoquinone was significantly faster in continuous flow microreactors and the desired products were obtained in higher yields compared to batch reactors.
Introduction
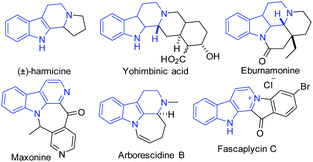
Structurally novel and multifarious heterocycles are very important in drug discovery programs for screening new hits against biological targets. Development of efficient synthetic transformations that allow the construction of complex molecular frameworks from relatively simple and easily accessible starting materials in atom economical and multiple bond forming events involving one-pot operations is very challenging. β-Carbolines are amongst the most important biologically valuable scaffolds found in a plethora of synthetic and natural products of medicinal significance.1 Many marine organisms, mammalian tissues and body fluids, plants, and insects contain numerous alkaloids or hormones having a β-carboline core unit (Fig. 1).1,2 β-Carboline containing molecules possess numerous biological activities such as antineoplastic,2 antimalarial,3a antimicrobial,3b antithrombotic,3c hypnotic and anxiolytic,3d,e anticonvulsive,3f and anti-inflammatory.3g Therefore, constructing structurally novel libraries of fused β-carboline derivatives through efficient synthetic strategies has become an important task.4 In recent years, considerable attention has been focused on the development of clean and sustainable energy mediated synthetic strategies due to the exhaustion and non-renewability of fossil fuels. In these perspectives, visible light photoredox catalysis5 has been found to be a promising alternative approach. Despite significant advances in this field, the full spectrum of the synthetic utility of visible light photoredox catalysis is yet to be explored.A careful survey of the literature revealed that most of the photoredox catalysis deals with the functionalization of the relatively unreactive C–H bonds adjacent to N-atoms and a single C–C bond is formed in the overall process. Examples of photoredox catalysis, where multiple C–C bonds are formed, are only a few.6 As part of our research program towards the development of newer heterocyclic libraries of medicinal importance,7 herein we report the construction of fused β-carbolines via a photoredox catalyzed oxidation/[3 + 2] cycloaddition/oxidative aromatization reaction cascade in batch and flow microreactors (Scheme 1). Tetrahydro-β-carbolines were functionalized in multiple C–C bond forming events that proceeded via photoredox generation of azomethine ylides and the subsequent [3 + 2] dipolar cycloaddition reaction. It is noteworthy that the synthetic strategy described herein does not require any additional step or reagent to yield aromatized products. Thus, an efficient, atom economical, and high yielding methodology involving oxidation, 1,3-dipolar cycloaddition, and aerobic oxidative aromatization for fused β-carbolines was developed.
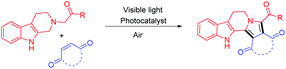 | ||
| Scheme 1 Visible light photoredox catalyzed oxidation/[3 + 2] cycloaddition/oxidative aromatization reaction cascade for the synthesis of fused β-carboline derivatives. | ||
Results and discussion
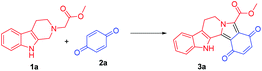
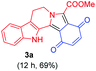
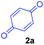
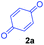
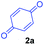
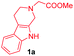
At the onset, we commenced our investigations by exploring the coupling of methyl 2-(1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)acetate 1a with 1,4-benzoquinone 2a. The substrate 1a was obtained by N-alkylation of tryptoline with methyl α-bromoacetate. Preliminary results revealed that in the presence of catalytic amount of the photoredox catalyst [Ru(bpy)3Cl2]·6H2O and visible light (white LED) the aromatized cycloadduct 3a could be obtained in good yields (Table 1).| Entry | Lightb | Photo-catalyst | Loading (mol%) | Time (h) | Yield of 3ac (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), photo-catalyst, MeCN (5 mL), air. b 11 W white LED bulb kept at a distance of 10 cm (approx.) from the reaction vessel. c Yields of the isolated products after column chromatography. d The reaction was performed under an oxygen atmosphere. e The reaction was performed under a nitrogen atmosphere. f t-BuOOH (10 eq.) was added to the reaction mixture. ND = the desired product was not detected on TLC. NI = not isolated. | |||||
