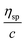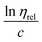Solvent optimization for bacterial extracellular matrices: a solution for the insoluble†
Thomas Seviour*a,
Piyarat Weerachanchaibc,
Jamie Hinksa,
Dan Roizmana,
Scott A. Ricead,
Linlu Baic,
Jong-Min Lee*c and
Staffan Kjellebergae
aSingapore Centre on Environmental Life Sciences Engineering (SCELSE), Nanyang Technological University, SBS-01N-27, 637551, Singapore. E-mail: twseviour@ntu.edu.sg; Fax: +65 6515 6751; Tel: +65 6592 7902
bNanyang Environment and Water Research Institute (NEWRI), Nanyang Technological University, Singapore
cSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 637459, Singapore. E-mail: jmlee@ntu.edu.sg; Fax: +65 6794 7553; Tel: +65 6513 8129
dSchool of Biological Sciences (SBS), Nanyang Technological University, 637551, Singapore
eCentre for Marine BioInnovation and School of Biotechnology and Biomolecular Sciences, University of New South Wales, Sydney, NSW 2052, Australia
First published on 19th December 2014
Abstract
Microbial biofilm systems are of industrial, environmental and medical concern. The existence of a structured matrix of extracellular polymeric substances (EPS) distinguishes biofilms from other bacterial communities. We contend that a lack of a cohesive framework for achieving solubilization of biofilm matrices contributes to suboptimal biofilm control strategies and a rudimentary understanding of important extracellular processes, such as cell–cell signaling and horizontal gene transfer. Here, we demonstrate that ionic liquids enable nonpolar systems for biofilm dissolution and allow the solubility parameter concept to be applied to a range of biofilms to identify optimum solvents. Solubilization was measured in terms of intrinsic solute viscosity (η), and Hildebrand solubility parameters (δ) for Pseudomonas aeruginosa rugose small colony variant biofilms and two distinct types of activated sludge biofilms were determined to be 24.8, 26.0 and 25.8 MPa1/2 respectively. Chromatographic separation of the matrix components of each biofilm was achieved in a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v blend of 1-ethyl-3-methylimidazolium acetate in N,N-dimethylacetamide, with partitioning of individual molecular weight fractions of each biofilm into the mobile phase accompanied by clear chromatographic peaks. While each biofilm may require its own specific solvent mixture, the work presented here provides a conceptual framework to enable the identification of that solvent mixture which will ultimately allow for the fractionation, isolation and characterization of hitherto intractable biofilm polymers.
60 v/v blend of 1-ethyl-3-methylimidazolium acetate in N,N-dimethylacetamide, with partitioning of individual molecular weight fractions of each biofilm into the mobile phase accompanied by clear chromatographic peaks. While each biofilm may require its own specific solvent mixture, the work presented here provides a conceptual framework to enable the identification of that solvent mixture which will ultimately allow for the fractionation, isolation and characterization of hitherto intractable biofilm polymers.
Introduction
Most microorganisms can self-assemble to form structures called biofilms by secreting extracellular polymeric substances (EPS) that bind them to each other and to solid substrata.1 Biofilms are prominent in many engineered and natural habitats,2 and allow cells the benefits of communal living, including mutualistic interactions, cell–cell communication,3 protection against predators,4 and resistance to toxic compounds.5Central to the concept of biofilms is the existence of an extracellular matrix. This is largely comprised of polymers including polysaccharides, protein adhesins and eDNA, among other compounds.6–8 Because of its physical and chemical properties, the extracellular matrix can function as an extension of the cell in which key processes take place. Horizontal gene transfer,9 pre-processing of complex substrates, and their detoxification,10 and trafficking of cell–cell signaling molecules11 are all mediated by the extracellular matrix. Nevertheless, our current understanding of this matrix is rudimentary. This contrasts with the detailed and clear descriptions that exist for intracellular processes, functional compartmentalization, molecular organization and intermolecular interactions.12 The secretion of extracellular polymeric substances (EPS) likely reflects an adaptive response by cells to extracellular conditions.13,14 Yet the extent to which microbially mediated processes are regulated extracellularly is unknown. Furthermore, our understanding of interactions between organization and compartmentalization of individual EPS components is limited to general and non-specific electrostatic interactions and hydrogen bonds.15
Certain EPS have been isolated from biofilms and their structure–function relationships described. They include granulan, which is found as antiparallel polysaccharide double helices stabilized by complementary hydrogen bonds and builds the matrix of activated sludge biofilms.16 However, characterizations of EPS from environmental and clinical biofilms still suffer from uncertainty about how best to solubilize them for detailed structural studies. While some biofilms appear to be fully soluble in alkali aqueous solutions,17 others such as those of Pseudomonas aeruginosa and certain activated sludge granular biofilms are only partially soluble in aqueous solvents.18,19 Direct compositional and functional analyses of biofilm EPS are therefore biased towards constituents that are soluble in aqueous solvents. Biofilms, almost by definition, however, are poorly soluble in aqueous solvents, and therefore many structurally important biofilm EPS are being overlooked.
P. aeruginosa is a model organism for studying biofilm formation and the EPS matrix.20 Its extracellular matrix is believed to consist of three polysaccharides, Pel, Psl and alginate.18,21 It also contains a large protein CdrA of 150 kDa,8 functional amyloid of Pseudomonas fibrils22 and filamentous phage.23 Yet, even for P. aeruginosa biofilm EPS, detailed information on the less soluble components is lacking. Friedman and Kolter discovered two genetic loci in P. aeruginosa (strain ZK2870) responsible for encoding carbohydrate-rich compounds (i.e. Pel and Psl).18 To the authors' best knowledge, no method for purifying Pel has been published and there is no structural information about it beyond it having a high-glucose content.24 While a structure for Psl has been published,21 the size fraction of Psl that was isolated and characterized was selected on the basis of higher spectral resolution, which results from increased solubility25 and it is clear that there are other components of Psl which remain unpurified, limiting a complete understanding of the characteristics of this EPS component. The size and structure of the extracellular protein adhesin CdrA render it insoluble and descriptions of CdrA are currently limited to theoretical studies based on its genetic homology to other adhesion proteins.8,26
Despite the wide-spread use of P. aeruginosa as a model system, low solubility has restricted establishing structure–function relationships for many of its EPS to indirect approaches (e.g. genetic knockdowns) rather than from the direct characterization of biofilm-isolated polymers. This limitation is also encountered with other biofilms, particularly for multi-species biofilms that are important in natural, medical and industrial settings, mediating, for example, complex biodegradation processes (e.g. activated sludge) and resistance to antimicrobials.27,28
We submit that poor biofilm solubility is a major obstacle to establishing structure–functional relationships for biofilm EPS. However, solubility is a function of interactions between solute (i.e. biofilm) and solvent.29 It may therefore be possible to make soluble biofilm constituents otherwise considered insoluble from their behavior in aqueous solvents. For example, cellulose is completely insoluble in water yet soluble in some organic solvents and ionic liquids.30 In this study, a method is established that selects for solvents that achieve maximum biofilm solubility, based on compatibility of solubility parameters (i.e. solubility parameter concept). We demonstrate complete biofilm solubilization for P. aeruginosa and two mixed microbial biofilms using nonpolar solvents and ionic liquids, and show that this can be used as the basis for subsequent purifications, such as separating extracellular and intracellular storage polysaccharides, and to fractionate high molecular weight biofilm constituents.
