Emission properties of dye-doped cationic nanoparticles: size, surfactant and monomeric composition effects
Eduardo Encisoa,
Luis Cerdán*b,
Leire Gartzia-Riveroc,
Jorge Bañuelosc,
Angel Costelab,
Iñigo López-Arbeloac and
Inmaculada García-Morenob
aFacultad de Ciencias Químicas, Universidad Complutense de Madrid, Av. Complutense s/n, 28040, Madrid, Spain
bInstituto de Química Física “Rocasolano”, Consejo Superior de Investigaciones Científicas, Serrano 119, 28006, Madrid, Spain. E-mail: lcerdan@iqfr.csic.es
cFacultad de Ciencia y Tecnología, Universidad del País Vasco/EHU, Apdo. 644, 48080, Bilbao, Spain
First published on 3rd December 2014
Abstract
Cationic nanoparticles (NPs) demonstrate advantages over similar anionic systems in relevant applications such as nanocarriers and biomarkers. To harness their unique properties and full potential in bioimaging, a clear understanding of the factors controlling the emission performance of dye-doped cationic NPs, especially under drastic pumping conditions such as those involved in high-resolution microscopy, is required. Herein, we present, for the first time, a comprehensive analysis of the photophysical and laser properties of Rhodamine 6G (Rh6G) doped cationic NPs in colloidal suspensions and self-assembled monoliths. Composition and morphological parameters such as dye content, weigh proportion of NPs in the solution, NPs size, dielectric constant of the surrounding medium, monomeric composition, and surfactant structure, have been mapped. The fluorescence capacity of Rh6G is mainly ruled by the size of the NP, as well as by its monomer and surfactant composition. The laser properties are more sensitive to compositional changes, since laser efficiencies ranging from 50% to 0% (no lasing) are measured. Hence, a most careful selection of monomers and surfactants must be carried out in order to boost their emission and photonic properties.
1. Introduction
The synthesis and functionalization of nanomaterials and nanocarriers has allowed new and sophisticated applications in all branches of technology ranging from photonic research and electronic and chemical industries, to environmental engineering and medicine. Diagnosis plays a key role in medicine to warrant a successful prevention and efficient treatment of diseases. The advantage of nanostructure-based diagnostic tools lies in their higher sensitivity and selectivity compared to classic methods.1 Different nanotechnology platforms have been developed to allow for the simultaneous and real-time evaluation of a broad range of disease markers in an accurate, non-invasive, and cost-effective manner.2–6 In this sense, fluorescent dyes represent an important class of in vitro and in vivo imaging tools.7 The main disadvantage of those dyes is their low water solubility and their low resistance to photodegradation. The fluorescence yield rapidly fades upon repeated excitation, restricting the range of applications in which they can be used. However, dyes with poor water solubility can be made more biocompatible by being placed inside a nanocarrier.8 At the same time, dye-doped nanocarriers or, more specifically, nanoparticles (NPs), show a number of advantages such as amplified signal intensities, and reduced photobleaching.9–12 Besides the biological imaging applications, dye-doped NPs are being used as biomarker,13 immunoassay reagents,14 standardization reagents for flow cytometry,15 and active medium for laser devices.10–12,16,17 In addition, arrays of fluorescent NPs that differ in light intensity, size or excited-state lifetime are extensively used to follow proteins, nucleic acids and molecular machines and assemblies within living bio-systems, simplifying the testing process and shortening the detection time.18It is well-known that in some of these relevant applications cationic NPs present advantages over similar anionic systems.19–21 As an example, both the laser efficiency and photostability of colloidal suspensions of Rhodamine 6G doped NPs are significantly higher in cationic systems.19 Moreover, cationic nanocarriers have a higher removal velocity from the bloodstream than those with negative charge.20 In addition, cationic NPs play a key role in emerging biomedical technologies, as their positive charge facilitates NPs cellular uptake and endocytosis, thus allowing the transport of loads that otherwise could not permeate the cell membrane, such as hydrophobic drugs or DNA molecules.21
Multiple studies have shown that the mechanism and rate of endocytosis and exocytosis processes, as well as intracellular trafficking of dye-doped NPs, strongly depends on parameters such as size, shape, composition, and concentration.22–25 However, up to date, there is no systematic study on the influence of these parameters on the emission properties of the system, especially under drastic pumping conditions such as those involved in high-resolution microscopy. In this sense, to harness the unique properties and full potential of cationic polymeric NPs in bioimaging, a representative and comprehensive research addressing the dependence of the emission properties on the morphology and composition of cationic polymeric NPs is highly in demand and thus acquires special relevance. Then, the present work is focused on studying the photophysical and, specially, the laser properties of Rh6G doped cationic NPs in colloidal suspensions and the random laser properties of photonic materials based on self-assembled dye-doped cationic NPs. Compositional and morphological parameters such as dye content, weigh proportion of NPs in the solution, NPs size, dielectric constant of the surrounding medium, monomeric composition, and surfactant structure, are modified in order to map their influence on the emission properties and thus to unveil the optimal composition for both laser and bioimaging applications.