| 1 | √ | — | — | 48 | ND |
| 2 | X | — | — | 48 | ND |
| 3 | √ | [Ru(bpy)3Cl2]·6H2O | 1.0 | 12 | 68 |
| 4 | X | [Ru(bpy)3Cl2]·6H2O | 1.0 | 48 | ND |
| 5 | √ | [Ru(bpy)3Cl2]·6H2O | 0.5 | 12 | 69 |
| 6 | √ | [Ru(bpy)3Cl2]·6H2O | 0.25 | 24 | 61 |
| 7 | √ | Ru(bpy)3(PF6)2 | 0.5 | 12 | 69 |
| 8 | √ | Rose bengal | 0.5 | 48 | NI |
| 9 | √ | Rose bengal | 5 | 48 | 33 |
| 10 | √ | Rose bengal | 10 | 48 | 30 |
| 11 | √ | Eosin Y | 5 | 48 | ND |
| 12 | √ | Rhodamine B | 5 | 48 | ND |
| 13d | √ | [Ru(bpy)3Cl2]·6H2O | 0.5 | 12 | 69 |
| 14e | √ | [Ru(bpy)3Cl2]·6H2O | 0.5 | 12 | ND |
| 15e,f | √ | [Ru(bpy)3Cl2]·6H2O | 0.5 | 24 | 65 |
It was found that 0.5 mol% of [Ru(bpy)3Cl2]·6H2O was sufficient to give high yields of 3a in a reasonable reaction time (Table 1, entries 3, 5 and 6). [Ru(bpy)3Cl2]·6H2O and Ru(bpy)3(PF6)2 were equally effective for the reaction whereas the organic dyes (rose bengal, eosin Y, rhodamine B) were less effective (Table 1, entries 5, 7, 8, 11 and 12). Among the organic dyes screened, rose bengal was somewhat effective but at higher loadings (Table 1, entries 8–10). Performing the reaction under an oxygen atmosphere did not improve the product yield whereas no product was obtained when the reaction was performed under a nitrogen atmosphere (Table 1, entries 13 and 14). However, even under a nitrogen atmosphere, a good yield of the desired product 3a was obtained using t-BuOOH as an oxidant for the reaction (Table 1, entry 15).
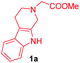
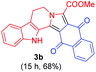
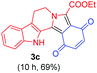
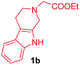
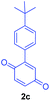
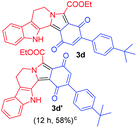
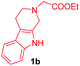
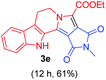
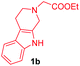
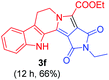
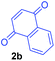
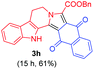
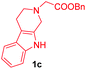
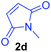
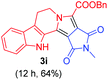
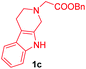
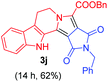
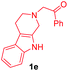
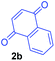
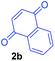
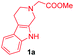
Having the optimal reaction conditions in hand, the generality of the reaction was investigated (Table 2). The scope of tetrahydro-β-carboline was studied by taking methyl, ethyl, benzyl, p-NO2-benzyl esters of 2-(1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)acetate (1a–d) and ketones (1e–f). The scope of the dipolarophile was studied by taking 1,4-benzoquinone (2a), 1,4-naphthoquinone (2b), 2-[4-(1,1-dimethylethyl)phenyl]-2,5-cyclohexadiene-1,4-dione (2c), N-methylmaleimide (2d), N-ethylmaleimide (2e), and N-benzylmaleimide (2f). The reaction was found to work well with a variety of β-carbolines and dipolarophiles. In most of the cases, the reaction gave good yields of the fused β-carbolines (3a–n). As expected, the reaction of tetrahydro-β-carboline 1b with 2-[4-(1,1-dimethylethyl)phenyl]-2,5-cyclohexadiene-1,4-dione 2c gave two products in nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
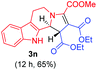
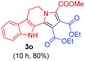
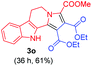