Results and discussion
Designer solvents achieve high solubility of biofilms
Our current understanding of biofilm solubility is based on their behavior in aqueous solvents. For the purposes of demonstrating a method for optimizing solvent selection, three biofilms were selected as representatives of different degrees of dissolution under mildly alkaline conditions. These were (1) pellicles formed by a rugose small colony variant (RSCV) of Pseudomonas aeruginosa, chosen on the basis of its high EPS production and partial solubility in alkali aqueous solvents (Fig. S1B†), a feature characteristic of P. aeruginosa biofilms,18 (2) Glycogen Accumulating Organism (GAO) – enriched activated sludge granules, that displays full solubility in a mild sodium hydroxide solution (pH > 9.5) as reported in Seviour et al.31 (Fig. S2B†), and (3) activated sludge granules enriched for Denitrifying Polyphosphate-Accumulating Organisms (DPAO), shown in preliminary studies to be completely insoluble in sodium hydroxide (Fig. S3B†). Additionally, GAOs and DPAOs store glycogen intracellularly and hence biofilms 2 and 3 were chosen to investigate whether intra- and extracellular polysaccharides can be separated on the basis of differential solubility.32A range of organic solvents and ionic liquids were initially screened for their ability to solubilize biofilms. Ionic liquids are “green” alternatives to organic solvents with inherent diversities that allow them to be blended and fine-tuned to optimize EPS yield, selectivity and substrate solubility.33 They are increasingly being applied as solvents for recalcitrant polysaccharides, including chitosan and cellulose.34–36 Samples were placed in the different solvents and a visual assessment of their solubility was made based on conversion of the biofilm from a solute into the solvent.
P. aeruginosa pellicle biofilms separated into two clear phases, biofilm (or solute) and solvent, at t = 0 (Fig. S1†) in the solvents tested, with the biofilm either separating to the bottom and surface of the solvent (e.g. butanol), dispersed throughout the solvent to give a turbid appearance (e.g. bicarbonate solution), or both (e.g. 1-ethyl-3-methylimidazolium acetate, or EMIM-Ac).
There was no visible change in the state of the P. aeruginosa pellicle biofilm following 3 d immersion at 50 °C in phosphate buffer solution (PBS) pH 7 (Fig. S1A†), Luria Bertani (LB) broth (Fig. S1C†) or 1-butanol (Fig. S1E†). Thus, biofilms were classified as having very low solubility in these solvents. After immersion in N,N-dimethylacetamide (DMAc), there was a slight coloration of the solvent concomitant with decolorization of the biofilm, indicating some transfer from solute to solvent (i.e. low solubility). While there was a visible reduction in biofilm volume following immersion in ethanolamine (Fig. S1D†), along with coloration of the solvent, there were still traces of undissolved biofilm (i.e. medium solubility). However, following immersion in 1-ethyl-3-methylimidazolium acetate (EMIM-Ac) (Fig. S1G†) and 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v EMIM-Ac/DMAc (Fig. S1H†), there were no signs of undissolved biofilm, and at the same time there was a darkening of the solvent and increase in solvent viscosity, suggestive of full biofilm dissolution (i.e. high solubility).
60 v/v EMIM-Ac/DMAc (Fig. S1H†), there were no signs of undissolved biofilm, and at the same time there was a darkening of the solvent and increase in solvent viscosity, suggestive of full biofilm dissolution (i.e. high solubility).
Similar results were observed for the other two biofilm types (Table S1†). Image-based analysis (Fig. S2 and S3†) indicated the degrees of solubilization of GAO-enriched and DPAO-enriched granular biofilms.
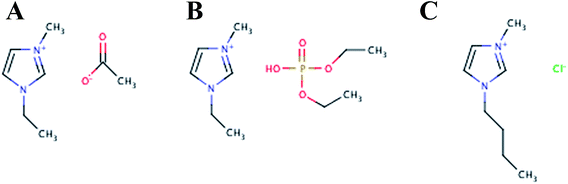
In addition to EMIM-Ac, the ionic liquids 1-ethyl-3-methylimidazolium diethyl phosphate (EMIM-DEP) and 1-butyl-3-methylimidazolium (BMIM-Cl) were shown here to be suitable for solubilizing all three biofilms. This is consistent with what has been observed for the recalcitrant polysaccharides chitin and cellulose.37,38 Representative structures of these ionic liquids are presented in Fig. 1. As with cellulose and chitin, some dissolution of all three biofilms was also observed in DMAc.39 Ethanolamine also achieved moderate solubilization, but most ionic liquids and organic solvents tested achieved negligible solubilization. For glycogen, complete solubilization was achieved in additional solvents including dimethyl sulfoxide, dimethylformamide, 2-pyrollidone and allyl alcohol.
An antimicrobial effect of ionic liquids was reported by Ruegg et al., who attributed their inhibition of microbial growth to membrane permeabilization or extreme osmotic shock.40 Ionic liquid-treated P. aeruginosa PAO1 RSCV cells in our study were reduced in diameter by 12% compared to cells in the growth media (LB), from 1.21 ± 0.142 μm to 1.07 ± 0.16 μm diameter, compared to 1.26 ± 0.19 μm diameter in PBS (Fig. 2A and B respectively), suggesting osmotic stress.41,42 A high level of the lipopolysaccharide compound 2-keto-3-deoxyoctonate was observed following exposure of the biofilms accompanying ionic liquid treatment of cells (0.005 μg per cell), consistent with cell lysis.19
Dissolving biofilms with ionic liquids may therefore result in contamination of recovered EPS with intracellular constituents, depending on whether these intracellular constituents are also soluble in the ionic liquids used. For example, glycogen is soluble in EMIM-Ac and EMIM-DEP (Table S1†), and an ionic liquid-based EPS extraction protocol for biofilms comprising glycogen-accumulating cells (i.e. GAO-enriched and DPAO granules) will probably have to contend with glycogen removal as well as other polymeric intracellular constituents. Nonetheless, EPS solubilization is an absolute precondition for any subsequent chemical analyses. An understanding of differential solubilities will inform how to distinguish intra- and extracellular molecules.
