Luminescence and energy transfer of 432 nm blue LED radiation-converting phosphor Ca4Y6O(SiO4)6:Eu2+, Mn2+ for warm white LEDs†
Panlai Li*,
Zhijun Wang*,
Qinglin Guo and
Zhiping Yang
Hebei Key Lab of Optic-electronic Information and Materials, College of Physics Science & Technology, Hebei University, Baoding 071002, China. E-mail: li_panlai@126.com; wangzj1998@126.com
First published on 27th November 2014
Abstract
A series of Ca4Y6O(SiO4)6:Eu2+, Mn2+ phosphors are synthesized by a solid state method. Ca4Y6O(SiO4)6:Eu2+, Mn2+ can be excited at wavelength ranging from 250 to 500 nm, which is well matched with ultraviolet-visible light emitting diode. Under 432 nm excitation, Ca4Y6O(SiO4)6:Eu2+, Mn2+ can create warm white emission by energy transfer from Eu2+ to Mn2+. A warm white light emitting diode is fabricated by combining a 432 nm blue LED with a single phase warm white emitting phosphor Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+, which has CIE chromaticity coordinates (0.336, 0.319), correlated color temperature (CCT) 4326 K and color rendering index (Ra) 86, respectively. The results indicate Ca4Y6O(SiO4)6:Eu2+, Mn2+ may serve as potential warm white emitting phosphor for blue LED based white LEDs.
1. Introduction
White light emitting diodes (LEDs) are widely used in solid-state lighting because of their advantages, such as energy savings, no mercury pollution, long life, short response time, small size, good hardness and high energy efficiency.1–3 White LEDs can be fabricated by blue chip-pumped yellow YAG:Ce3+, but the color rendering index (CRI) of white light made by complementary blue and yellow emission is deficient because of the lack of contribution from red light.4 Therefore, to offset the deficiency, several red emitting phosphors have been developed to add into the YAG:Ce3+.5 Unfortunately, the extreme difference in degradation between the different host phosphors can produce color aberrations. Design of single-phase phosphor pumped by ultraviolet (UV) or near-UV chips is of significance for white LEDs because single-phase phosphor has excellent color rendering indexes and electro-optical design makes it simple to control different colors in comparison with mixed phosphors.6–13 However, the efficiency of UV or near-UV chip chips is still lower than that of blue chip. Accordingly, exploration of single phase phosphor with green-to-red emission bands for blue LEDs would be useful.14 A phosphor could emit radiation couple co-doped activators with f–f or d–d electron configurations, such as Eu2+/Mn2+, Ce3+/Mn2+ and Ce3+/Eu2+, and then energy transfer would occur between activator/co-activator couples by effective resonant type via a multipolar interaction, and the energy transfer to Mn2+ would be an exchange interaction.6–13,15–17 However, as far as we know, co-doped single-phase phosphors with blue absorption or for blue LEDs rarely have been investigated.14 Moreover, for indoor illumination, warm white light, similar to most luminescence lamps, CCT (CCT ≤ 4500 K or even CCT ≤ 3500 K), is more favorable for human sight and better recommended.18–20 Therefore, there is an urgent need for novel and highly efficient warm white emitting phosphor that can be excited by blue LEDs.Ternary rare-earth-metal silicate Ca4Y6O(SiO4)6 is an efficient host lattice for luminescence of various rare earth ions and mercury-like ions.21,22 The oxy-apatite host lattice consists of two cationic sites, that is, the 9-fold coordinated 4f site with C3 point symmetry and 7-fold coordinated 6h site with Cs point symmetry. Both sites are suitable and easily accommodate a great variety of rare earth and transitional-metal ions.23 Luminescent properties of Sb3+, Pb2+, Eu3+, Tb3+ and Dy3+ in Ca4Y6O(SiO4)6 have been reported.21,22,24,25 Moreover, Lin et al. explored white emission properties of Ca4Y6O(SiO4)6:Ce3+/Tb3+/Mn2+, which can be excited effectively by 284 and 358 nm UV radiation.23 Nonetheless, Ca4Y6O(SiO4)6:Ce3+/Tb3+/Mn2+ has a low absorption in the blue region. Therefore, in the present work, a warm white emitting phosphor Ca4Y6O(SiO4)6:Eu2+, Mn2+ which can be excited by blue light is explored, and the energy transfer property from Eu2+ to Mn2+ is studied.