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio whose structures were tentatively assigned as 3d (28%) and 3d′ (30%). In both the products (3d/3d′), the less substituted double bond of the quinone dipolarophile underwent cycloaddition with the azomethine ylide. Using diethylacetylene dicarboxylate 2g as a dipolarophile, the reaction yielded a partly oxidised product 3n under standard conditions (Table 2, entry 14). Structural elucidation and relative stereochemical assignments of 3n were carried out by 2D DQFCOSY, NOESY and HSQC experiments. However, when the reaction of diethylacetylene dicarboxylate was conducted under an oxygen atmosphere, it gave very good yield of desired aromatized product 3o (Table 2, entry 15). The product 3o was also obtained in fairly good yield under the standard reaction conditions (under air) for an extended period of time (Table 2, entry 16). β-Nitrostyrenes and ethyl acrylate were not found as good dipolarophiles for the reaction as they gave complex reaction mixtures under our standard conditions.
1 ratio whose structures were tentatively assigned as 3d (28%) and 3d′ (30%). In both the products (3d/3d′), the less substituted double bond of the quinone dipolarophile underwent cycloaddition with the azomethine ylide. Using diethylacetylene dicarboxylate 2g as a dipolarophile, the reaction yielded a partly oxidised product 3n under standard conditions (Table 2, entry 14). Structural elucidation and relative stereochemical assignments of 3n were carried out by 2D DQFCOSY, NOESY and HSQC experiments. However, when the reaction of diethylacetylene dicarboxylate was conducted under an oxygen atmosphere, it gave very good yield of desired aromatized product 3o (Table 2, entry 15). The product 3o was also obtained in fairly good yield under the standard reaction conditions (under air) for an extended period of time (Table 2, entry 16). β-Nitrostyrenes and ethyl acrylate were not found as good dipolarophiles for the reaction as they gave complex reaction mixtures under our standard conditions.
| Entry | Tetrahydro-β-carboline 1 | Dipolarophile 2 | Product 3; Rxn. time; yieldb (%) |
|---|---|---|---|
| a Reaction conditions: tetrahydro-β-carboline 1 (0.1 mmol), dipolarophile 2 (0.1 mmol), [Ru(bpy)3Cl2]·6H2O (0.5 mol%), MeCN (5 mL), 11 W white LED bulb, air. b Yields of the isolated products after column chromatography. c Combined yield for 3d (28%) and 3d′(30%). d The reaction was performed under an oxygen atmosphere. | |||
| 1 |
|

|
|
| 2 |
|
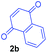
|
|
| 3 |
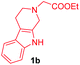
|
|
|
| 4 |
|
|
|
| 5 |
|
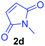
|
|
| 6 |
|
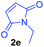
|
|
| 7 |
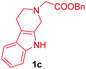
|
|
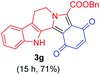
|
| 8 |
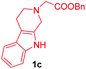
|
|
|
| 9 |
|
|
|
| 10 |
|
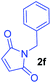
|
|
| 11 |
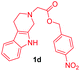
|
|
|
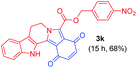
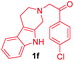
| 12 |
|
|
|
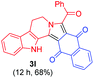
| 13 |
|
|
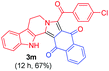
|
| 14 |
|
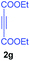
|
|
| 15d |
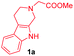
|
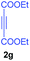
|
|
| 16 |
|
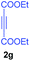
|
|
All the reactions gave several spots on TLC at the beginning but after a longer reaction run a clean spot (corresponding to our desired product) appeared on TLC and by-products were minimized. Although moderate to good yields (58–80%) of the desired products were obtained, no other by-product could be isolated using column chromatography and characterized. In other similar photoredox [3 + 2] cycloadditions, an external oxidant (other than O2) is required to obtain aromatized products.6 However, it is noteworthy that most of the reactions we performed yielded aromatized products using air as the green oxidant. It is also noticeable that the reaction is compatible with tetrahydro-β-carbolines containing a free amine (NH) group, therefore all the products can be derivatized easily through N-alkylation/acylation.