Determination of biofilm solubility parameters
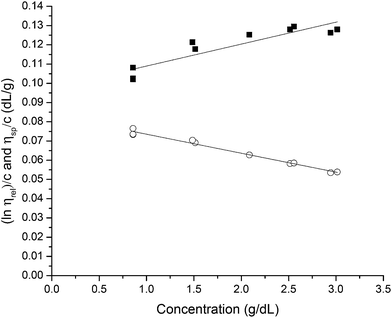
The solubility parameter concept states that two materials with corresponding solubility parameters will be miscible within each other due to balancing molecular forces.43 This parameter can be used to select the best solvent for any polymer networks, such as biofilms, however it is only applicable to nonpolar systems. Ionic liquids therefore enable the application of the solubility parameter concept to identify the optimal solvents for the three representative biofilms. For the three biofilm in this study we used the Huggins constant (kH), Kraemer constant (kK) and Hildebrand solubility parameter (δ) to illustrate how to match solute to solvent:EPS transfer from solute (i.e. biofilm) into solvent was measured as an increase in solvent viscosity. There is a direct correlation between the amount of biomacromolecule solubilized and viscosity.44 Viscosity is thus a functional output that reflects the extent of solubilization of important biofilm constituents. Specific viscosity (ηsp) and relative viscosity (ηrel) were then calculated at each concentration in solvents spanning the solubility parameter range (i.e. mg dry solid/mL solvent) and the Huggins and Kraemer relationships plotted (eqn (1) and (2)). This is illustrated for P. aeruginosa RSCV pellicle biofilm in EMIM-Ac/ethanolamine, with ηsp/c and (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ηrel)/c plotted versus concentration (Fig. 3). Thus, from the common intercept of the Huggins and Kraemer relationships, [kH], and [kK] for P. aeruginosa RSCV pellicle biofilm dissolved in EMIM-Ac/ethanolamine are 1.9 and 1.8, respectively.
ηrel)/c plotted versus concentration (Fig. 3). Thus, from the common intercept of the Huggins and Kraemer relationships, [kH], and [kK] for P. aeruginosa RSCV pellicle biofilm dissolved in EMIM-Ac/ethanolamine are 1.9 and 1.8, respectively.
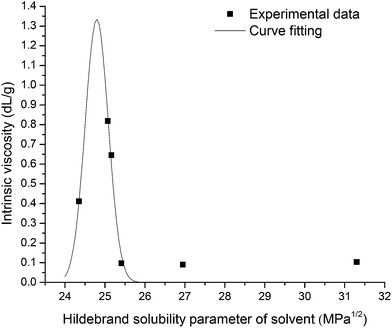
Linear Huggins and Kraemer relationships were observed for all samples, indicating that aggregation of EPS was not occurring.45 Fig. 4 shows the plot of intrinsic viscosity [η] of P. aeruginosa RSCV pellicle biofilm in the different solvents as a function of [δsolvent] values ([δsolvent] ranging from 24.35 to 31.30 MPa1/2). The Hildebrand regular solution theory states that the plot of [η] against [δsolvent] should be a smooth curve. Thus, as the midpoint of the solubility parameter range, [δsample] of P. aeruginosa RSCV pellicle biofilm is 24.8 (Fig. 4).
[kH], [kK] and [η] values of biofilms in the various solvents investigated are summarized in Table 1. The ability for a biofilm to be dissolved by a solvent is a precondition for determining solubility parameters and therefore limits the spread of results for data fitting purposes. Only five of the twenty-three solvents achieved any biofilm dissolution (Table S1†). A clear [ηmax] was observed for all biofilms, however, suggesting [δsample] values for the RSCV pellicle, GAO-enriched granular and DPAO granular biofilms of approximately 24.8, 26.0 and 25.8 MPa1/2 respectively. Irregularities in the relationship between [η] and [δsample] probably arose from the confounding effects of hydrogen bonding between solvent and solute.45 Solute [η] is proportional to intermolecular forces between solute and solvent. Hence, the solvent of greatest solute intrinsic viscosity provides for greatest dissolution.46 [kH] has also been found to decrease with increasing solvent power,47 which supports the results from this study with [ηmax] also corresponding to minima in [kH] (Table 1). Thus, the ionic liquid or the ionic liquid–organic solvent blend that gives either the highest intrinsic viscosity or lowest [kH] could be the best solvent for biofilm dissolution.
| Solvent | δsolvent | kH | kK | η |
|---|---|---|---|---|
| P. aeruginosa RSCV pellicle biofilm | ||||
| Bmim-Cl/DMAc | 24.35 | 0.52 | 0.07 | 0.41 |
Emim-AC/DMAc (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
25.07 | 0.25 | 0.16 | 0.82 |
| Emim-Ac | 25.16 | 0.79 | −0.06 | 0.65 |
| Emim-DEP | 25.41 | 6.93 | −5.19 | 0.10 |
Emim-Ac/ethanolamine (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
26.95 | 1.93 | 1.80 | 0.09 |
| Ethanolamine | 31.30 | 4.75 | −3.01 | 0.10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| GAO-enriched granular biofilm | ||||
Bmim-Cl/DMAc (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v:v) 40, v:v) |
24.35 | 0.37 | 0.10 | 0.66 |
Emim-AC/DMAc (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v:v) 40, v:v) |
25.07 | 0.50 | 0.09 | 1.53 |
| Emim-Ac | 25.16 | 0.27 | 0.15 | 1.85 |
| Emim-DEP | 25.41 | 0.21 | 0.19 | 1.33 |
Emim-Ac/ethanolamine (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
26.95 | 0.79 | 0.06 | 1.37 |
| Ethanolamine | 31.30 | 0.59 | 0.08 | 0.27 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| DPAO granular biofilm | ||||
Bmim-Cl/DMAc (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
24.35 | 0.85 | −0.12 | 1.00 |
Emim-AC/DMAc (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
25.07 | 0.72 | 0.03 | 1.79 |
| Emim-Ac | 25.16 | 29.27 | 1.04 | 0.15 |
| Emim-DEP | 25.41 | −0.03 | −0.92 | 1.67 |
Emim-Ac/ethanolamine (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
26.95 | 0.14 | 0.23 | 1.31 |
| Ethanolamine | 31.30 | 21.23 | −13.55 | 0.22 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Glycogen | ||||
Bmim-Cl/DMAc (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
24.35 | 8.24 | 7.89 | 0.17 |
Emim-AC/DMAc (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
25.07 | 0.07 | 0.34 | 1.01 |
| Emim-Ac | 25.16 | 0.75 | 0.74 | 0.97 |
| Emim-DEP | 25.41 | 0.92 | 0.94 | 0.82 |
Emim-Ac/ethanolamine (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) 40, v/v) |
26.95 | 1.22 | 1.47 | 0.53 |
| Ethanolamine | 31.30 | 18.77 | 10.61 | 0.04 |
Solubilization by ionic liquids provides separation of extra- and intracellular biofilm polysaccharides
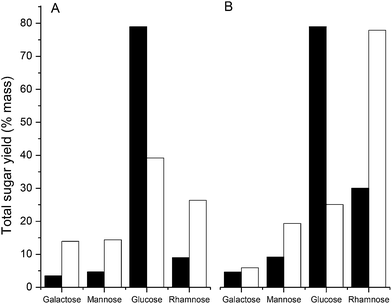