2. Experimental section
2.1. Sample preparation
A series of Ca4−x−yY6O(SiO4)6:xEu2+, yMn2+ (x, y: mole concentration) samples were synthesized by a high temperature solid state method. Initial materials CaCO3 (A.R.), Y2O3 (A.R.), SiO2 (A.R.), MnCO3 (99.99%) and Eu2O3 (99.99%), weighted in stoichiometric proportion, were thoroughly mixed and ground by an agate mortar and pestle for more than 30 min till they were uniformly distributed. The obtained mixtures were put into crucibles and heated at 900 °C for 2 h in an atmosphere. After that, the samples were thoroughly ground and sintered at 1350 °C for 4 h in a reducing atmosphere (5% H2/95% N2), then slowly cooled down to room temperature. The samples were then ground into powder for taking measurements.2.2. Materials characterization
Phase formation was determined by X-ray diffraction (XRD) in a Bruker AXS D8 advanced automatic diffractometer (Bruker Co., Germany) with Ni-filtered Cu Kα1 radiation (λ = 0.15406 nm), and a scan rate of 0.02° s−1 was applied to record the patterns in the 2θ range from 10° to 60°. Steady time resolved photoluminescence spectra were detected by a FLS920 fluorescence spectrometer, with the excitation source being a 450 W Xe lamp. Curve fittings are performed on the luminescence decay curves to confirm the decay time. Commission International de I'Eclairage (CIE) chromaticity coordinates were measured by a PMS-80 spectra analysis system. Quantum efficiency was analyzed with a photoluminescence quantum efficiency measurement system (C9920-02, Hamamatsu Photonics, Shizuoka) containing a 150 W xenon lamp. All measurements were carried out at room temperature. High-temperature photoluminescence spectra were detected by a fluorescence spectrophotometer (Hitachi F-4600) with a TAP-02 high temperature control system, scanning wavelength range from 400 to 700 nm, spectral resolution 0.2 nm, and excitation source a 450 W Xe lamp.3. Results and discussion
3.1. Phase formation
XRD patterns of Ca4−x−yY6O(SiO4)6:xEu2+, yMn2+ were recorded and similar diffraction patterns were observed for each sample. As a representative, the XRD patterns of Ca3.95Y6O(SiO4)6:0.05Eu2+, Ca3.95Y6O(SiO4)6:0.05Mn2+ and Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ are shown in Fig. 1. When the diffraction data were compared with the standard JCPDS card (no. 27-0093), there was no difference between the doped impurity Ca4Y6O(SiO4)6 and Ca4Y6O(SiO4)6. The uniform diffraction patterns indicate that the phase formation of Ca4Y6O(SiO4)6 is not influenced by small amounts of Eu2+, Mn2+ and Eu2+/Mn2+. Ca4Y6O(SiO4)6 has a hexagonal crystal structure, with a space group P63/m (176), and cell parameters a = b = 0.9356 nm, c = 0.6793 nm, α = β = 90°, and γ = 120°. However, as shown in Fig. S1,† it is noted that the diffraction peaks (2θ) shift to a small (Eu2+) or large (Mn2+) degree compared with those of the standard PDF card, which implies diversification of lattice parameters. According to the equation 1/d2 = (h2 + k2 + l2)/a2, the lattice constant a can be achieved. For Ca3.95Y6O(SiO4)6:0.05Mn2+ and Ca3.95Y6O(SiO4)6:0.05Eu2+, the a values are calculated to be 0.9317 nm and 0.9396 nm, respectively. The values are a little smaller (or bigger) than that of blank hexagonal Ca4Y6O(SiO4)6 phase (a = 0.9356 nm). The contraction may be attributed to the ionic radius of replacement ions being smaller than that of host ions, whereas the expansion may because the ionic radius of replacement ions is larger than that of host ions. Therefore, in view of the similar ion radius and valence, Eu2+ (0.130 nm) ions are expected to substitute Ca2+ (0.118 nm) sites in the compound Ca4Y6O(SiO4)6.26 According to the same principle and ref. 23, Ca2+ sites are expected to be replaced by Mn2+ (0.090 nm) ions in the same crystal structure.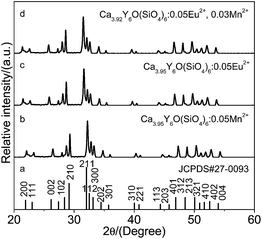 | ||
