Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Transition metal-catalyzed direct remote C–H functionalization of alkyl groups via C(sp3)–H bond activation
Guanyinsheng
Qiu
a and
Jie
Wu
*bc
aCollege of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China
bDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200433, China. E-mail: jie_wu@fudan.edu.cn; Fax: +86 21 6564 1740; Tel: +86 21 6510 2412
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Road, Shanghai 200032, China
First published on 6th May 2015
Abstract
Correction for ‘Transition metal-catalyzed direct remote C–H functionalization of alkyl groups via C(sp3)–H bond activation’ by Guanyinsheng Qiu, et al., Org. Chem. Front., 2015, 2, 169–178.
In this review, two important contributions reported by Sahoo and co-workers were not included in the text.1,2 As part of the scientific research community, we should respect every contribution. The details, which should have been included on page 8 of the original manuscript, are as follows.
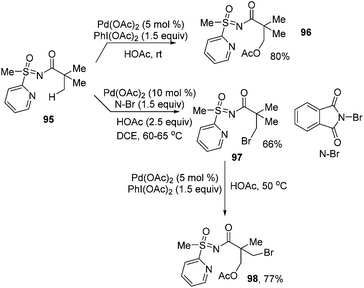
In 2012, Sahoo and co-workers developed a novel and reusable directing group, S-methyl-S-2-pyridyl-sulfoximine (MPyS). This directing group could facilitate acyloxylation of primary β-Csp3–H bonds under mild conditions. Interestingly, sequential bromination/chlorination and acetoxylation were achieved with the assistance of the MPyS group by slightly changing the reaction conditions.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Notes and references
- R. K. Rit, M. R. Yadav and A. K. Sahoo, Org. Lett., 2012, 14, 3724 CrossRef CAS.
- R. K. Rit, M. R. Yadav, K. Ghosh, M. Shankar and A. K. Sahoo, Org. Lett., 2014, 16, 5258 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2015 |