2. Experimental section
2.1. Materials
Methyl methacrylate (MMA) (Aldrich, 99%) was purified with a 0.1 M sodium hydroxide solution to remove inhibitor. Rhodamine 6G (Rh6G) (Fluka), the selected monomers, 2-hydroxyethyl methacrylate (HEMA) (Aldrich, 97%), glycidyl methacrylate (Fluka, 97%), butylacrylate (BA) (Aldrich 99%), acrylamide (AA) (Sigma 99%), 2,2′-azobis(2-methyl propionamide) dihydrochloride (AIBA) (Aldrich 97%), and the selected surfactants, cetyl trimethyl ammonium bromide (CTAB) (Sigma 98%), tetradecyl trimethyl ammonium bromide (TTAB) (Sigma 99%), dodecyl trimethyl ammonium bromide (DTAB) (Sigma 98%), and tetraethylammonium bromide (TOAB) (Aldrich 98%) were used without further purification. As reaction and suspension media, deionized water obtained from a Direct QTM 5 Millipore, and methanol (MeOH) (Sigma Aldrich, 99,8%), ethanol (EtOH, 99,8%) and n-propanol (PrOH) (Sigma Aldrich 99.5%) mixtures were considered.2.2. Synthesis of NPs
Rh6G doped polymeric micro-nanoparticles were prepared from a batch dispersion polymerization of MMA by modifying the Nagao et al. recipe.12,26 Taking into account that the laser action of xanthene dyes is enhanced in protic but not very polar media,16 the original recipe was modified by copolymerizing hydrophobic monomers MMA and BA with more hydrophilic monomers, such as HEMA, GMA or AA, and exploring different monomer weight ratios. CTAB, TTAB, DTAB and TOAB were added into the feed mixture as colloidal cationic surfactant stabilizers in terms of controlling initial nuclei stabilization which influence in the final particle size and polydispersity. After oxygen degassing with a nitrogen flux, the free radical polymerization was carried out at 65 °C with monomer content in the feed less than 8 wt% related to the total mass of suspension. The reaction was initiated by adding 1 wt% of a water soluble cationic initiator such as AIBA with respect to the monomer present in the feed mixture.2.3. Nanoparticles characterization
The monomer conversion was determined gravimetrically by removing 2 ml aliquots of the suspensions after 3 hours of polymerization. The diameters of the particles were measured by dynamic light scattering (DLS) in a 90 Plus Particle Analyzer (Brookhaven Instruments); the relative standard deviation of the particle size distribution indicates the polydispersity of the samples, since a value below 0.1 is considered a fairly monodisperse system. The morphology and microscopic structures of the latex monoliths were observed using field-emission scanning electron microscopy (FESEM, JEOL, JMS-6700F) with an acceleration voltage of 5 kV and an emission current of 10 mA. The samples were fragments of thin films obtained after drying the suspension in an oven at 40 °C.2.4. Photophysical properties
The photophysical properties were registered in diluted aqueous suspensions of latex NPs doped with Rh6G, by diluting the original ones down to a latex content of 0.03–0.04 wt%, with the Rh6G concentration in the suspension being thus reduced from 3 × 10−4 M to 2 × 10−6 M. UV-Vis absorption and fluorescence spectra were recorded on a Cary 4E spectrophotometer and on a SPEX Fluorolog 3-22 spectrofluorimeter, respectively. The absorption spectra were recorded using a blank suspension of latex in water as reference. Fluorescence quantum yields (ϕ) were evaluated from corrected spectra, using a diluted solution (10−6 M) of Rh6G dye in water (ϕ = 0.59) as reference. The inherent uncertainty in our experimental set-up is ϕ ± 5–10%. Radiative decay curves were registered by the time correlated single-photon counting technique (Edinburgh Instruments, model FL920) equipped with a microchannel plate detector (Hamamatsu C4878) of picosecond time-resolution (20 ps). Fluorescence emission was monitored at the maximum emission wavelength after excitation at 470 nm by means of a diode laser (PicoQuant, model LDH470) with 150 ps FWHM (full width at half maximum) pulses. The fluorescence lifetime (τ) was obtained from the slope after the deconvolution of the instrumental response signal from the recorded decay curves by means of an iterative method. The goodness of the exponential fit was controlled by statistical parameters (χ2, Durbin–Watson and the analysis of the residuals). The registration of the photophysics of the large-sized NPs (diameter > 80 nm) was problematic. Thus, in most cases the baseline was subsequently corrected manually to appropriately adjust the zero of absorbance. Besides, the measurement of the relative fluorescence quantum yield was doubtful due to uncertainty in the registration of the absorbance at the excitation wavelength. To avoid such problems, the absolute fluorescence quantum yield was calculated from the homogeneous powder attained by drying the solution and posterior grinding of the sample, and using an integrating sphere coupled to the spectrofluorimeter. The absorbance at the excitation wavelength was achieved comparing the scatter signal of the dye-doped NP and a Teflon disk as reference (with a diffuse reflectance of 100%).2.5. Laser properties