In the past decade, continuous flow microreactors have received considerable attention for performing organic transformation in safer and efficient ways.8 Due to their small dimensions, microreactors are associated with high heat and mass transfer efficiency, very high surface to volume ratio and enhanced illumination homogeneity. These features make microreactors a very good tool for photochemistry.9 Considering these aspects, a continuous flow protocol for the photoredox catalyzed coupling of tetrahydro-β-carbolines with dipolarophile was developed. Handling gaseous reagents (oxygen), and precisely controlling their flow rates in segmented flow microfluidic conditions are somewhat tricky. Therefore, in order to have an operationally simple microfluidic set-up, we preferred t-BuOOH as an oxidant over gaseous oxygen. The reagents, photo-catalyst and oxidant were introduced through two syringe pumps into visible light transparent capillary microreactors (PFA tubing, id = 500 μm, length = 5 m, volume = 0.98 mL) which were wrapped over a visible light source (11 W white LED). Schematic illustration of the photochemical reaction in flow microreactors is depicted in Fig. 2. The results of this study are given in Table 3. The short length scale and high illumination homogeneity in the microreactor provide increased photon flux. It resulted in an acceleration of the coupling reaction; full conversion was observed in a residence time of 8 minutes (Table 3, entry 3). Moreover the yields were slightly better in microreactors (75%) than in batch conditions (69%). Decreasing the stoichiometry of oxidant (t-BuOOH) from 10 eq. to 5 eq. resulted in low yields of the desired product (Table 3, entry 5). The daily outcome of the flow reactor was calculated to be 2.16 mmol of the product per day which shows that the microreactor has the potential for scale up production.
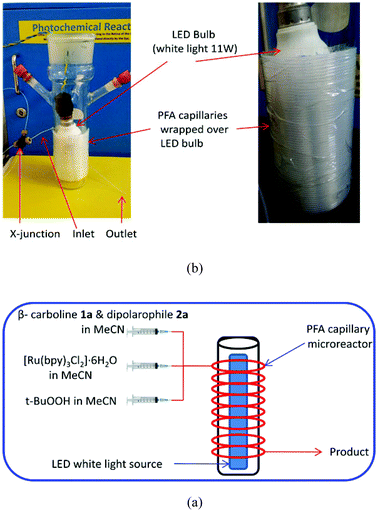 | ||
| Fig. 2 (a) Schematic illustration of the photoredox coupling in flow microreactors. (b) Picture of the capillary microreactor wrapped over a visible light source (LED). | ||
The formation of fused heterocycles through the photoredox coupling of tetrahydro-β-carbolines with dipolarophiles can be explained by a plausible mechanism depicted in Scheme 2. [Ru(bpy)3Cl2]·6H2O gets activated by visible light and accepts an electron from β-carboline 1 to give a cation radical 4. Next the intermediate 4 loses a proton to yield iminium ion 5 which further loses another proton to yield an azomethine ylide 6. The azomethine ylide 6 undergoes an 1,3-dipolar cycloaddition reaction with dipolarophile 2 to yield a cycloadduct 7 which readily aromatizes to final product 3.