| Fig. 1 XRD patterns of Ca3.95Y6O(SiO4)6:0.05Eu2+, Ca3.95Y6O(SiO4)6:0.05Mn2+ and Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ with the standard data of Ca4Y6O(SiO4)6 (JCPDS no. 27-0093). | ||
3.2. Photoluminescence properties
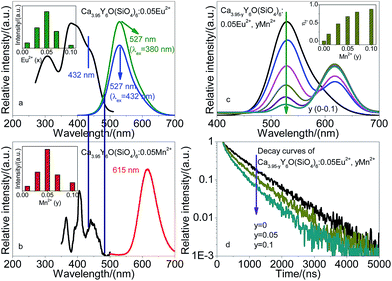
Fig. 2(a) shows that Ca3.95Y6O(SiO4)6:0.05Eu2+ has green emission under 380 nm and 432 nm excitation. Monitored at 527 nm, the excitation spectrum of Ca4Y6O(SiO4)6:Eu2+ has two obvious excitation bands, which are mainly caused by the transitions of Eu2+ from the 4f7 ground state to the 4f65d1 excited state.7,8 Eu2+ ions have many excited states, and consequently, they have an unresolved broad excitation spectrum. The excitation spectrum shows that excitation wavelength can extend from 250 to 500 nm, although the peak is at 380 nm; however, there is an obvious excitation intensity in the blue region (such as at 432 nm). As shown in Fig. 2(a), Ca4Y6O(SiO4)6:Eu2+ can be excited effectively by 432 nm blue light, and produces green emission. The inset of Fig. 2(a) shows that emission intensities of Ca4Y6O(SiO4)6:Eu2+ enhance with increasing Eu2+ concentration to a point, then decrease with further increasing Eu2+ concentration, The optimal Eu2+ concentration is x = 0.05.Fig. 2(b) shows typical emission and excitation spectra for Ca3.95Y6O(SiO4)6:0.05Mn2+ under 405 nm excitation. As is well known, d–d transitions of Mn2+ are spin- and parity-forbidden, thus excitation transition of Mn2+ doped Ca4Y6O(SiO4)6 is difficult to pump and the emission intensity is very weak. A broad emission band peak at 615 nm is attributed to the spin-forbidden 4T1(4G)–6A1(6S) transition. Its excitation spectrum consists of several bands peaking at 365, 405, and 441 nm corresponding to the transitions from 6A1(6S) to 4T2(4D), [4A1(4G), 4E(4G)] and 4T2(4G), respectively.7–10 The inset of Fig. 2(b) shows that the Mn2+ optimal concentration is y = 0.05.
As shown in Fig. 2(a) and (b), there is a spectral overlap between the emission band of Ca4Y6O(SiO4)6:Eu2+ and the excitation band of Ca4Y6O(SiO4)6:Mn2+. Accordingly, an energy transfer from Eu2+ to Mn2+ is expected in Ca4Y6O(SiO4)6. Under 432 nm excitation, emission spectra of Ca3.95−yY6O(SiO4)6:0.05Eu2+, yMn2+ with increasing Mn2+ concentration were measured, and the variation in emission spectra and emission intensity of samples are shown in Fig. 2(c). The results show that the emission intensity of Mn2+ increases systematically from y = 0.01 to y = 0.05, and reaches saturation at y ≥ 0.05. In other words, there is an energy transfer from Eu2+ to Mn2+ in Ca4Y6O(SiO4)6. To aid understanding of the energy transfer process, energy transfer efficiency (ηT) from Eu2+ to Mn2+ of samples was calculated. The ηT from Eu2+ to Mn2+ can be expressed according to Paulose et al.27
| ηT = 1 − (IS/IS0) | (1) |
It is known that if energy is transferred from a donor to an acceptor, the temporal decay of donor decreases. Monitored at 527 nm emission, the temporal decay of Eu2+ as a function of Mn2+ concentration was investigated, and the results are shown in Fig. 2(d). For Eu2+ and Mn2+ co-doped samples, if Eu2+ and Mn2+ work independently, and there is no energy transfer between them, the photoluminescence lifetime of both activators will be as same as in the single-doped samples. If energy transfer from Eu2+ to Mn2+ exists in Ca4Y6O(SiO4)6, the decay of Eu2+ excitation state will be accelerated by this energy transfer channel, and consequently the lifetime of Eu2+ will be shortened. As shown in Fig. 2(d), shortening the lifetime of Eu2+ confirmed the energy transfer between Eu2+ and Mn2+ ions. In other words, the energy transfer Eu2+ → Mn2+ exists in Ca4Y6O(SiO4)6.