Original suspensions, without dilution, were contained in 1 cm optical path quartz cells carefully sealed to avoid solvent evaporation. The samples were transversely pumped at 532 nm with 5.5 mJ, 6 ns FWHM pulses from a frequency-doubled Q-switched Nd:YAG laser (Monocrom STR-2+) at 10 Hz repetition rate. The exciting pulses were line-focused onto the front face of the cell, providing pump fluences on the active medium of 180 J cm−2. The oscillation cavity (2 cm length) consisted of a 90% reflectivity aluminum mirror, with the end lateral face of the cell as output coupler.The photostability of the gain medium was evaluated by irradiating under lasing conditions 10 μL of solution. The solutions were contained in cylindrical Pyrex tubes (1 cm height, 1 mm inner diameter) carefully sealed. Monitoring of sample photolysis was carried out by recording the laser-induced fluorescence emission from the dye solutions in the capillary, which were placed horizontally and excited along the axis with the same pump pulses from the Nd:YAG laser used for producing dye laser emission, as a function of the pump pulses at 10 Hz repetition rate. The fluorescence emission was monitored perpendicular to the exciting beam, collected by an optical fiber, imaged onto the input slit of a monochromator (Acton Research Corp.) and detected with a charge-coupled device (CCD) (SpectruMM:GS128B). The fluorescence emission was recorded by feeding the signal to a boxcar (Stanford Research, model 250) to be integrated before being digitized and processed by a computer. Each experience was repeated at least three times. The estimated error of the energy measurements was 10% and the experimental error in the photostability measurements was estimated to be on the order of 7%. Details of the experimental setup can be found elsewhere.16
2.6. Self-assembled NPs
Polystyrene cylindrical boxes 1 inch in diameter were filled with 2 ml of colloidal suspension with a 5 wt% of NPs previously prepared and the solutions were left to dry in an oven at 40 °C until complete water evaporation. Pieces from the center of the sample were selected to avoid meniscus effects in the sample edge, and were stuck to a microscope cover glass (2 cm × 2 cm) to allow sample manipulation. The thickness of the samples was measured by using a Digital Comparator (Mahr Extramess 2001) with a resolution of up to 0.2 μm.The random laser emission from the self-assembled NPs were analyzed pumping the solid samples at 532 nm with 20 ns FWHM pulses from a frequency-doubled Q-switched Nd:YAG laser (Lotis TII SL-2132) at 15 Hz repetition rate. The pump radiation was vertically polarized, and the light incident on the sample was focalized onto the sample with a spherical quartz lens (f = 15 cm), at an angle of incidence of 34° with respect to the normal to the surface of the sample. The spot on the surface of the sample was elliptical with major and minor axis of 2 mm and 1.6 mm, respectively. The RL emission was collected normal to the sample with a 5 cm focal length spherical lens, focused onto a fibre bundle and detected with a spectrograph/monochromator equipped with a thermoelectrically cooled CCD detector. Neutral density filters were used to avoid CCD detector saturation. The integration time in the CCD was set at 1000 ms so that all the measurements were averaged over fifteen pulses.
3. Results and discussion
3.1. Structural characterization of NPs
Fig. 1 shows SEM images of some of the samples synthesized in this work. The resulting monoliths do not present NP ordering due to the level of polydispersity of the samples and the fast drying method used to obtain the monoliths. This disordered self-assembling will be responsible for the appearance of incoherent random lasing in the irradiated monoliths (see Section 3.4).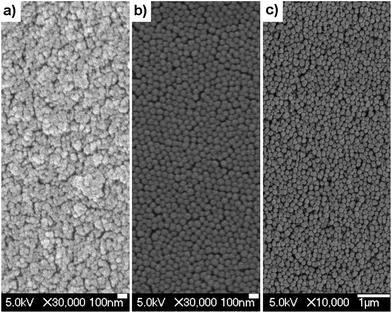 | ||
| Fig. 1 SEM photographs of representative samples: (a) S3 (dNP = 42 nm), (b) SU-5 (dNP = 93 nm), and (c) DC9 (dNP = 187 nm). Note the differences in the scale bars. | ||
Tables 1–4 show the particle size and polydispersity of the synthesized samples, which can be correlated with the different feed mixture compositions considered in this work. Table 1 shows the particle size reduction by increasing the CTAB surfactant concentration. The smaller NPs show large polydispersities, since the surfactant can stabilize a broad range of oligomer nuclei sizes. The reduction of the weight percentage of surfactant increases the NP size by diminishing the solubility of the oligomers in the suspension media and achieving an earlier nucleation point with low levels of oligomer concentrations. Given the high diffusion of the monomers to the seed particles, an inhibition of new nuclei formation can be considered. Hence, the growth of all the particle seed is produced at the same time. This explains the observed low polydispersity in the largest NPs suspensions.