As discussed above, an ionic liquid-based EPS solubilization may also need to differentiate between the extracellular matrix and intracellular polysaccharide polymers observed as a consequence of cell lysis. For example, glycogen, present as an intracellular storage polysaccharide in the GAO-enriched granular and DPAO granuar biofilms, is soluble in a wide range of ionic liquids and organic solvents (Table S1†). Based on the Hildebrand regular solution theory, glycogen has a [δsample] value of 25.8 MPa1/2, which could partially explain the values of the GAO-enriched and DPAO granular biofilms, as these are of similar magnitude (26.0 and 25.8 MPa1/2 respectively). High glucose levels were recorded in both DPAO and GAO-enriched granules relative to RSCV pellicle biofilms, consistent with the presence of intracellular glycogen, a multi-branched polymer of glucose monomers (Fig. 5). Treating GAO-enriched and DPAO granules with ionic liquids will therefore result in a mixture of solubilized EPS and intracellular storage glycogen.Glycogen is soluble in water (Table S1†), suggesting that water can be used as an anti-solvent to separate glycogen from the EPS. To illustrate how understanding solubilities in organic and aqueous solvents can be used to segregate EPS from intracellular storage polymers, GAO-enriched and DPAO granules were treated with EMIM-Ac and water as solvent and anti-solvent respectively. Galactose, rhamnose, glucose and mannose contents of GAO-enriched and DPAO granular biofilms were measured before and after treatment with EMIM-Ac and water (Fig. 5). Galactose, rhamnose, glucose and mannose have been demonstrated as major EPS constituents in GAO-enriched granular biofilms.48 Treatment with EMIM-Ac and water substantially enriched the galactose, mannose and rhamnose contents in both biofilms and a significant reduction in the concentration of glucose detected. While it is possible that some water soluble, glucose-rich extracellular polysaccharides were also removed, given the high glycogen content of the DPAO- and GAO-enriched granules,49,50 the reduction in glucose is likely explained by the fact that both glycogen and biofilm EPS were brought into solution by EMIM-Ac but that only the glucose remained in solution in water while the EPS components were precipitated by the addition of water. Thus, the combination of ionic liquid and water solubilization can be used to differentiate between EPS and intracellular polysaccharide biopolymers.
Biofilm solubility parameter determination identifies the appropriate solvent for constituent fractionation and isolation
The solvent capabilities of ionic liquids EMIM-DEP and EMIM-Ac are illustrated above. Coupled with their other properties, including high thermal/chemical stability and wide liquid range, these ionic liquids offer a means to isolate EPS previously overlooked due to low solubility. However, there are challenges to using ionic liquids to purify high molecular weight macromolecules. These include their high cost and viscosity. Ionic liquids are 2–3 times more viscous than organic solvents, which precludes their use as mobile phases in liquid chromatography.51The [δsolvent] of ionic liquid mixtures does not correlate linearly with their concentration, and therefore does not follow Kay's mixing rule.52 The [δsolvent] values of ionic liquid–organic solvent mixes tend to be closer to those of the ionic liquid than the organic solvent. According to the solubility parameter concept it should therefore be possible to maintain the [δsample] values of ionic liquid-based solvents by blending these with a high fraction of compatible organic solvent to reduce the cost and viscosity without compromising EPS solubility.
Based on maximum intrinsic viscosities, EMIM-Ac/DMAc is the best solvent for all three biofilms used in this study (Table 1). According to the solvent compatibility concept, EMIM-Ac/DMA is optimum for RSCV pellicle and EMIM-DEP for GAO-enriched and DPAO granular biofilms. [kH] is another measure of polymer–solvent interactions, with lower [kH] indicating a higher degree of solvent–solute interactions and hence solvent compatibility. On this basis the prediction of optimum solvent for these biofilms based on [kH] values supports the prediction obtained by solvent compatibility. Matching solvent to biofilms on the basis of their solubility parameters will thus likely result in a range of solvents being identified as optimum for different biofilms.
Gel permeation chromatography (GPC) was used here to determine whether the increase in viscosity elicited by the transfer of EPS from biofilm into solution (Fig. 3) promoted the solvation of new constituents, and to observe whether these constituents could be resolved chromatographically. This would indicate the suitability of GPC coupled with an ionic liquid-based mobile phase in subsequent studies for isolating and characterizing key structural extracellular polymers, including those previously ignored due to low solubility. EMIM-Ac/DMAc was selected as the most appropriate mobile phases of those tested (with chromatography-compatible viscosities) for fractionating the RSCV pellicle biofilm based on matching [δsolvent] and [δsample] values.
Contrary to the prediction based on the non-linear correlation between [δsolvent] and ionic liquid content, incomplete dissolution of all biofilms tested here was observed at EMIM-Ac fractions less than 40% v/v. This is likely due to changes in polar and hydrogen-bonding interactions with the increasing organic solvent content.44 To ensure that all constituents were included in the analysis, a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 blend of EMIM-Ac and DMAc was used as the mobile phase for determining the molecular weight (MW) distribution by GPC.
60 blend of EMIM-Ac and DMAc was used as the mobile phase for determining the molecular weight (MW) distribution by GPC.
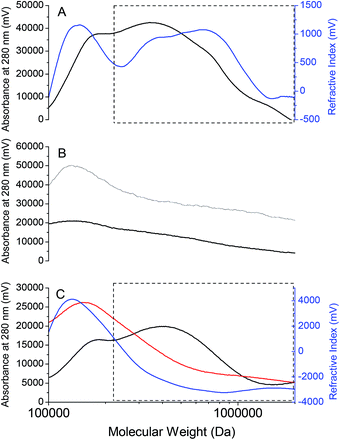
A representative MW profile of an RSCV pellicle biofilm solubilized in 40% vol/vol EMIM-Ac in DMAc in Fig. 6A shows that there are probably four chromatographic peaks corresponding to distinct MW fractions. These appeared at 1.0 × 106, 7.0 × 105, 3.5 × 105 and 1.0 × 105 Da, as referenced against MW standards of pullulan, a polysaccharide of maltotriose commonly used to determine MW distribution of microbial exopolymers.53 Based on overlapping UV absorbance (at 280 nm) and Refractive Index (RI) signals it is likely that all constituents contain some amino acids. This would suggest that the dominant materials are not polysaccharides although they could still be glycoproteins or peptidoglycans.17 Therefore, biofilm solubilization by an ionic liquid–organic solvent blend selected on the basis of compatibility of solubility parameters allows for MW fractionation of P. aeruginosa RSCV pellicle biofilm EPS, and subsequently isolation of individual EPS constituents.