Generally, there are two aspects to the resonant energy-transfer mechanism: one is exchange interaction and the other is multipolar interaction. It is well known that if energy transfer results from the exchange interaction, the critical distance between sensitizer and activator should be shorter than 5 Å. In many cases, concentration quenching results from energy transfer from one activator to another until an energy sink is reached in the lattice. The critical distance (Rc) for energy transfer from Eu2+ to Mn2+ ions can be calculated using the concentration quenching method, and the critical distance REu–Mn between Eu2+ and Mn2+ can be estimated by28
| REu–Mn = 2[3V/(4πxcN)]1/3 | (2) |
On the basis of Dexter's energy transfer formula for exchange and multipolar interactions, the following relation can be obtained30–35
| ln(η0/η) ∝ C | (3) |
| (η0/η) ∝ Cα/3 | (4) |
The relationships of ln(IS0/IS) ∝ C and (IS0/IS) ∝ Cα/3 are illustrated in Fig. 3. By consulting the fitting factor R, the relation (IS0/IS) ∝ C8/3 has the best fitting, implying that the dipole–quadrupole interaction is applied for energy transfer from Eu2+ to Mn2+. The results also prove the conclusion using the critical distance (Rc) of energy transfer.
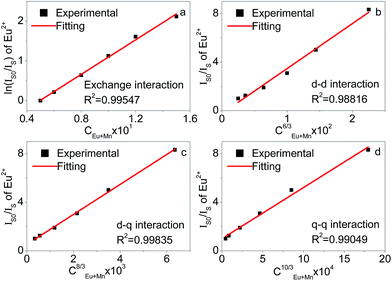 | ||
| Fig. 3 Dependence of ln(IS0/IS) of Eu2+ on (a) C and IS0/IS of Eu2+ (b) C6/3, (c) C8/3, and (d) C10/3. | ||
For white LED application, quantum efficiency (QE) is an important parameter for phosphors. Tunable white light emission with suitable QE can be obtained in Ca3.95−yY6O(SiO4)6:0.05Eu2+, yMn2+ by energy transfer from Eu2+ to Mn2+ ions, as shown in Table 1. For warm white light emitting phosphor Ca3.95−yY6O(SiO4)6:0.05Eu2+, yMn2+, the QE can reach 39.6%. The data indicate that Ca3.95−yY6O(SiO4)6:0.05Eu2+, yMn2+ has relatively appropriate QE and warm white light emission, therefore it could be used as phosphor for white LEDs.
| Samples | CIE coordinates (X, Y) | CCT (K) | Quantum efficiency (QE) |
|---|---|---|---|
| y = 0 | (0.306, 0.502) | 7119 | 37.1% |
| y = 0.01 | (0.319, 0.436) | 5619 | 38.3% |
| y = 0.03 | (0.336, 0.319) | 4326 | 39.6% |
| y = 0.05 | (0.356, 0.313) | 3601 | 39.1% |
| y = 0.07 | (0.438, 0.335) | 3209 | 38.3% |
| y = 0.1 | (0.581, 0.366) | 2653 | 38.1% |
Color coordinates an important factor for evaluating the performance of phosphors. Table 1 includes the measured Commission Internationale de I'Eclairage (CIE 1931) chromaticity coordinates and CCT of Ca3.95−yY6O(SiO4)6:0.05Eu2+, yMn2+. Eu2+ concentration is fixed at 0.05 as the concentration of Mn2+ increases from 0 to 0.1, with the corresponding color of phosphor shifting from green to white light, then to red. In particular, CCT of white light can also be tuned by appropriately tuning Mn2+ concentration. It is clear that warm white light can be created for different practical applications by varying Mn2+ concentration in Ca3.95−yY6O(SiO4)6:0.05Eu2+, yMn2+.