| Sample | CSa (mM) | ηb (%) | CNPc (mM) | dNPd (nm) | λabe (nm) | εmaxf (M−1 cm−1) | λflug (nm) | ϕh | τi (ns) | Effj (%) | λlak (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a CS: surfactant concentration.b η: polymerization yield and percentage of polymer in colloid (in brackets).c CNP: dye concentration inside NP.d dNP: NP mean diameter and standard deviation (in brackets).e λab: absorption peak wavelength.f εmax: molar extinction coefficient at λab.g λflu: fluorescence peak wavelength.h ϕ: fluorescence quantum yield.i τ: fluorescence lifetime.j Eff: laser energy conversion efficiency.k λla: laser peak wavelength. | |||||||||||
| S1 | 6.9 | 95 [4.3] | 7.6 | 21 [0.17] | 528 | 6.7 × 104 | 554 | 0.80 | 4.44 | 49 | 575 |
| S2 | 3.4 | 91 [3.8] | 8.3 | 29 [0.13] | 528 | 7.8 × 104 | 555 | 0.78 | 4.37 | 48 | 576 |
| S3 | 2.1 | 92 [4.0] | 7.8 | 42 [0.10] | 529 | 7.0 × 104 | 556 | 0.78 | 4.41 | 39 | 578 |
| S4 | 1.2 | 92 [3.8] | 8.1 | 53 [0.09] | 528 | 6.9 × 104 | 556 | 0.81 | 4.36 | 29 | 578 |
| Sample | R–OH (%) | η (%) | CNP (mM) | dNP (nm) | λab (nm) | εmax (M−1 cm−1) | λflu (nm) | ϕ | τ (ns) | Eff (%) | λla (nm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Recorded in powder samples.b Large amount of coagulum. | ||||||||||||
| DC-1 | — | 0 | 95 [4.3] | 7.6 | 21 [0.17] | 528 | 6.7 × 104 | 554 | 0.80 | 4.49 | 49 | 575 |
| DC-2 | MeOH | 7 | 85 [3.9] | 8.3 | 26 [0.19] | — | — | — | — | — | 49 | 576 |
| DC-3 | MeOH | 10 | 96 [4.4] | 7.6 | 26 [0.16] | 529 | 8.4 × 104 | 556 | 0.75 | 4.50 | 48 | 575 |
| DC-4 | EtOH | 4 | 91 [4.1] | 8.1 | 23 [0.17] | 529 | 7.6 × 104 | 556 | 0.76 | 4.59 | 49 | 576 |
| DC-5 | EtOH | 7.5 | 95 [4.2] | 7.6 | 30 [0.13] | 529 | 8.6 × 104 | 556 | 0.75 | 4.56 | 48 | 575 |
| DC-6 | EtOH | 10 | 89 [3.9] | 8.3 | 48 [0.11] | 529 | 9.2 × 104 | 555 | 0.77a | 4.53 | 34 | 577 |
| DC-7 | EtOH | 12.5 | 96 [4.6] | 7.3 | 87 [0.05] | 530 | 8.1 × 104 | 557 | 0.73a | 4.53 | 4 | 575 |
| DC-8 | EtOH | 13.2 | 93 [4.2] | 7.8 | 109 [0.08] | 530 | 7.5 × 104 | 557 | 0.75a | 4.41 | 2 | 577 |
| DC-9 | EtOH | 15.4 | 94 [4.7] | 7.8 | 187 [0.04] | 531 | 7.1 × 104 | 557 | 0.73a | 4.43 | 0 | — |
| DC-10 | EtOH | 18 | 91 [4.1] | 7.8 | 269 [0.08] | 530 | 7.8 × 104 | 557 | 0.67a | 4.42 | 0 | — |
| DC-11 | PrOH | 7 | 93 [4.1] | 7.8 | 39 [0.09] | 529 | 8.8 × 104 | 557 | 0.75 | 4.40 | 42 | 577 |
| DC-12 | PrOH | 10 | 89 [4.1] | 8.5 | 91 [0.07] | 530 | 8.5 × 104 | 557 | 0.78a | 4.40 | 2 | 575 |
| DC-13 | PrOH | 12 | 46 [2.8] | b | 311 [0.07] | 530 | 12.4 × 104 | 557 | 0.69a | 4.35 | 0 | — |
| Sample | Composition MMA/HEMA/X | η (%) | CNP (mM) | dNP (nm) | λab (nm) | εmax (M−1 cm−1) | λflu (nm) | ϕ | τ (ns) | Eff (%) | λla (nm) | Inb (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
a Recorded in powder samples.b In [%]: percent intensity of the laser output after 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pump pulses referred to as the initial intensity I0; In [%] = (I0/In) × 100. 000 pump pulses referred to as the initial intensity I0; In [%] = (I0/In) × 100. |
|||||||||||||
| M1 | X = — | 75/25/0 | 92 [4.0] | 8.3 | 32 [0.21] | 527 | 7.4 × 104 | 555 | 0.75 | 4.35 | 45 | 575 | |
| M2 | X = GMA | 65/25/10 | 95 [4.3] | 7.6 | 21 [0.20] | 528 | 6.7 × 104 | 554 | 0.80 | 4.49 | 49 | 575 | 93 |
| M3 | X = GMA | 56/25/19 | 88 [3.9] | 8.3 | 23 [0.16] | 528 | 7.1 × 104 | 555 | 0.79 | 4.66 | 55 | 576 | |
| M4 | X = GMA | 37.5/25/37.5 | 90 [4.0] | 8.1 | 28 [0.16] | 529 | 7.8 × 104 | 556 | 0.78 | 4.66 | 55 | 576 | 40 |
| M5 | X = GMA | 0/25/75 | 98 [4.3] | 7.3 | 43 [0.06] | 529 | 7.9 × 104 | 556 | 0.75a | 4.61 | 34 | 578 | 25 |
| M6 | X = AA | 57/25/18 | 84 [4.0] | 8.3 | 43 [0.12] | 527 | 8.7 × 104 | 556 | 0.76a | 4.37 | 42 | 576 | |
| M7 | X = AA | 51/25/24 | 91 [4.0] | 8.3 | 68 [0.08] | 527 | 9.3 × 104 | 556 | 0.76a | 4.37 | 22 | 579 | 55 |