The MW peaks at 1.0 × 106, 7.0 × 105 and 3.5 × 105 Da were present at much lower intensities or were absent in the representative chromatograms and MW profiles of P. aeruginosa RSCV planktonic cells in 40% EMIM-Ac in DMAc, and RSCV pellicle biofilm in 10% EMIM-Ac in DMAc (Fig. 6B). Neither solubilization of free cells in 40% EMIM-Ac nor the RSCV pellicle biofilm in 10% EMIM-Ac elicited the increase in solvent viscosity seen with the RSCV pellicle biofilm in 40% vol/vol EMIM-Ac in DMAc. The low MW peak was observed in all RSCV samples and therefore probably corresponds to small hydrophobic molecules expressed by P. aeruginosa planktonic cells and biofilms alike that are weakly bound and partition easily into organic solvents. These include the redox mediator pyocyanin,54 quorum sensing molecules 2-heptyl-3-hydroxy-4-quinolone (i.e. Pseudomonas quinolone signal, or PQS),55 and the AHLs N-3-oxo-dodecanoyl-L-homoserine lactone and N-butanoyl-L-homoserine lactone.11 The higher MW peaks for RSCV pellicle biofilm solubilized in 40% vol/vol EMIM-Ac are therefore associated with the transfer of biofilm EPS into the solvent (i.e. transition from insoluble to soluble state). The MW fractions 1.0 × 106, 7.0 × 105 and 3.5 × 105 are all likely to be components of the RSCV pellicle biofilm EPS that can be fractionated and isolated. The absence of these peaks in the chromatogram of the planktonic cells indicates negligible contamination of the high MW content of the pellicle biofilm by intracellular constituents.
The chromatograms for the GAO-enriched and DPAO granular biofilms are presented in Fig. 6C. As with the RSCV pellicle biofilm, partitioning of the higher MW peaks (>3.0 × 105 Da) only occurred with 40% EMIM-Ac in DMAc as solvent (i.e. not 10% EMIM-Ac in DMAc). For the GAO-enriched granular biofilm, three clear chromatographic peaks were seen, with the high MW peak observable only by RI detection, indicating its polysaccharide nature. This outcome is identical to that observed for the same granules when analyzed using aqueous GPC (0.1 M NaOH).17 For the DPAO granular biofilms, a single broad peak appeared at approximately 1 × 106 Da with the transfer of biofilm EPS into solvent, confirmed by the increase in solvent viscosity. MW distributions of all three biofilms in DMAc, with no ionic liquid present show the complete absence of peaks corresponding to high MW compounds (i.e. > 2 × 105 Da) (Fig. S4†).
Comments
It is becoming clear that the biofilm matrix is not just a glue-like material that holds the biofilm cells together or allows for colonization of populations and communities on solid surfaces, but is an active extension of the cells, allowing reactions to proceed that are not achievable intracellularly because of toxicity or transport limitations. However, our understanding of how the matrix functions as an active biofilm component and what processes occur within is confounded by the likelihood that EPS expression has multiple secondary functions, including for example signaling communication.56 A shift towards direct functional assignment is therefore required, to complement the genetic methods currently used (e.g. knockdown methods). Some of the unanswered questions about the biofilm domain include the extent to which extracellular processes are regulated, the roles of beneficial compounds, and degrees of functional compartmentalization, arrangement of and interactions between different EPS components. To address these questions a molecular level understanding of EPS composition and interactions is required.Even for P. aeruginosa biofilms, a model biofilm organism with a well-characterized extracellular domain, most of the extracellular polysaccharides known to be synthesized have not been completely isolated and characterized from P. aeruginosa biofilms, including Pel and alginate which have only been recovered from agar plate colonies following a saline solution wash.18,57 We demonstrate here that it is possible to select solvents that can achieve full solubilization of biofilms by matching the biofilm solubility parameter with complementary ionic liquid–organic solvent blends. Furthermore, we illustrate the potential use of ionic liquid-based solvents to fractionate the constituents of the EPS from several different biofilms on the basis of their molecular weights.
Improved methods for biofilm solubilization and fractionation will benefit not only the study of extracellular polysaccharides but also extracellular protein adhesins. The low solubility of CdrA of P. aeruginosa and Fibrillar Hemagglutinin Adhesin protein of Bordetella pertussis has restricted their functional and structural assignment to computational methods and truncated forms only.8,58 Direct characterization of extracellular protein adhesins in their natural states will only be possible with improvements in the current isolation methods. Indeed, the three biofilm-associated MW constituents of P. aeruginosa RSCV pellicle biofilm detected in this study from GPC are all likely to be proteins, either free or bound to sugars as glycoproteins or proteoglycans, since each absorbed UV at 280 nm.17 While there are examples of extracellular polymers that have been isolated from biofilms,48 to achieve their successful isolation is still not routine practice with many biofilm EPS. Identifying the means to solubilize all biofilm components will enable the isolation of and direct functional assignment to a range of biofilm constituents including polysaccharides, proteins or other biomacromolecules not previously considered due to the perceived low solubility.
Mixed microbial communities can deliver complex tasks more efficiently and under more extreme conditions compared to monocultures.59–61 Considerable industrial benefit will emerge by understanding the EPS domain of mixed microbial biofilm systems, such as activated sludge, which represents the largest biotechnology industry in the world.28 Extracellular polymers are commonly cited as impairing hydraulic loading rates through solid–liquid separators in activated sludge systems.62,63 However, the absence of a unified approach for assessing activated sludge solubility limits our understanding of the roles of EPS across activated sludge systems, with only aqueous phase extraction techniques considered and solubilization methods largely empirically-derived.
There is a growing list of biological applications for ionic liquids, including fractionation and deconstruction of lignocellulosic biomass,64 primarily for microbial production of biofuels. The potential for 1-alkyl-3-methylimidazolium as an antimicrobial agent has been demonstrated.65 The work described here is the first to target specifically the EPS of biofilms. Enabling organic solvents has advantages in biopolymer separation by GPC by removing interactions between molecules and column packing material. Proteins typically contain charged moieties that interact ionically with surface-charged sites of stationary phases causing retention time shifts, contribute to peak tailing or asymmetry, and modify protein conformation.66
Describing the basis for achieving EPS solubilization and isolation, from single and mixed microbial biofilms is therefore an important step towards understanding composition and organization within the extracellular domain and thus improving the control of a wide range of industrial and environmental biofilm-based processes.
Experimental
Pseudomonas aeruginosa PAO1 pellicle biofilm
A Pseudomonas aeruginosa PAO1 strain, deficient in Pyoverdine expression was transformed to consecutively express a fluorescent eYFP-Gmr tag.67,68 A rugose small colony variant (RSCV) was then isolated from effluent of a 3 d culture of PAO1-PVD-eYFP biofilm fed minimal glucose-containing M9-media.69 Isolates of this PAO1-RSCV were grown and selected on Lysogeny Broth (LB) containing agar plates and stored in 20% glycerol-containing LB media at −80 °C.Pellicles of PAO1-RSCV were then grown in LB media at room temperature, initially as 10 mL aliquots in 50 mL tubes grown without agitation for 48 h until pellicle biofilms formed. These were transferred to 2 L Erlenmeyer bottles containing 400 mL of LB agar media each and grown for 10–12 d until a 2 cm-thick pellicle formed. Samples were collected and centrifuged for 10 min at 6000 rpm in a Beckman JLA-8.1 rotor at 10 °C, washed in PBS and consecutively centrifuged for 10 min at 8000 rpm in an Eppendorf tabletop centrifuge at 10 °C. Samples were then freeze-dried under vacuum for 48 h in a GAO-enriched media.
Candidatus ‘Competibacter phosphatis’ glycogen accumulating organism (GAO)-enriched activated sludge granules
GAO-enriched activated sludge granules were collected from a laboratory-scale sequencing batch reactor (SBR) operating in an enhanced biological phosphorus removal (EBPR) process configuration to treat abattoir wastewater. Refer to Yilmaz et al. and Lemaire et al. for reactor details.70,71 Average influent chemical oxygen demand (COD), total nitrogen (N) and total phosphorus (P) were approximately 600, 230 and 35 mg L−1 respectively. The exopolysaccharide content of these granules was attributed to Candidatus ‘Competibacter phosphatis’ GAO in Seviour et al.49Denitrifying polyphosphate accumulating activated sludge granules
Activated sludge granules were sampled from a laboratory-scale SBR treating synthetic wastewater and achieving stable EBPR, as described by Tan et al. The reactor operated with two continuous phases of feeding, anaerobic, and aerobic/anoxic periods over a 6 h cycle. Carbon was provided as a mixture of acetate and propionate (COD 200 mg L−1), with NH4–N and PO43−–P feed concentrations of 20 and 10 mg L−1 respectively.Sugar analysis
Glycosyl composition analysis was performed by combined gas chromatography/mass spectrometry (GC/MS) of the per-O-trimethylsilyl (TMS) derivatives of the monosaccharide methyl glycosides produced from the sample by acidic methanolysis.Between 300 μg and 500 μg of biofilm was used for the analysis. The samples were placed into test tubes and 20 μg of inositol was added. Methyl glycosides were then prepared from the dry samples by methanolysis in 1 M HCl in methanol at 80 °C (18 h), followed by re-N-acetylation with pyridine and acetic anhydride in methanol (for detection of amino sugars). The samples were then per-O-trimethylsilylated by treatment with Tri-Sil (Pierce) at 80 °C (0.5 h). These procedures were carried out as previously described in York et al.,72 and Merkle and Poppe.73 GC/MS analysis of the TMS methyl glycosides was performed on an Agilent 7890A GC interfaced to a 5975C MSD, using an Agilent DB-1 fused silica capillary column (30 m × 0.25 mm ID).
Epifluorescent microscopy
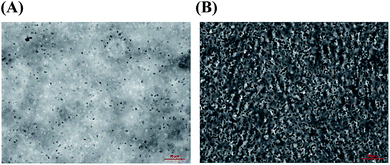
Images were taken with a ZEISS Z1 Axio-Observer Microscope, in phase contrast mode 3, under transmitted light using an EC Plan-Neofluar 100×/1.30 oil objective lens. Following freeze-drying, PAO1-RSCV samples were solubilized 50 mg in 750 μL of solvent each, in 60 °C, as follows: (A) PBS solution pH = 7.8; (B) PBS–NaOH solution at pH = 10; and (C) ionic liquid EMIM-acetate. Finally 20 μL of each, were applied to glass slides, and imaged. Image analysis was conducted using Zeiss Zen software, with average cell diameters determined from a random selection of 60 bacteria.Huggins, Kraemer and Hildebrand determination
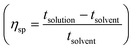 per concentration and natural logarithm of relative viscosity
per concentration and natural logarithm of relative viscosity 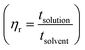 per concentration versus concentration (C; g dL−1). kH and kK are Huggins, and Kraemer constants, respectively. tsolution and tsolvent are the efflux times of solution and solvent, respectively.
per concentration versus concentration (C; g dL−1). kH and kK are Huggins, and Kraemer constants, respectively. tsolution and tsolvent are the efflux times of solution and solvent, respectively.
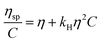 | (1) |
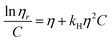 | (2) |
The Hildebrand solubility parameter (δ) for polymers is the mid-point of the solubility parameter range where the polymer is soluble. The [δ] values of solvents and solvent mixtures (i.e. δsolvent) were determined as described in Weerachanchai et al. [η] values derived from the biofilms dissolved in various solvents were plotted against [δsolvent] to obtain the [δ] values of the biofilms (i.e. δsample), which is equal to the [δsolvent] value corresponding to a maximum in intrinsic viscosity (ηmax).
The plot of [η] vs. [δsolvent] was then fitted by the Mangaraj equation (eqn (3)) to determine the Hildebrand solubility parameters of the samples at room temperatures (25 °C):
η = ηmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−A(δsolvent − δsample)2] exp[−A(δsolvent − δsample)2]
| (3) |
Gel permeation chromatography
Biofilms (20 mg) were dissolved in 1 mL of 40% v/v EMIM-Ac in DMAc and maintained at 50 °C for 8 h. Chromatographic separation was achieved in a Shimadzu system comprising DGU-20A 3r Prominence Degasser and LC-20AD Solvent Delivery Unit, fitted with an Agilent PLgel 10 μm column of 105 Å pore size for separation across the MW range 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 1
000 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700
700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da. The eluent flow rate was 0.5 mL min−1 and the sample injection volume was 20 μL, with detection by Shimadzu RID-10A Refractive Index detector and RF-20A XS Prominence fluorescence detector. Molecular weights are referenced against Pullulan 110, 400 and 800 kDa molecular weight standards (Sigma Aldrich).
000 Da. The eluent flow rate was 0.5 mL min−1 and the sample injection volume was 20 μL, with detection by Shimadzu RID-10A Refractive Index detector and RF-20A XS Prominence fluorescence detector. Molecular weights are referenced against Pullulan 110, 400 and 800 kDa molecular weight standards (Sigma Aldrich).