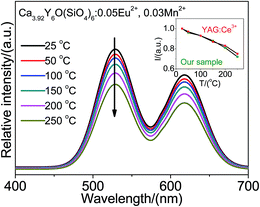
For application of high power LEDs, the thermal stability of a sample is an important issue for consideration. Fig. 4 shows temperature dependence of emission spectra for Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+, under 432 nm excitation. The inset shows the temperature quenching characteristics of commercial YAG:Ce and Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ in the temperature range from 25 °C to 250 °C. Compared with YAG:Ce, the emission intensity of Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ is very close to that of YAG:Ce when the temperature is raised up to 200 °C. The intensity of the sample drops to about 81% when the temperature is 200 °C, while that of YAG:Ce decreases to 83% of the initial value. The results indicate that the sample has good thermal quenching properties.
To further understand the temperature dependence, Arrhenius fitting was conducted following the below equation36
IT = I0/[1 + c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/kT)] exp(−ΔE/kT)]
| (5) |
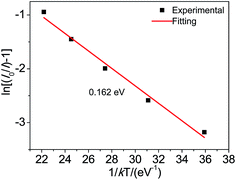 | ||
| Fig. 5 Arrhenius fitting of emission intensity of Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ and the calculated activation energy (ΔE) for thermal quenching. | ||
3.3. Application of Ca4Y6O(SiO4)6:Eu2+, Mn2+
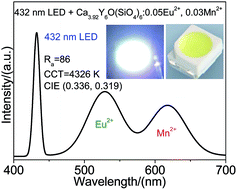
To demonstrate application of Ca4Y6O(SiO4)6:Eu2+, Mn2+, a white LED was fabricated by coating Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ phosphor on a 432 nm UV-chip driven by a 20 mA current. Fig. 6 shows the emission spectrum of a white LED, with CIE chromaticity coordinates (0.336, 0.319) and correlated color temperature (CCT) 4326 K, and color rendering index (Ra) 86. Performance of white LEDs based on Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ is superior to that of white LEDs fabricated by coating YAG:Ce yellow phosphor on blue chips [(0.292, 0.325), CCT = 7756 K] because the former shows higher color rendering index and lower CCT value.4 Moreover, Table S1† depicts the CIE chromaticity coordinates and correlated color temperature of Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+ samples in a white LED device with current 20–200 mA. The results show that the white LEDs have a relatively appropriate color stability.4. Conclusion
In summary, Ca4Y6O(SiO4)6:Eu2+, Mn2+ can be effectively excited by 432 nm blue LED, and produce warm white emission by energy transfer from Eu2+ to Mn2+, with QE about 39.6%. A warm white light emitting diode is fabricated by combining a 432 nm blue LED with the warm white emitting phosphor Ca3.92Y6O(SiO4)6:0.05Eu2+, 0.03Mn2+. The white LEDs have CIE coordinates (0.336, 0.319), correlated color temperature (CCT) 4326 K and color rendering index (Ra) 86. The results indicate that Ca4Y6O(SiO4)6:Eu2+, Mn2+ may be a potential single-phase white emitting phosphor for blue based LEDs.Acknowledgements
The work is supported by the National Natural Science Foundation of China (no. 50902042), the Natural Science Foundation of Hebei Province, China (no. A2014201035; E2014201037) and the Education Office Research Foundation of Hebei Province, China (no. ZD2014036; QN2014085).References
- M. Shang, C. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372–1386 RSC.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Mater. Sci. Eng., R, 2010, 71, 1–34 CrossRef PubMed.
- C. C. Lin and R.-S. Liu, J. Phys. Chem. Lett., 2011, 2, 1268–1277 CrossRef CAS.
- C.-W. Yeh, W.-T. Chen, R.-S. Liu, S.-F. Hu, H.-S. Sheu, J.-M. Chen and H. T. Hintzen, J. Am. Chem. Soc., 2012, 134, 14108–14117 CrossRef CAS PubMed.
- S. E. Brinkley, N. Pfaff, K. A. Denault, Z. Zhang, H. T. (Bert) Hintzen, R. Seshadri, S. Nakamura and S. P. DenBaars, Appl. Phys. Lett., 2011, 99, 241106 CrossRef PubMed.
- K. Li, D. Geng, M. Shang, Y. Zhang, H. Lian and J. Lin, J. Phys. Chem. C, 2014, 118, 11026–11034 CAS.
- Y. Chen, Y. Li, J. Wang, M. Wu and C. Wang, J. Phys. Chem. C, 2014, 118, 12494–12499 CAS.
- Y. Li, Y. Shi, G. Zhu, Q. Wu, H. Li, X. Wang, Q. Wang and Y. Wang, Inorg. Chem., 2014, 53, 7668–7675 CrossRef CAS PubMed.