| M8 | X = AA | 48/25/27 | 88 [4.0] | 8.1 | 115 [0.08] | 527 | 8.1 × 104 | 554 | 0.79a | 4.31 | 10 | 578 | |
| M9 | X = AA | 44/25/31 | 99 [4.4] | 7.6 | 115 [0.08] | 526 | 7.8 × 104 | 556 | 0.77a | 4.30 | 18 | 579 | |
| M10 | X = AA | 41/25/34 | 99 [4.5] | 7.3 | 67 [0.21] | 526 | 7.6 × 104 | 554 | 0.77a | 4.28 | 28 | 580 | 35 |
| M11 | X = AA | 37.5/25/37.5 | 99 [4.4] | 7.6 | — | 525 | 7.2 × 104 | 554 | 0.77 | 4.23 | 18 | 580 | |
| M12 | X = BA | 64/25/11 | 94 [4.2] | 7.8 | 24 [0.19] | 526 | 6.6 × 104 | 553 | 0.72 | 4.18 | 52 | 577 | |
| M13 | X = BA | 50/25/25 | 94 [4.2] | 7.8 | 25 [0.18] | 525 | 6.5 × 104 | 553 | 0.70 | 4.12 | 50 | 576 | |
| M14 | X = BA | 37.5/25/37.5 | 91 [4.1] | 8.1 | 26 [0.16] | 525 | 7.2 × 104 | 553 | 0.71 | 4.10 | 52 | 577 | 52 |
| M15 | X = BA | 0/25/75 | 90 [3.8] | 9.3 | 26 [0.18] | 525 | 6.9 × 104 | 554 | 0.70 | 4.11 | 50 | 577 | 40 |
| Sample | Surfactant (mM) | η (%) | CNP (mM) | dNP (nm) | λab (nm) | εmax (M−1 cm−1) | λflu (nm) | ϕ | τ (ns) | Eff (%) | λla (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Recorded in powder samples. | |||||||||||
| SU-1 | [CTAB] = 2.5 | 88 [4.0] | 8.3 | 21 [0.20] | 528 | 6.9 × 104 | 554 | 0.75 | 4.36 | 51 | 575 |
| SU-2 | [CTAB] = 6.3 | 91 [4.0] | 8.5 | 21 [0.19] | — | — | — | — | — | 48 | 576 |
| SU-3 | [TTAB] = 2.2 | 91 [3.9] | 8.0 | 30 [0.11] | 529 | 6.9 × 104 | 555 | 0.72 | 4.46 | 53 | 575 |
| SU-4 | [TTAB] = 5.8 | 94 [4.3] | 7.8 | 27 [0.16] | — | — | — | — | — | 54 | 576 |
| SU-5 | [DTAB] = 2.0 | 85 [3.7] | 8.5 | 93 [0.09] | 532 | 8.8 × 104 | 559 | 0.73a | 4.42 | 4 | 577 |
| SU-6 | [DTAB] = 4.0 | 95 [4.3] | 7.8 | 98 [0.09] | — | — | — | — | — | 3 | 575 |
| SU-7 | [TOAB] = 1.4 | 94 [3.9] | 8.1 | 186 [0.04] | 528 | 5.1 × 104 | 556 | 0.64a | 4.23 | 0 | — |
The effect of the solvent dielectric constant has been considered in the suspensions shown in Table 2 by synthesizing NPs with different alcohol–water mixtures. Particles sizes in the range of 20–300 nm can be easily prepared by controlling the ethanol concentration of the solvent mixture. Due to the presence of alcohol, the hydrophilic oligomers reduce their solubility in the suspension media, and the particle nuclei are formed at an earlier stage of polymerization. As explained above, this leads to an increase in the particle size and monodispersity. Nevertheless, upon drying of the samples, the SEM images show some level of NP fusion (Fig. 1c), most probably resulting from the drying process itself.
The dye concentration does not influence appreciably the NP size, since a change on the dye concentration in a range of 0.03–0.91 wt% respect to the monomer feed offers a random variation of the particle size in a range of 31–42 nm. Table 3 shows the parameters of different suspensions where the monomer compositions were modified. In this suspension set a 25 wt% of HEMA was introduced in all preparations, given the large affinity of Rh6G by alcohol functional group; however, the concentrations of MMA and a third introduced monomer, chosen from GMA, AA and BA, were changed. The shift of MMA composition does not change so much the particle diameter in the case of GMA and BA monomers; however, the introduction of AA, a monomer more hydrophilic than MMA, increases the particle size, probably by solvent swelling of the formed particles. Furthermore, after reaching a maximum, the average diameters are reduced, and no particles are formed anymore after crossing a given AA threshold wt%. Accordingly, the light dispersion of those suspensions are dramatically reduced.
Finally, Table 4 shows the large effect of the surfactant chemical composition effects on the NP sizes. Large hydrophobic tails in the surfactant better stabilize the oligomer nuclei, and lead to smaller NPs. The octyl hydrophobic tails of TOAB cannot stabilize enough the oligomer nuclei, and the obtained NPs show the largest sizes of this suspension set.