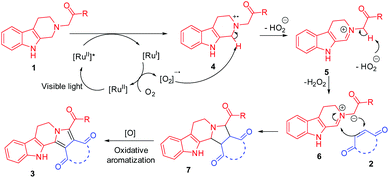 | ||
| Scheme 2 A plausible mechanism for the visible light photoredox catalyzed oxidation/[3 + 2] cycloaddition/oxidative aromatization reaction cascade for the synthesis of fused β-carbolines. | ||
Conclusions
In conclusion, we have developed an efficient photoredox catalyzed oxidation/[3 + 2] cycloaddition/oxidative aromatization reaction cascade to yield structurally diverse heterocyclic frameworks. Multiple new C–C bonds were formed during the photoredox catalysis via generation of azomethine ylides from tetrahydro-β-carbolines followed by the [3 + 2] cycloaddition reaction with a variety of dipolarophiles. The synthetic strategy we developed allows the formation of fused β-carboline derivatives in a manner that is atom economical, green and sustainable. The reaction efficiency was studied in batch and continuous flow microreactors. The use of continuous flow microreactors led to an improved irradiation over the reaction mixture, and offered considerably shorter reaction time and better yields of products compared to batch reactors.Acknowledgements
R.A.M. is thankful to the Department of Science & Technology, India for financial support (GAP 0378 & GAP 0470). Financial support in part from 12th Five Year Plan Project “Affordable Cancer Therapeutics (ACT-CSC-0301)” is also acknowledged. J. S. K. and G. S. S. are thankful to NIPER Hyderabad, and S. B. is thankful to UGC India for his fellowship.Notes and references
- (a) T. A. Mansoor, C. Ramalhete, J. Molnar, S. Mulhovo, M. J. U. Ferreira and A.-C. Tabernines, J. Nat. Prod., 2009, 72, 1147 CrossRef CAS PubMed; (b) R. Cao, W. Peng, Z. Wang and A. Xu, Curr. Med. Chem., 2007, 14, 479 CrossRef CAS PubMed; (c) K. Higuchi and T. Kawasaki, Nat. Prod. Rep., 2007, 24, 843 RSC; (d) T. Kawasaki and K. Higuchi, Nat. Prod. Rep., 2005, 22, 761 RSC; (e) G. M. Carbrera and A. M. Seldes, J. Nat. Prod., 1999, 62, 759 CrossRef PubMed; (f) M. M. Airaksinen and I. Kari, Med. Biol., 1981, 59, 190 CAS.
- (a) J. R. Allen and B. R. Holmstedt, Phytochemistry, 1979, 19, 1573 CrossRef; (b) V. Bazika, T.-W. Lang, S. Pappelbaum and E. Corday, Am. J. Cardiol., 1966, 17, 227 CrossRef CAS PubMed; (c) Y. Boursereau and I. Coldham, Bioorg. Med. Chem. Lett., 2004, 14, 5841 CrossRef CAS PubMed; (d) M. R. Uskokovic and G. Grethe, The Alkaloids, ed. R. H. F. Manske, Academic Press, New York, 1973, vol. 14, p. 181 Search PubMed; (e) S. Schwikkard and R. V. Heerden, Nat. Prod. Rep., 2002, 19, 675 RSC; (f) J. C. P. Steele, N. C. Veitch, G. C. Kite, M. S. J. Simmonds and D. C. Warhurst, J. Nat. Prod., 2002, 65, 85 CrossRef CAS PubMed.
- (a) T. B. Beghyn, J. Charton, F. Leroux, G. Laconde, A. Bourin, P. Cos, L. Maes and B. Deprez, J. Med. Chem., 2011, 54, 3222 CrossRef CAS PubMed; (b) P. Molina, P. M. Fresnda, S. Gareia-Zafra and P. Almendros, Tetrahedron Lett., 1994, 35, 8851 CrossRef CAS; (c) M. Zhao, L. Bi, W. Bi, C. Wang, Z. Yang, J. Ju and S. Peng, Bioorg. Med. Chem., 2006, 14, 4761 CrossRef CAS PubMed; (d) M. Ozawa, Y. Nakada, K. Sugimachi, F. Yabuuchi, T. Akai, E. Mizuta, S. Kuno and M. Yamaguchi, Jpn. J. Pharmacol., 1994, 64, 179–187 CrossRef CAS PubMed; (e) G. Biggio, A. Concas, S. Mele and M. G. Corda, Brain Res. Bull., 1987, 19, 301 CrossRef CAS PubMed; (f) G. Dorey, G. Poissonnet, M. C. Potier, L. P. D. Carvalho, P. Venault, G. Chapouthier, J. Rossier, P. Potier and R. H. Dodd, J. Med. Chem., 1989, 32, 1799 CrossRef CAS PubMed; (g) A. M. Deveau, M. A. Labroli and C. M. Dieckhaus, Bioorg. Med. Chem. Lett., 2001, 11, 1251 CrossRef CAS PubMed.