Acknowledgements
SCELSE is funded by Singapore's National Research Foundation, Ministry of Education, Nanyang Technological University (NTU) and National University of Singapore (NUS) and hosted by NTU in partnership with NUS. The Competitive Research Programme (NFR/CRP/5/2009/03) of National Research Foundation and Academic Research Fund (RGT27/13) of Ministry of Education in Singapore provided additional support for this project.Notes and references
- H.-C. Flemming and J. Wingender, Nat. Rev. Microbiol., 2010, 8, 623–633 CAS.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Nat. Rev. Microbiol., 2004, 2, 95–108 CrossRef CAS PubMed.
- K. L. Visick and C. Fuqua, J. Bacteriol., 2005, 187, 5507–5519 CrossRef CAS PubMed.
- A. Chavez-Dozal, C. Gorman, M. Erken, P. D. Steinberg, D. McDougald and M. K. Nishiguchi, Appl. Environ. Microbiol., 2013, 79, 553–558 CrossRef CAS PubMed.
- B. Halan, A. Schmid and K. Buehler, Appl. Environ. Microbiol., 2011, 77, 1563–1571 CrossRef CAS PubMed.
- I. W. Sutherland, Water Sci. Technol., 2001, 43, 77–86 CAS.
- E. S. Gloag, L. Turnbull, A. Huang, P. Vallotton, H. Wang, L. M. Nolan, L. Mililli, C. Hunt, J. Lu, S. R. Osvath, L. G. Monahan, R. Cavaliere, I. G. Charles, M. P. Wand, M. L. Gee, R. Prabhakar and C. B. Whitchurch, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 11541–11546 CrossRef CAS PubMed.
- B. R. Borlee, A. D. Goldman, K. Murakami, R. Samudrala, D. J. Wozniak and M. R. Parsek, Mol. Microbiol., 2010, 75, 827–842 CrossRef CAS PubMed.
- Y. Lee, S. EL Andaloussi and M. J. A. Wood, Hum. Mol. Genet., 2012, 21, R125–R134 CrossRef CAS PubMed.
- A. Ghosh, K. Chakrabarti and D. Chattopadhyay, Microbiology, 2009, 155, 2049–2057 CrossRef CAS PubMed.
- E. C. Pesci, J. B. J. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg and B. H. Iglewski, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 11229–11234 CrossRef CAS.
- M. T. Madigan, J. M. Martinko, K. S. Bender, D. H. Buckley and D. A. Stahl, Brock Biology of Microorganisms, Pearson, Boston, 2014 Search PubMed.
- T. J. Battin, W. T. Sloan, S. Kjelleberg, H. Daims, I. M. Head, T. P. Curtis and L. Eberl, Nat. Rev. Microbiol., 2007, 5, 76–81 CrossRef CAS PubMed.
- M. Dimopoulou, M. Vuillemin, H. Campbell-Sills, P. M. Lucas, P. Ballestra, C. Miot-Sertier, M. Favier, J. Coulon, V. Moine, T. Doco, M. Roques, P. Williams, M. Petrel, E. Gontier, C. Moulis, M. Remaud-Simeon and M. Dols-Lafargue, PLoS One, 2014, 9, e98898 Search PubMed.
- C. Mayer, R. Moritz, C. Kirschner, W. Borchard, R. Maibaum, J. Wingender and H.-C. Flemming, Int. J. Biol. Macromol., 1999, 26, 3–16 CrossRef CAS.
- T. Seviour, A. K. Malde, S. Kjelleberg, Z. Yuan and A. E. Mark, Biomacromolecules, 2012, 13, 1965–1972 CrossRef CAS PubMed.
- T. Seviour, B. C. Donose, M. Pijuan and Z. Yuan, Environ. Sci. Technol., 2010, 44, 4729–4734 CrossRef CAS PubMed.
- L. Friedman and R. Kolter, J. Bacteriol., 2004, 186, 4457–4465 CrossRef CAS PubMed.
- S. S. Adav and D.-J. Lee, J. Hazard. Mater., 2008, 154, 1120–1126 CrossRef CAS PubMed.
- M. Klausen, A. Aaes-Jørgensen, S. Molin and T. Tolker-Nielsen, Mol. Microbiol., 2003, 50, 61–68 CrossRef CAS.
- M. S. Byrd, I. Sadovskaya, E. Vinogradov, H. Lu, A. B. Sprinkle, S. H. Richardson, L. Ma, B. Ralston, M. R. Parsek, E. M. Anderson, J. S. Lam and D. J. Wozniak, Mol. Microbiol., 2009, 73, 622–638 CrossRef CAS PubMed.
- M. S. Dueholm, M. Søndergaard, M. Nilsson, G. Christensen, A. Stensballe, M. Overgaard, M. Givskov, T. Tolker-Nielsen, D. Otzen and P. H. Nielsen, Microbiology, 2013, 2, 365–382 CAS.
- M. J. Mooij, E. Drenkard, M. A. Llamas, C. M. J. E. Vandenbroucke-Grauls, P. H. M. Savelkoul, F. M. Ausubel and W. Bitter, Microbiology, 2007, 153, 1790–1798 CrossRef CAS PubMed.
- A. Ghafoor, I. D. Hay and B. H. A. Rehm, Appl. Environ. Microbiol., 2011, 77, 5238–5246 CrossRef CAS PubMed.
- S. Braun, H.-O. Kalinowski and S. Berger, 150 and More Basic NMR Experiments: a Practical Course, Wiley-VCH, Weinheim, 1998 Search PubMed.
- N. H. Tolia and L. Joshua-Tor, Nat. Methods, 2006, 3, 55–64 CrossRef CAS PubMed.
- S. P. Lopes, H. Ceri, N. F. Azevedo and M. O. Pereira, Int. J. Antimicrob. Agents, 2012, 40, 260–263 CrossRef CAS PubMed.
- Microbial Ecology of Activated Sludge, ed. R. J. Seviour and P. H. Nielsen, IWA Publishing, London, 2010 Search PubMed.
- S. H. Lee and S. B. Lee, Chem. Commun., 2005, 3469–3471 RSC.
- P. Weerachanchai and J.-M. Lee, ACS Sustainable Chem. Eng., 2013, 1, 894–902 CrossRef CAS.
- T. Seviour, M. Pijuan, T. Nicholson, J. Keller and Z. Yuan, Water Res., 2009, 43, 4469–4478 CrossRef CAS PubMed.
- Y. Zhou, M. Pijuan, R. J. Zeng, H. Lu and Z. Yuan, Water Res., 2008, 42, 2361–2368 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- H. Xie, S. Zhang and S. Li, Green Chem., 2006, 8, 630–633 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- P. Weerachanchai, S. S. J. Leong, M. W. Chang, C. B. Ching and J.-M. Lee, Bioresour. Technol., 2012, 111, 453–459 CrossRef CAS PubMed.