3.2. Photophysical properties
An in-depth analysis of the photophysical properties of the cationic NPs in aqueous solutions was carried out as a function of the NP size, monomeric composition, surfactant structure, as well as dye concentration. All the photophysical results are listed in Tables 1–4The absorption and fluorescence bands of Rh6G were consistently peaked at ∼529 and 554 nm (Fig. 2), respectively, with slight shifts (±1 nm) due to compositional and concentration effects. These spectral bands match with those exhibited by Rh6G solved in a liquid solution mimicking the monomeric environment (a mixture of monomers MMA, HEMA and GMA in the same molar ratio considered in the NPs).16 In addition, the absorption and fluorescence spectra of the new system are very similar to those registered in dilute solutions of the dye, indicating the low tendency to self-associate of Rh6G in the synthesized cationic NPs, despite the high dye loadings inside the same (up to 2.2 × 10−2 M). In contrast, anionic NPs with similar size and composition rendered absorption and emission peaks at ∼533 and 558 nm, respectively, red-shifted with respect to those of the cationic NPs.16 The molar absorption coefficients in cationic NPs range from 6.7 × 104 to 11 × 104 M−1 cm−1, which approach those measured in the anionic NPs and in solutions mimicking the monomeric environment. The fluorescence quantum yield in cationic NPs reaches values as high as 0.80 (sample S1, Table 1), improving those recorded for analogous anionic NPs (up to 0.75).16 As explained elsewhere, this increase in the fluorescence ability of Rh6G is ascribed to the decrease in the dye content in the NP surface, thus overcoming the deleterious effects of the polymer/water interphase.19 This population redistribution within the NP is revealed as well through the analysis of the fluorescence lifetime. The decay rate registered in cationic NPs are properly adjusted to mono-exponentials (∼4.4 ns), losing the bi-exponential behavior observed at high concentrations in analogous anionic NPs.16
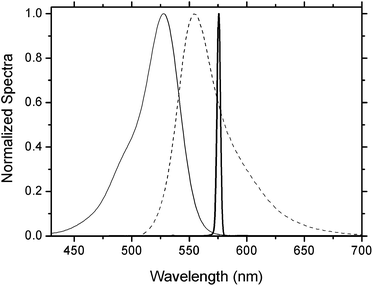 | ||
| Fig. 2 Normalized absorption (solid line), fluorescence (dashed line) and laser (thick solid line) spectra of Rh6G in a suspension of cationic NPs (sample S1, Table 1). | ||
Although in broad terms the photophysical properties are similar in all the evaluated samples, there are some differences related to the particular compositions and structural properties. Nonetheless, the herein reported fluorescence quantum yields have to be taken with care due to the uncertainty introduced by the experimental apparatus (ϕ ± 5–10%) and owing to the difficulty in reproducing exactly the synthetic route, which can alter the final properties of the NPs (Fig. 3).
3.3. Laser properties in colloidal suspensions
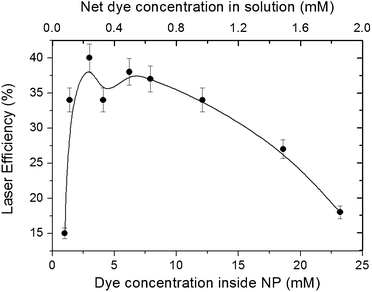
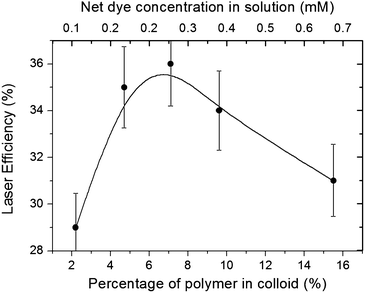
As shown in our previous paper,19 this kind of laser gain medium presents stunning laser efficiencies and photostabilities. Hence, it is important to assess how these properties depend on the compositional properties of the NPs. The laser behavior of Rh6G exhibits a stronger dependence on the size, concentration, and composition than that shown by the photophysical properties.3.4. Random laser properties in self-assembled NPs
These promising results prompted us to investigate further the scope and limitations of these dye-sensitized cationic NPs not contained in a suspension but self-assembled as disordered solid monoliths. The solid samples, of ∼100 μm thickness, were obtained by drying 2 ml of suspension at room temperature. Under front-face irradiation at a pump intensity of 490 kW cm−2 (see Methods), random laser (RL) emission from the randomly self-assembled Rh6G-sensitized cationic NPs (see Fig. 1) was obtained due to multiple scattering and amplification of light among NPs.28,32–34As we reported in our previous work on self-assembled monoliths, where anionic NPs were used, the NP size drastically affected the RL properties.28 The use of cationic NPs does not influence these trends in the emission properties when the size is changed from 20 nm up to 269 nm (Table 2). The peak intensity rises abruptly as the NP size is increased, reaching an emission maximum for NPs close to 100 nm in size (samples DC-7 and DC-8), where the Full Width at Half Maximum (FWHM) reaches a minimum of ca. 5 nm (Fig. 5a). For NPs larger than this value the emission intensity and its FWHM are drastically worsened. Hence, in terms of amplification, the RL emission properties of Rh6G inside cationic latexes compare well to or slightly enhance those previously achieved with analogous anionic NPs.28
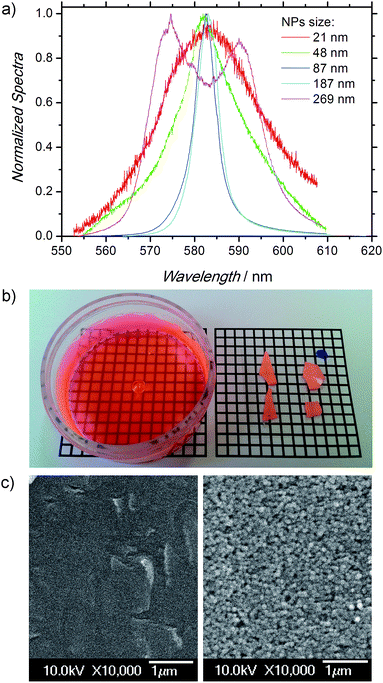 | ||
| Fig. 5 (a) Normalized emission spectra from samples of self-assembled NPs (MMA/HEMA/GMA 65/25/10) with different sizes (Table 2). (b) Photographs of thin films from samples with same NP size (110–115 nm) but different monomeric composition: left, sample M9 (Table 3); right, sample DC-8 (Table 2). (c) SEM micrographs of samples in (b). | ||