- (a) N. Srinivasan and A. Ganesan, Chem. Commun., 2003, 916 RSC; (b) C. Liu and R. A. Widenhoefer, J. Am. Chem. Soc., 2004, 126, 10250 CrossRef CAS PubMed; (c) A. S. Karpov, T. Oeser and T. J. J. Müller, Chem. Commun., 2004, 1502 RSC; (d) S. Shirakawa, K. Liu, H. Ito and K. Maruoka, Chem. Commun., 2011, 47, 1515 RSC; (e) S. Zhao, X. Liao and J. M. Cook, Org. Lett., 2002, 4, 687 CrossRef CAS PubMed; (f) G. Varchi, A. Battaglia, C. Samori, E. Baldelli, B. Danieli, G. Fontana, A. Guerrini and E. Bombardelli, J. Nat. Prod., 2005, 68, 1629 CrossRef CAS PubMed; (g) D. Sawant, R. Kumar, P. R. Maulik and B. Kundu, Org. Lett., 2006, 8, 1525 CrossRef CAS PubMed.
- (a) R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872 CrossRef CAS PubMed; (b) D. P. Hari and B. Konig, Chem. Commun., 2014, 50, 6688 RSC; (c) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (d) D. Ravelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97 RSC; (e) H. Jiang, C. Huang, J. Guo, C. Zeng, Y. Zhang and S. Yu, Chem. – Eur. J., 2012, 18, 15158 CrossRef CAS PubMed; (f) L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC; (g) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS PubMed; (h) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102 RSC.
- (a) Y.-Q. Zou, L.-Q. Lu, L. Fu, N.-J. Chang, J. Rong, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2011, 50, 7171 CrossRef CAS PubMed; (b) M. Rueping, D. Leonori and T. Poisson, Chem. Commun., 2011, 47, 9615 RSC; (c) C. Vila, J. Lau and M. Rueping, Beilstein J. Org. Chem., 2014, 10, 1233 CrossRef PubMed; (d) L. Huang and J. Zhao, Chem. Commun., 2013, 49, 3751 RSC; (e) S. Guo, H. Zhang, L. Huang, Z. Guo, G. Xiong and J. Zhao, Chem. Commun., 2013, 49, 8689 RSC; (f) H. Hou, S. Zhu, F. Pan and M. Rueping, Org. Lett., 2014, 16, 2872 CrossRef CAS PubMed; (g) Q.-H. Deng, Y.-Q. Zou, L.-Q. Lu, Z.-L. Tang, J.-R. Chen and W.-J. Xiao, Chem. – Asian J., 2014, 9, 2432 CrossRef CAS PubMed; (h) J. Xie, Q. Xue, H. Jin, H. Li, Y. Cheng and C. Zhu, Chem. Sci., 2013, 4, 1281 RSC; (i) X. Ju, D. Li, W. Li, W. Yu and F. Bian, Adv. Synth. Catal., 2012, 354, 3561 CrossRef CAS; (j) D. Lenhart and T. Bach, Beilstein J. Org. Chem., 2014, 10, 890 CrossRef PubMed; (k) S. Zhu, A. Das, L. Bui, H. Zhou, D. P. Curran and M. Rueping, J. Am. Chem. Soc., 2013, 135, 1823 CrossRef CAS PubMed.
- (a) A. Kamal, C. N. Reddy, M. Satyaveni, D. Chandrasekhar, J. B. Nanubolu, K. K. Singarapu and R. A. Maurya, Chem. Commun., 2015, 51, 10475 RSC; (b) A. Kamal, K. N. V. Sastry, D. Chandrasekhar, G. S. Mani, P. R. Adiyala, J. B. Nanubolu, K. K. Singarapu and R. A. Maurya, J. Org. Chem., 2015, 80, 4325 CrossRef CAS PubMed; (c) R. A. Maurya, P. R. Adiyala, D. Chandrasekhar, C. N. Reddy, J. S. Kapure and A. Kamal, ACS Comb. Sci., 2014, 16, 466 CrossRef CAS PubMed.
- For selected recent articles and reviews over flow microreactors, see: (a) T. Lebleu, J. Maddaluno and J. Legros, Org. Chem. Front., 2015, 2, 324 RSC; (b) A. Xolin, A. Stévenin, M. Pucheault, S. Norsikian, F.-D. Boyer and J.-M. Beau, Org. Chem. Front., 2014, 1, 992 RSC; (c) B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688 CrossRef CAS PubMed; (d) R. A. Maurya, C. P. Park, J. H. Lee and D.-P. Kim, Angew. Chem., Int. Ed., 2011, 50, 5952 CrossRef CAS PubMed; (e) S. Sharma, R. A. Maurya, K.-I. Min, G.-Y. Jeong and D.-P. Kim, Angew. Chem., Int. Ed., 2013, 52, 7564 CrossRef CAS PubMed; (f) K. C. Basavaraju, S. Sharma, R. A. Maurya and D.-P. Kim, Angew. Chem., Int. Ed., 2013, 52, 6735 CrossRef CAS PubMed; (g) S. T. R. Mueller and T. Wirth, ChemSusChem, 2015, 8, 245 CrossRef CAS PubMed; (h) C. Wiles and P. Watts, Green Chem., 2014, 16, 55 RSC.
- For selected recent articles and reviews over photochemical reactions in flow microreactors, see: (a) Y. Su, N. J. W. Straathof, V. Hessel and T. Noel, Chem. – Eur. J., 2014, 20, 10562 CrossRef CAS PubMed; (b) C. P. Park, R. A. Maurya, J. H. Lee and D.-P. Kim, Lab Chip, 2011, 11, 1941 RSC; (c) X. Wang, G. D. Cuny and T. Noel, Angew. Chem., Int. Ed., 2013, 52, 7860 CrossRef CAS PubMed; (d) K. Asano, Y. Uesugi and J.-i. Yoshida, Org. Lett., 2013, 15, 2398 CrossRef CAS PubMed; (e) M. Neumann and K. Zeitler, Org. Lett., 2012, 14, 2658 CrossRef CAS PubMed; (f) R. A. Maurya, C. P. Park and D. P. Kim, Beilstein J. Org. Chem., 2011, 7, 1158 CrossRef CAS PubMed; (g) J. W. Tucker, Y. Zhang, T. F. Jamison and C. R. J. Stephenson, Angew. Chem., Int. Ed., 2012, 51, 4144 CrossRef CAS PubMed; (h) E. K. Lumley, C. E. Dyer, N. Pamme and R. W. Boyle, Org. Lett., 2012, 14, 5724 CrossRef CAS PubMed; (i) D. Cantillo, O. de Frutos, J. A. Rincón, C. Mateos and C. O. Kappe, J. Org. Chem., 2014, 79, 8486 CrossRef CAS PubMed; (j) J. W. Beatty and C. R. J. Stephenson, J. Am. Chem. Soc., 2014, 136, 10270 CrossRef CAS PubMed; (k) D. Cantillo, O. de Frutos, J. A. Rincón, C. Mateos and C. O. Kappe, Org. Lett., 2014, 16, 896 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00207a |
| This journal is © the Partner Organisations 2015 |