- N. Guo, H. You, C. Jia, R. Ouyang and D. Wu, Dalton Trans., 2014, 43, 12373–12379 RSC.
- C.-K. Chang and T.-M. Chen, Appl. Phys. Lett., 2007, 91, 081902 CrossRef PubMed.
- C.-H. Huang, W.-R. Liu and T.-M. Chen, J. Phys. Chem. C, 2010, 114, 18698–18701 CAS.
- G. Li, D. Geng, M. Shang, C. Peng, Z. Cheng and J. Lin, J. Mater. Chem., 2011, 21, 13334–13344 RSC.
- P. Li, Z. Wang, Z. Yang and Q. Guo, J. Mater. Chem. C, 2014, 2, 7823–7829 RSC.
- W.-J. Yang and T.-M. Chen, Appl. Phys. Lett., 2007, 90, 171908 CrossRef PubMed.
- D. Geng, M. Shang, Y. Zhang, H. Lian and J. Lin, Dalton Trans., 2013, 42, 15372–15380 RSC.
- M. Jiao, Y. Jia, W. Lü, W. Lv, Q. Zhao, B. Shao and H. You, J. Mater. Chem. C, 2014, 2, 90–97 RSC.
- M. Shang, D. Geng, Y. Zhang, G. Li, D. Yang, X. Kang and J. Lin, J. Mater. Chem., 2012, 22, 19094–19104 RSC.
- N. Guo, Y. Zheng, Y. Jia, H. Qiao and H. You, J. Phys. Chem. C, 2012, 116, 1329–1334 CAS.
- Z. Wang, P. Li, Q. Guo and Z. Yang, Mater. Res. Bull., 2014, 52, 30–36 CrossRef CAS PubMed.
- G. Zhu, S. Xin, Y. Wen, Q. Wang, M. Que and Y. Wang, RSC Adv., 2013, 3, 9311–9318 RSC.
- J. Lin and Q. Su, J. Mater. Chem., 1995, 5, 603–606 RSC.
- W. L. Wanmaker, J. W. ter Vrugt and J. G. Verlijsdonk, J. Solid State Chem., 1971, 3, 452–457 CrossRef CAS.
- G. Li, Y. Zhang, D. Geng, M. Shang, C. Peng, Z. Cheng and J. Lin, ACS Appl. Mater. Interfaces, 2012, 4, 296–305 CAS.
- M. J. Lammers and G. Blasse, J. Electrochem. Soc., 1987, 134, 2068–2072 CrossRef CAS PubMed.
- J. P. M. van Vliet and G. Blasse, Mater. Res. Bull., 1990, 25, 391–394 CrossRef CAS.
- C.-H. Huang, Y.-C. Chen, T.-M. Chen, T.-S. Chan and H.-S. Sheu, J. Mater. Chem., 2011, 21, 5645–5649 RSC.
- P. I. Paulose, G. Jose, V. Thomas, N. V. Unnikrishnan and M. K. R. Warrier, J. Phys. Chem. Solids, 2003, 64, 841–846 CrossRef CAS.
- G. Blass, Philips Res. Rep., 1969, 24, 131–144 Search PubMed.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer, Berlin, 1994 Search PubMed.
- D. L. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063–1070 CrossRef CAS PubMed.
- N. Guo, Y. Huang, H. You, M. Yang, Y. Song, K. Liu and Y. Zheng, Inorg. Chem., 2010, 49, 10907–10913 CrossRef CAS PubMed.
- R. Reisfeld, E. Greenberg, R. Velapoldi and B. Barnett, J. Chem. Phys., 1972, 56, 1698–1705 CrossRef CAS PubMed.
- U. Caldiňo, J. L. Hernández-Pozos, C. Flores, A. Speghini and M. Bettinelli, J. Phys.: Condens. Matter, 2005, 17, 7297–7306 CrossRef.
- R. Martínez-Martínez, M. García, A. Speghini, M. Bettinelli, C. Falcony and U. Caldiňo, J. Phys.: Condens. Matter, 2008, 20, 395205 CrossRef.
- C.-H. Huang, T.-W. Kuo and T.-M. Chen, ACS Appl. Mater. Interfaces, 2010, 2, 1395–1399 CAS.
- P. Dorenbos, J. Phys.: Condens. Matter, 2005, 17, 8103–8111 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08283d |
| This journal is © The Royal Society of Chemistry 2015 |