Among the methods adopted for assembling NPs on a flat substrate, evaporation-driven connective self-assembly has emerged as an extremely simple, fast and cost-effective strategy.35 Nevertheless, the lack of full control over the evaporation avoids achieving quality structures over large areas. In fact, the large change in volume upon drying causes large mechanical stress, cracking the resulting films or monoliths. The use of NPs with a more “plastic” composition could inhibit the mechanical fracture due to an increase in monolith elasticity. The introduction of monomers (GMA, BA or AA) with lower glass transition temperatures (Tg) than MMA leads to a more plastic material with lower elastic limit and better damage resistance. As expected, the viscoelastic properties of these co-monomers avoided the cracking in the self-assembled samples, attaining homogeneous and stable films of areas similar to those of the confined geometries selected to carry out the evaporation process (Fig. 5b). But for the same reason, upon drying the solutions, the deposited NPs becomes fused together resulting in a dense amorphous network (Fig. 5c) with a refractive index contrast clearly reduced, giving place to translucid samples in which the excitation of RL is impaired,36 even for NPs as large as 115 nm. On the contrary, equally large NPs, but with a less plastic composition, give place to cracked, but highly opaque (Fig. 5b), samples with well-defined NPs (Fig. 5c), in which RL is readily obtained (Fig. 5a). These results indicate that, in order to further improve the assembling of dye-doped cationic NPs to support laser emission, the polymeric composition has to be such that the best compromise between refractive index contrast and viscoelastic properties of the medium are attained.
4. Conclusions
In summary, the present work has provided a comprehensive analysis of the photophysical and laser properties of Rhodamine 6G doped cationic NPs in colloidal suspensions and the random laser properties of photonic materials based on self-assembled dye-doped cationic NPs. To the best of our knowledge, it is the first time that the effects of compositional and morphological parameters such as dye concentration, weigh proportion of NPs in the solution, NPs size, dielectric constant of the surrounding medium, monomeric composition, and surfactant structure, have been assessed. In general terms, such cationic framework ameliorates the fluorescence and laser performance of Rh6G with respect to anionic NPs. The photophysical properties of all the synthesized cationic NPs are similar, with the highest differences having been observed when changing the size of the NP, and, in lower extent, the monomer and surfactant compositions. The laser properties are more sensitive to compositional changes – overall due to an increase in the size of the NPs – since laser efficiencies from 50% to 0% (no lasing) are measured. Once dried, the self-assembled NPs only show RL emission in the cases were a less plastic monomeric composition is used, as otherwise the NPs become fused together resulting in a dense amorphous network with a refractive index contrast clearly reduced.Hence, to reach the full potential of these dye-doped NPs as biomarkers, a compromise must be reached between the composition and morphology of the system that optimizes their biological behaviour with those promoting their emission properties. From the point of view of their photonic behaviour, the best records, considering emitting efficiency and stability, are achieved with NPs 20–40 nm in diameter, based on linear-structured (no-globular) surfactants and on copolymers (no homopolymers) of MMA with more polar monomers such as GMA and BA, affording high dye concentrations inside the NPs as well as high concentrations of NPs in the medium. These results are critical in building the knowledge foundations required to design dye-doped cationic NPs to be efficiently and specifically used in laser and bioimaging applications.
Acknowledgements
This work was supported by Projects TRACE2009-0144 and MAT2010-20646-C04-01 and -04 of the Spanish Ministerio de Economía y Competitividad (MINECO) and IT339-10 of the Gobierno Vasco. L. Gartzia thanks the Gobierno Vasco for a predoctoral fellowship.Notes and references
- K. Riehemann, S. W. Schneider, T. A. Luger, B. Godin, M. Ferrari and H. Fuchs, Angew. Chem., Int. Ed., 2009, 48, 872 CrossRef CAS PubMed.
- D. Hanahan and R. A. Weinberg, Cell, 2000, 100, 57 CrossRef CAS.
- M. Ferrari, Nat. Nanotechnol., 2006, 1, 8 CrossRef PubMed.
- G. Neuert, C. Albrecht, E. Pamir and H. E. Gaub, FEBS Lett., 2006, 580, 505 CrossRef CAS PubMed.
- D. K. Maurya, N. Y. Ng, K. A. Mahabadi, Y. N. Liang and I. Rodriguez, Biotechnol. J., 2007, 2, 1381 CrossRef CAS PubMed.
- K. Kato, M. Toda and H. Iwata, Biomaterials, 2007, 28, 1289 CrossRef CAS PubMed.
- See, for example, catalogs from Molecular Probes, Thermo Scientific and Bangs Laboratories Inc.