- J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC.
- P. S. Barber, C. S. Griggs, G. Gurau, Z. Liu, S. Li, Z. Li, X. Lu, S. Zhang and R. D. Rogers, Angew. Chem., Int. Ed., 2013, 125, 12576–12579 CrossRef.
- M. Poirier and G. Charlet, Carbohydr. Polym., 2002, 50, 363–370 CrossRef CAS.
- T. L. Ruegg, E.-M. Kim, B. A. Simmons, J. D. Keasling, S. W. Singer, T. Soon Lee and M. P. Thelen, Nat. Commun., 2014, 5, 3490 Search PubMed.
- J. Hinks, Y. Wang, W. H. Poh, B. C. Donose, A. W. Thomas, S. Wuertz, S. C. J. Loo, G. C. Bazan, S. Kjelleberg, Y. Mu and T. Seviour, Langmuir, 2014, 30, 2429–2440 CrossRef CAS PubMed.
- W. W. Baldwin, M. J. Sheu, P. W. Bankston and C. L. Woldringh, J. Bacteriol., 1988, 170, 452–455 CAS.
- D. J. David and T. F. Sincock, Polymer, 1992, 33, 4505–4514 CrossRef CAS.
- P. Weerachanchai, Y. Wong, K. H. Lim, T. T. Y. Tan and J.-M. Lee, ChemPhysChem, 2014, 15, 3580–3591 CAS.
- P. Bustamante, J. Navarro-Lupión and B. Escalera, Eur. J. Pharm. Sci., 2005, 24, 229–237 CrossRef CAS PubMed.
- V. Malpani, P. A. Ganeshpure and P. Munshi, Ind. Eng. Chem. Res., 2011, 50, 2467–2472 CrossRef CAS.
- S. R. Samanta, J. Appl. Polym. Sci., 1992, 45, 1635–1640 CrossRef CAS.
- T. Seviour, L. K. Lambert, M. Pijuan and Z. Yuan, Environ. Sci. Technol., 2010, 44, 8964–8970 CrossRef CAS PubMed.
- T. Seviour, L. K. Lambert, M. Pijuan and Z. Yuan, Appl. Microbiol. Biotechnol., 2011, 92, 1297–1305 CrossRef CAS PubMed.
- C. H. Tan, K. S. Koh, C. Xie, M. Tay, Y. Zhou, R. Williams, W. J. Ng, S. A. Rice and S. Kjelleberg, ISME J., 2014, 8, 1186–1197 CrossRef CAS PubMed.
- G. Yu, D. Zhao, L. Wen, S. Yang and X. Chen, AIChE J., 2012, 58, 2885–2899 CrossRef CAS.
- P. Weerachanchai, Z. Chen, S. S. J. Leong, M. W. Chang and J.-M. Lee, Chem. Eng. J., 2012, 213, 356–362 CrossRef CAS PubMed.
- J. W. Lee, W. G. Yeomans, A. L. Allen, F. Deng, R. A. Gross and D. L. Kaplan, Appl. Environ. Microbiol., 1999, 65, 5265–5271 CAS.
- R. O. Fernández and R. A. Pizarro, J. Chromatogr. A, 1997, 771, 99–104 CrossRef.
- M. W. Calfee, J. P. Coleman and E. C. Pesci, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11633–11637 CrossRef CAS PubMed.
- F.-A. Herbst, M. T. Søndergaard, H. Kjeldal, A. Stensballe, P. H. Nielsen and M. S. Dueholm, J. Proteome Res., 2014 DOI:10.1021/pr500938x.
- L. R. Evans and A. Linker, J. Bacteriol., 1973, 116, 915–924 CAS.
- A. V. Kajava, N. Cheng, R. Cleaver, M. Kessel, M. N. Simon, E. Willery, F. Jacob-Dubuisson, C. Locht and A. C. Steven, Mol. Microbiol., 2001, 42, 279–292 CrossRef CAS.
- K. Brenner, L. You and F. H. Arnold, Trends Biotechnol., 2008, 26, 483–489 CrossRef CAS PubMed.
- X. Z. Li, J. S. Webb, S. Kjelleberg and B. Rosche, Appl. Environ. Microbiol., 2006, 72, 1639–1644 CrossRef CAS PubMed.
- J. J. Beun, A. Hendriks, M. C. M. van Loosdrecht, E. Morgenroth, P. A. Wilderer and J. J. Heijnen, Water Res., 1999, 33, 2283–2290 CrossRef CAS.
- M. S. Allen, K. T. Welch, B. S. Prebyl, D. C. Baker, A. J. Meyers and G. S. Sayler, Environ. Microbiol., 2004, 6, 780–790 CrossRef CAS PubMed.
- T. Seviour, Z. Yuan, M. C. M. van Loosdrecht and Y. Lin, Water Res., 2012, 46, 4803–4813 CrossRef CAS PubMed.
- A. Brandt, J. Grasvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- Y. Venkata Nancharaiah, G. K. K. Reddy, P. Lalithamanasa and V. P. Venugopalan, Biofouling, 2012, 28, 1141–1149 CrossRef CAS PubMed.
- P. Hong, S. Koza and E. S. P. Bouvier, J. Liq. Chromatogr. Relat. Technol., 2012, 35, 2923–2950 CAS.
- B. W. Holloway and A. F. Morgan, Annu. Rev. Microbiol., 1986, 40, 79–105 CrossRef CAS PubMed.
- K.-H. Choi and H. P. Schweizer, Nat. Protoc., 2006, 1, 153–161 CrossRef CAS PubMed.
- L. Yang, M. Nilsson, M. Gjermansen, M. Givskov and T. Tolker-Nielsen, Mol. Microbiol., 2009, 74, 1380–1392 CrossRef CAS PubMed.
- R. Lemaire, Z. Yuan, L. L. Blackall and G. R. Crocetti, Environ. Microbiol., 2008, 10, 354–363 CrossRef CAS PubMed.
- G. Yilmaz, R. Lemaire, J. Keller and Z. Yuan, Biotechnol. Bioeng., 2008, 100, 529–541 CrossRef CAS PubMed.
- W. S. York, A. G. Darvill, M. McNeil, T. T. Stevenson and P. Albersheim, in Methods in Enzymology, ed. H. W. Arthur Weissbach, Academic Press, 1986, vol. 118, pp. 3–40 Search PubMed.
- R. K. Merkle and I. Poppe, in Methods in Enzymology, ed. G. W. H. William and J. Lennarz, Academic Press, 1994, vol. 230, pp. 1–15 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10930a |
| This journal is © The Royal Society of Chemistry 2015 |