- X. Hong, Z. Wang, J. Yang, Q. Zheng, S. Zong, Y. Sheng, D. Zhu, C. Tang and Y. Cui, Analyst, 2012, 137, 4140 RSC.
- A. Palma, L. A. Alvarez, D. Scholz, D. O. Frimannsson, M. Grossi, S. J. Qinn and D. F. O'Shea, J. Am. Chem. Soc., 2011, 133, 19618 CrossRef CAS PubMed.
- A. Monguzzi, M. Frigoli, C. Larpent and F. Meinardi, RSC Adv., 2012, 2, 11731 RSC.
- L. Cerdán, E. Enciso, V. Martin, J. Bañuelos, I. López-Arbeloa, A. Costela and I. García-Moreno, Nat. Photonics, 2012, 6, 621 CrossRef.
- E. Enciso, A. Costela, I. García-Moreno, V. Martín and R. Sastre, Langmuir, 2010, 26, 6154 CrossRef CAS PubMed.
- M. R. Lorenz, V. Holzapfel, A. Musyanovych, K. Nothelfer, P. Walther, H. Frank, K. Landfester, H. Schrezenmeier and V. Mailänder, Biomaterials, 2006, 27, 2820 CrossRef CAS PubMed.
- L. Wang and W. Tan, Nano Lett., 2006, 6, 84 CrossRef CAS PubMed.
- M. A. Iannone, T. G. Consler, K. H. Pearce, J. B. Stimmel, D. J. Parks and J. G. Gray, Cytometry, 2001, 44, 326 CrossRef CAS.
- V. Martin, J. Bañuelos, E. Enciso, I. Lopez Arbeloa, A. Costela and I. García-Moreno, J. Phys. Chem. C, 2011, 115, 3926 CAS.
- S. Kedia, R. Vijaya, A. K. Ray and S. Sinha, J. Nanophotonics, 2010, 4, 049506 CrossRef PubMed.
- H. Ow, D. R. Larson, M. Srivastava, B. A. Baird, W. W. Webb and U. Wiesner, Nano Lett., 2005, 5, 113 CrossRef CAS PubMed.
- L. Cerdán, L. Gartzia-Rivero, E. Enciso, J. Bañuelos, I. Lopez Arbeloa, A. Costela and I. García-Moreno, Laser Phys. Lett., 2014, 11, 015901 CrossRef.
- L. Zhang, F. X. Gu, J. M. Chan, A. Z. Wang, R. S. Langer and O. C. Farokhzad, Clin. Pharmacol. Ther., 2008, 83, 761 CrossRef CAS PubMed.
- J. Ramos, J. Forcada and R. Hidalgo-Alvarez, Chem. Rev., 2014, 114, 367 CrossRef CAS PubMed.
- Z.-G. Yue, W. Wei, P.-P. Lv, H. Yue, L.-Y. Wang, Z.-G. Su and G.-H. Ma, Biomacromolecules, 2011, 12, 2440 CrossRef CAS PubMed.
- J. Ramos, J. Forcada and R. Hidalgo-Alvarez, Chem. Rev., 2014, 114, 367 CrossRef CAS PubMed.
- J. Li and A. Alexander-Katz, ACS Nano, 2013, 12, 10799 Search PubMed.
- S. Li and N. Malmstadt, Soft Matter, 2013, 9, 4969 RSC.
- D. Nagao, N. Anzai, Y. Kobayashi, S. Gu and M. J. Konno, J. Colloid Interface Sci., 2006, 298, 232 CrossRef CAS PubMed.
- F. Lopez Arbeloa, T. Lopez Arbeloa and I. Lopez Arbeloa, Handbook of Advanced Electronic and Photonic Materials and Devices, Academic Press, New York, 2001, vol. 7, pp. 209–245 Search PubMed.
- L. Cerdán, A. Costela, E. Enciso and I. Garcia-Moreno, Adv. Funct. Mater., 2013, 23, 3916 CrossRef.
- A. Costela, I. García-Moreno, L. Cerdán, V. Martin, O. García and R. Sastre, Adv. Mater., 2009, 21, 4163 CrossRef CAS.
- L. Cerdán, A. Costela, I. García-Moreno, V. Martín and M. E. Pérez-Ojeda, J. Quantum Electron., 2011, 47, 907 CrossRef.
- J. Krause, M. Imlau, T. Woike and D. Schaniel, Opt. Mater. Express, 2012, 2, 71 CrossRef CAS.
- X. Wu, A. Yamilov, H. Noh, H. Cao, E. W. Seelig and R. P. H. Chan, J. Opt. Soc. Am. B, 2004, 21, 159 CrossRef CAS.
- S. Gottardo, R. Sapienza, P. D. García, A. Blanco, D. S. Wiersma and C. López, Nat. Photonics, 2008, 2, 429 CrossRef CAS.
- Y. Chen, J. Herrnsdorf, B. Guilhabert, Y. Zhang, A. L. Kanobolotsky, P. Skabara, E. Gu, N. Laurand and M. D. Dawson, Org. Electron., 2012, 13, 1126 Search PubMed.
- J. F. Galisteo-López, M. Ibisate, R. Sapienza, L. S. Froufe-Pérez, A. Blanco and C. López, Adv. Mater., 2011, 23, 30 CrossRef PubMed.
- J. Yi, G. Feng, L. Yang, K. Yao, C. Yang, Y. Song and S. Zhou, Opt. Commun., 2010, 285, 5276 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |