Nitrogen-doped active carbon as a metal-free catalyst for acetylene hydrochlorination†
Chunli Zhanga,
Lihua Kangab,
Mingyuan Zhu*ab and
Bin Dai*ab
aSchool of Chemistry and Chemical Engineering of Shihezi University, Shihezi, Xinjiang 832000, P. R. China. E-mail: zhuminyuan@shzu.edu.cn; db_tea@shzu.edu.cn; Fax: +86 9932057210; Tel: +86 9932057270
bKey Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi, Xinjiang 832000, P. R. China
First published on 19th December 2014
Abstract
In this study, we report high catalytic activity for acetylene hydrochlorination by post-treatment of active carbon (AC) with polyaniline (PANI) followed by pyrolyzation at high temperature. The presence of PANI species in N-doped AC can be proven by transmission electron microscopy and X-ray photoelectron spectra. The N-doped AC catalyst with PANI had significantly improved catalytic activity and stability. Results demonstrated that the order of the nitrogen species' role in acetylene hydrochlorination is as follows: pyrrolic N > graphitic N > pyridinic N. It is a green, sustainable process for the polyvinyl chloride industry as a metal-free substitute for toxic HgCl2 because of its low cost, environmental stability, and excellent catalytic performance.
1. Introduction
The vinyl chloride monomer (VCM) is the monomer used to manufacture polyvinyl chloride (PVC), which is produced predominantly through acetylene hydrochlorination catalyzed by mercuric chloride (HgCl2) with active carbon (AC).1 However, the application of HgCl2 catalyst is restricted in industrial production because of its high volatility and toxicity. Therefore, a metal-free catalyst is necessary to prevent the problem of environment pollution caused by HgCl2 catalysts.Shinoda et al. found that the catalytic activity of metal chloride is closely related to its standard electrode potential and that AuCl3 is the optimal catalyst to replace HgCl2 for acetylene hydrochlorination because of its high activity since 1975.2,3 However, AuCl3 catalyst is easily deactivated in the acetylene hydrochlorination process, which is mostly ascribed to the reduction of Au3+ to lower valence state and coke deposition. Therefore, extensive work has been conducted to improve the stability of Au-based catalysts by inhibiting Au3+ species reduction. Bimetallic AuCl3–CuCl2/C catalyst revealed favorable catalytic activity; moreover, more enhanced stability of AuCl3–CuCl2/C than that of AuCl3/C was observed.4 In our previous study, both bimetallic Au–La/SAC catalyst and Au–Co/SAC catalyst improved the stability of Au catalyst by the slight occurrence of coke deposition and inhibited the valence change of gold. TiO2–AuCl3/AC catalyst also revealed outstanding stability in acetylene hydrochlorination.5–7 Au–Cs/AC catalyst recently delivered stable performance during a 500 h test, with the conversion of acetylene and the selectivity of vinyl chloride reaching 99.8%and 99.9%, respectively.8 However, the present noble-metal catalysts still face great challenges for industrial production because of their high cost and the limited reserves of noble metals. Thus, low-cost, environment-friendly, and high-efficiency non-mercury catalysts for acetylene hydrochlorination are necessary.
Nitrogen-doped carbon and carbon nitride materials have been receiving much attention for the past few years because of their unique chemical, mechanical, and electronic properties, which provide access to an even wider range of applications than carbon materials, making them interesting and promising metal-free catalysts.9 To date, attention has been focused on the use of N-doped carbon catalyst on carbon being active for acetylene hydrochlorination reaction. Nitrogen-doped level and type of nitrogen functionalities depend strongly on the materials and on synthesis conditions. Therefore, most studies thus far have focused on unique materials that contain special nitrogen species to examine the catalytic mechanism for acetylene hydrochlorination. For example, N-doped carbon nanotubes (N-CNTs) are active for acetylene hydrochlorination because of a positive correlation between quaternary nitrogen content and activity.10 Our previous experiment revealed that g-C3N4 catalyst on AC is active for gas phase hydrochlorination of acetylene by introducing pyridinic nitrogen.11 A nanocomposite of N-doped carbon derived from silicon carbide could make acetylene conversion twice that of SiC@N–C under the same conditions by using pyrrolic materials.12
Polyaniline (PANI), an interesting subject of research, was initiated with the discovery of conducting polymers because of their unique π-conjugated structures that lead to good mechanical performance, environmental stability, and reversible control of conductivity by charge-transfer doping and protonation.13 PANI has been well investigated as a catalyst for oxygen reduction reactions (ORRs). Borghei et al. reported that a post-treatment of few-walled carbon nanotubes with PANI and pyrolyzed at high temperature resulted in better ORR activity corresponding to higher graphitic N content (45%). Their theoretical calculations also supported their results.14,15 On the basis of the above studies, we speculate that N-doped carbon materials may enhance the activation of hydrogen chloride (HCl) and adsorb acetylene (C2H2) by introducing pyrrolic nitrogen and graphitic nitrogen. Therefore, in the present work, we prepared a doped AC derived from PANI to adsorb HCl and C2H2 directly for acetylene hydrochlorination in the absence of additional metal species. Furthermore, we identified that the order of nitrogen species' role for acetylene hydrochlorination is as follows: pyrrolic N > graphitic N > pyridinic N. Compared with toxic and energy-consuming conversational HgCl2 catalysts, this attribute in conjunction with the feasibility of low-cost monomer and large-scale production make it an ideal candidate for low-cost, environment-friendly, and sustainable non-mercury catalysts over acetylene hydrochlorination. To the best of our knowledge, synthesizing such carbon material for acetylene hydrochlorination using this method has not yet been reported.
2. Experiment section
2.1 Materials
Activated carbon powder (neutral, coconut carbon), C2H2 (acetylene gas, 98%), HCl (hydrogen chloride gas, 99%), C6H7N (aniline, 99.5%), and (NH4)2S2O8 (ammonium peroxydisulfate [APS], 98%) were used in the present study.2.2 Catalyst preparation
AC support was initially washed with diluted aqueous HCl (1 mol L−1) at 343 K for 5 h to remove metal impurities. In a typical approach, 10 mL aniline was first dispersed with 2.0 g AC. The suspension was kept below 283 K while the oxidant (APS) was added. The sample was vacuum dried using a rotary evaporator after constant mixing for 24 h to enable the polymerized PANI to uniformly mix. The subsequent heat treatment was performed at temperatures ranging from 773 K to 1273 K in an inert atmosphere of a nitrogen gas for 1 h. The heat-treated sample was pre-leached in 0.5 M H2SO4 at 353 K for 8 h to remove unstable and inactive species from the catalyst, after which it was thoroughly washed by ethanol and deionized water. Finally, the catalyst was heat treated in nitrogen gas atmosphere for 3 h, as described in the literature.16 The mass ratio between the carbon supports and the aniline was around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 1
5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 using the same method. The obtained samples were labeled as 1#PANI-AC, 2#PANI-AC, 3#PANI-AC, and 4#PANI-AC. Meanwhile, the samples of the different temperatures were coded as follows: PANI-AC500, PANI-AC600, PANI-AC700, PANI-AC800, PANI-AC900, and PANI-AC1000, respectively.
6 using the same method. The obtained samples were labeled as 1#PANI-AC, 2#PANI-AC, 3#PANI-AC, and 4#PANI-AC. Meanwhile, the samples of the different temperatures were coded as follows: PANI-AC500, PANI-AC600, PANI-AC700, PANI-AC800, PANI-AC900, and PANI-AC1000, respectively.
2.3 Catalyst characterization
Surface area analysis (BET) was conducted by obtaining nitrogen adsorption isotherm with Micromeritics ASAP 2020 instrument at 77 K. Transmission electron microscopy (TEM) was performed using a JEM2010 electron microscope at an accelerating voltage of 200 kV to examine the sample morphologies. An X-ray photoelectron spectroscopy (XPS) analysis was executed by Kratos AXIS Ultra DLD spectrometer with monochromatized Al-Kα X-ray source (225 W). Temperature-programmed desorption (TPD) was also analyzed with an AutoChem 2720 instrument (Micromeritics Instrument Corporation, USA). The samples were pretreated in hydrogen chloride or acetylene atmosphere at a reaction temperature of 453 K for 4 h. Then, high-purity nitrogen (40 mL min−1) was passed through the sample at 298 K for 30 min. The sample was heated from 298 K to 1173 K (heating rate, 10 K min−1) to collect the data. Thermogravimetric analysis (TGA) of the samples was conducted on the TGDSC simultaneous thermal analyzer (NETZSCH STA 449F3 Jupiter1, Germany). Approximately 7 mg of sample was used and heated to 1173 K under nitrogen atmosphere, flow rate of 30 mL min−1, and heating rate of 10 K min−1.Activity tests were performed in a fixed-bed microreactor (diameter, 10 mm). The temperature of the reactor was regulated by a CKW-1100 temperature controller produced by (Beijing, China). Nitrogen was used to purge the pipeline before the reaction to remove water and air from the system. Hydrogen chloride (1.04 mL min−1, 1 bar) and acetylene (1.14 mL min−1, 1 bar) were fed through a mixing vessel via calibrated mass flow controllers into a heated glass reactor containing catalyst (0.72 g) at a gas hourly space velocity of 36 h−1. The feed HCl–C2H2 volume ratio was 1.15, and a reaction temperature of 453 K was selected. The gas phase products were first passed through an absorption bottle containing NaOH solution and then analyzed online by GC equipped with a flame ionization detector.
3. Results and discussion
Nitrogen adsorption/desorption isotherms were used to confirm the physical and structural properties of PANI-AC catalysts. Table 1 presents the results, which listed the specific areas and the total pore volumes of the fresh and spent PANI-AC catalysts. The fresh specific surface of the PANI-AC catalysts decreased from 1073.2 m2 g−1 to 491.3 m2 g−1, and the specific pore volume decreased from 0.61 m3 g−1 to 0.28 m3 g−1 with the increase of precursor aniline content. The pore structure of the catalysts was destroyed to some extent, leading to a decrease in the BET surface area and the total pore volume. Meanwhile, the BET surface also decreased after the reaction, which indicated that the reason for inactivation should be acetylene polymerization resulting in carbon deposition and pore blocking.| Sample | SBET (m2 g−1) | V (cm3 g−1) | D (nm) | |||
|---|---|---|---|---|---|---|
| Fresh | Spent | Fresh | Spent | Fresh | Spent | |
| AC | 1073.2 | 973.9 | 0.61 | 0.55 | 2.28 | 2.26 |
| 1#PANI-AC | 906.3 | 779.3 | 0.52 | 0.43 | 2.28 | 2.20 |
| 2#PANI-AC | 853.8 | 765.9 | 0.48 | 0.43 | 2.27 | 2.27 |
| 3#PANI-AC | 592.2 | 417.1 | 0.35 | 0.25 | 2.35 | 2.44 |
| 4#PANI-AC | 491.3 | 109.5 | 0.28 | 0.07 | 2.28 | 2.50 |
The structure and morphology of AC, 1#PANI-AC, 2#PANI-AC, 3#PANI-AC, and 4#PANI-AC catalysts can be clearly observed in the TEM image in Fig. S1 of the ESI.† The homogeneous dispersion of PANI on the surface of the AC support was visible, meanwhile, the layer of PANI morphology became more obvious with the increase of precursor aniline content and result in the specific surface area from 1073.2 m2 g−1 to 491.3 m2 g−1 (Table 1). Which demonstrated that PANI interacted with the AC surface successfully, increasing the amount of active sites.
This study aimed to evaluate the effect of catalytic activity on acetylene hydrochlorination with the increase of precursor aniline content. Fig. 1 illustrates the reaction times for the acetylene conversion of AC, 1#PANI-AC, 2#PANI-AC, 3#PANI-AC, and 4#PANI-AC catalysts. Fig. 1a shows that the rate of acetylene conversion decreased from 76.27% to 72.30% after 9 h for the optimal catalyst of 3#PANI-AC, whereas the selectivity to VCM increased slightly from the initial 99.85% to 99.96% (Fig. 1b). By contrast, the maximum conversion of acetylene conversion for AC was 49.85% and the minimum was 37.73%. The highest selectivity to VCM was 99.90%, although its specific BET surface area was higher than that of 3#PANI-AC (Table 1). This result indicated that N-doped AC (PANI-AC) promoted the initial catalytic activity of the AC catalysts and significantly enhanced the catalyst stability, with the mass ratio between the carbon supports and the aniline increasing from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The increased activity of the PANI-AC catalyst was ascribed to the presence of PANI species in the AC, which changed the structure of the AC. Furthermore, XPS was systematically conducted to explore the relationship between the nitrogen content on the catalyst surface and their catalytic activity. Fig. 2 shows the as-obtained 3#PANI-AC sample, which had a nitrogen content of 2.39% and revealed excellent acetylene conversion (76.27%). By contrast, AC with a nitrogen content of 0.67% demonstrated a low level of acetylene conversion (49.85%). This result manifested that an increase in the nitrogen content significantly enhanced the catalytic activity of AC. However, no improvement was found for the catalytic performance of 4#PANI-AC with nitrogen content of 3.02% due to the pore structure of the catalyst, which was destroyed to some extent. These findings are consistent with the result of BET surface area and the total pore volume.
5. The increased activity of the PANI-AC catalyst was ascribed to the presence of PANI species in the AC, which changed the structure of the AC. Furthermore, XPS was systematically conducted to explore the relationship between the nitrogen content on the catalyst surface and their catalytic activity. Fig. 2 shows the as-obtained 3#PANI-AC sample, which had a nitrogen content of 2.39% and revealed excellent acetylene conversion (76.27%). By contrast, AC with a nitrogen content of 0.67% demonstrated a low level of acetylene conversion (49.85%). This result manifested that an increase in the nitrogen content significantly enhanced the catalytic activity of AC. However, no improvement was found for the catalytic performance of 4#PANI-AC with nitrogen content of 3.02% due to the pore structure of the catalyst, which was destroyed to some extent. These findings are consistent with the result of BET surface area and the total pore volume.
To investigated the content of nitrogen on the performance of PANI-AC catalyst for acetylene hydrochlorination with the mass ratio between the carbon supports and the aniline increasing from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, Elemental analysis was also examined to explore the C, H, N content for fresh and spent nitrogen-doped AC catalysts, the results listed in Table 2, the content of nitrogen increased gradually with the increase of precursor aniline content for fresh catalysts, These dates are consistent well with the result of XPS. However, after experiencing 9 h of reaction, the content of nitrogen decreased to some extent, it is worth mentioning that the content of nitrogen only decreased 0.134% for fresh catalyst and spent 3#PANI-AC catalyst, the acetylene conversion over 3#PANI-AC exhibited no obvious decrease after 9 h, which demonstrated that the content of nitrogen play an important role for the catalytic performance and stability in acetylene hydrochlorination.
6, Elemental analysis was also examined to explore the C, H, N content for fresh and spent nitrogen-doped AC catalysts, the results listed in Table 2, the content of nitrogen increased gradually with the increase of precursor aniline content for fresh catalysts, These dates are consistent well with the result of XPS. However, after experiencing 9 h of reaction, the content of nitrogen decreased to some extent, it is worth mentioning that the content of nitrogen only decreased 0.134% for fresh catalyst and spent 3#PANI-AC catalyst, the acetylene conversion over 3#PANI-AC exhibited no obvious decrease after 9 h, which demonstrated that the content of nitrogen play an important role for the catalytic performance and stability in acetylene hydrochlorination.
| Sample | Element Composition (%) | ||
|---|---|---|---|
| C | H | N | |
| 1#PANI-AC (fresh) | 92.79 | 0.630 | 1.525 |
| 2#PANI-AC (fresh) | 96.53 | 0.622 | 1.633 |
| 3#PANI-AC (fresh) | 87.40 | 0.757 | 1.943 |
| 4#PANI-AC (fresh) | 89.09 | 0.632 | 2.987 |
| 1#PANI-AC (spent) | 81.89 | 0.957 | 1.272 |
| 2#PANI-AC (spent) | 83.57 | 0.878 | 1.345 |
| 3#PANI-AC (spent) | 85.28 | 0.937 | 1.808 |
| 4#PANI-AC (spent) | 81.74 | 0.931 | 2.668 |
The above results manifested that the catalyst of 3#PANI-AC displayed the best performance when the mass ratio between the carbon supports and the aniline is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. In the next set of experiments, the effect of different temperature levels on the 3#PANI-AC catalyst was investigated. As shown in the TEM image (Fig. 3), the morphology and structure of AC, PANI-AC500, and PANI-AC900 catalysts can be clearly observed. The surface of the AC catalyst can be seen as the polymer layer. The oxidation of aniline is a classic redox reaction, in which electrons from aniline are withdrawn by an oxidant. AC serves as an electron transfer mediator between aniline (reductant) and APS (oxidant). The aniline monomer or oligomers are first adsorbed on the surface of AC. Then, the polymer starts to grow with the reaction. A very thin voile-like layer is formed on the AC surface with the continuing process of calcination at a high temperature.17 The layer of voile-like morphology became more obvious as the temperature increased from 773 K to 1173 K. The figures demonstrated that the AC interacted with the nitrogen species from aniline, allowing nitrogen atom doped into the apparent surface, increasing the amount of active sites.
5. In the next set of experiments, the effect of different temperature levels on the 3#PANI-AC catalyst was investigated. As shown in the TEM image (Fig. 3), the morphology and structure of AC, PANI-AC500, and PANI-AC900 catalysts can be clearly observed. The surface of the AC catalyst can be seen as the polymer layer. The oxidation of aniline is a classic redox reaction, in which electrons from aniline are withdrawn by an oxidant. AC serves as an electron transfer mediator between aniline (reductant) and APS (oxidant). The aniline monomer or oligomers are first adsorbed on the surface of AC. Then, the polymer starts to grow with the reaction. A very thin voile-like layer is formed on the AC surface with the continuing process of calcination at a high temperature.17 The layer of voile-like morphology became more obvious as the temperature increased from 773 K to 1173 K. The figures demonstrated that the AC interacted with the nitrogen species from aniline, allowing nitrogen atom doped into the apparent surface, increasing the amount of active sites.
The 3#PANI-AC catalyst was calcinated in a tube furnace under nitrogen atmosphere at temperatures ranging from 773 K to 1273 K. As shown in Fig. 4, the conversion of acetylene and catalytic stability were both enhanced as the calcination temperature increased. PANI-AC900 revealed the best catalytic activity with a highest acetylene conversion of 76.27%. However, the PANI-AC1000 revealed relatively lower activity, with the highest acetylene conversion of 69.01%. Temperature levels that exceed 1173 K resulted in the deactivation of the catalyst, which implied that precursor calcinations at extremely high temperatures may induce the decomposition of PANI species and then lead to decrease of nitrogen content.
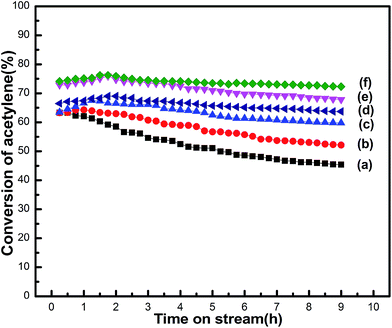 | ||
| Fig. 4 Acetylene conversion of 3#PANI-AC at different calcination temperatures: (a) 773 K, (b) 883 K, (c) 993 K, (d) 1273 K, (e) 1073 K, and (f) 1173 K. | ||
The structure of AC has been changed significantly at the temperature higher than 873 K. Therefore, we compared BET results between AC and 3#PANI-AC catalyst at different temperature, the results can be found in Table S1 of the ESI,† The specific surface area of the AC increased from 1062.2 m2 g−1 to 1397.5 m2 g−1 and the specific pore volume increased from 0.59 cm3 g−1 to 0.70 cm3 g−1 as the calcinations temperature increased. The N-doped AC catalysts have the similar results. It is worthwhile to note that the specific surface area of the N-doped AC decreased comparing with AC at the same temperature, which indicated that PANI interacted with AC successfully at different temperature, allowing PANI into the apparent surface of AC, increasing the amount of active sites, which is consistent well with results of TEM.
The previous result demonstrated that N-doped catalysts play an important catalytic role in acetylene hydrochlorination. Moreover, the higher the number of nitrogen doped, the higher the catalytic activity, which agreed well with our previous work.11 However, the detailed reaction mechanism is yet to be explored, especially the roles of different nitrogen species. Nitrogen species may function according to their kind in the acetylene hydrochlorination because the nitrogen doped into the carbon matrix presented several different species. Exploring the amount and kind of nitrogen species is critical to improve the catalytic activity of N-doped AC and to determine the involvement of the catalytic activity with the key nitrogen species. XPS deconvolution of N 1s on the samples with different temperatures was performed to examine the different species, including their binding energy, dispersion, and relative quantity. Fig. 5 and Table 3 present the results. As shown Fig. 5, there appear to be three types of nitrogen species. The pyridinic N (398.6 ± 0.3 eV) bonds with two carbon atoms in the six-membered rings with one p-electron localized in the π system, the pyrrolic N (400.5 ± 0.3 eV) bonds with two carbon atoms in the five-membered rings, and graphitic N (400.9 ± 0.3 eV) corresponds to nitrogen atoms that are linked with three carbon atoms in grapheme layer and thereby replacing the carbon atom in graphene hexagonal ring.14,18
 | ||
| Fig. 5 XPS deconvolution of N 1s for N-doped AC: (a) PANI-AC600, (b) PANI-AC700, (c) PANI-AC800, and (d) PANI-AC900. | ||
| Sample | Nitrogen content (wt %) | Area%, binding energy (eV) | ||
|---|---|---|---|---|
| Pyridinic N | Pyrrolic N | Graphitic N | ||
| PANI-AC500 | 4.01 | 42.31 (398.76) | 29.85 (400.36) | 27.84 (401.20) |
| PANI-AC600 | 3.62 | 40.84 (398.58) | 30.52 (400.33) | 28.65 (401.00) |
| PANI-AC700 | 2.92 | 32.08 (398.52) | 34.75 (400.46) | 33.17 (401.11) |
| PANI-AC800 | 2.52 | 27.09 (398.33) | 36.85 (400.34) | 36.06 (401.20) |
| PANI-AC900 | 2.39 | 21.29 (398.32) | 42.36 (400.41) | 36.35 (401.21) |
| PANI-AC1000 | 1.95 | 19.70 (398.30) | 46.16 (400.31) | 34.14 (401.21) |
The higher nitrogen concentration is likely due to a stronger interaction of PANI with the functionalized AC. However, nitrogen content gradually decreased with increasing pyrolysis temperature from 773 K to 1273 K (Table 3), possibly due to the decomposition of PANI species. We performed an annealing treatment for N-doped AC (at 1173 K) to obtain a PANI-AC900 sample with a total nitrogen content of 2.39%, but the content of nitrogen was 4.01% for PANI-AC500 catalyst. The results of catalytic activity revealed that the activity performance of PANI-AC900 is much higher than that of PANI-AC500 (Fig. 4). In other words, the activity increased while the content of nitrogen is decreased with increasing calcinations temperature. Obviously, the nitrogen species played a key role in acetylene hydrochlorination reaction rather than nitrogen content at this point. The relative content of pyrrolic N increased but the pyridinic nitrogen obviously decreased. Meanwhile, the relative content of graphitic N just changed from 27.84% to 34.14% as the temperature increased, which is consistent with their activity sequence displayed in Fig. 4. This result indicated that the catalytic performance of N-doped AC was closely linked with pyrrolic N; graphitic N was also involved in the catalytic performance. The order of nitrogen species' role for acetylene hydrochlorination is as follows: pyrrolic N > graphitic N > pyridinic N.
Meanwhile, TOF was calculated based on the nitrogen content and acetylene conversion at about 4.5 h. As is displayed in Table 4, TOF improved gradually as the temperature increased, indicating that PANI has good dispersion on the surface of AC. The PANI-AC900 catalyst, which had the best catalytic performance, displayed the higher pyrrolic N (42.36%) and graphitic N (36.35%). For the PANI-AC1000 catalyst, the relative amounts of pyrrolic N (46.16%) and graphitic N (34.14%), as well as the TOF, were higher than those of the PANI-AC900 catalyst. However, its nitrogen content was obviously lower than that of PANI-AC900. Thus, the PANI-AC1000 catalytic activity was inferior to the catalyst of PANI-AC900.
| Sample | Nitrogen content (wt %) | Conversion (%) | TOF (min−1) |
|---|---|---|---|
| PANI-AC500 | 4.01 | 51.27 | 0.01265 |
| PANI-AC600 | 3.62 | 58.55 | 0.01601 |
| PANI-AC700 | 2.92 | 63.71 | 0.02159 |
| PANI-AC800 | 2.52 | 71.52 | 0.02798 |
| PANI-AC900 | 2.39 | 73.80 | 0.03056 |
| PANI-AC1000 | 1.95 | 66.34 | 0.03367 |
The study of the thermal stability of PANI-AC in nitrogen atmosphere is necessary because PANI-AC catalyst is synthesized by the calcination process at temperatures ranging from 773 K to 1273 K. Fig. 6 illustrates the TGA and DTA results of PANI-AC without calcinations and TGA of AC. The peak shows a two-stage weight loss: (i) the first obvious peak observed at the temperature range of 373 K to 623 K corresponding to the loss of moisture evaporation before 413 K and removal of hydrochloric acid dopant at about 557.7 K;19 and (ii) a broad peak at the higher temperature range of 623 K to 923 K, accounting for the structural decomposition of polymer.20 From the analysis of TGA, precursor calcinations at higher temperatures may induce the decomposition of PANI species and then gradually lead to a decrease of nitrogen content, which is consistent with the result of acetylene conversion (Fig. 4) and the content of nitrogen in Table 3.
 | ||
| Fig. 6 Analysis of (a) DTG of PANI-AC without calcinations, (b) TGA of PANI-AC without calcinations, and (c) TGA analysis of AC in nitrogen atmosphere. | ||
To further understand the role of nitrogen functionalities in HCl and C2H2 activation, we conducted TPD of HCl and C2H2 because the peak area corresponds to the adsorption amount of HCl and C2H2, and the desorption temperature reflects the adsorption strength. Fig. 7 shows the HCl-TPD (Fig. 7a) and C2H2-TPD (Fig. 7b) profiles for N-doped catalysts at different temperature. As seen in Fig. 7a, the HCl desorption temperature increased from 398 K to 421 K for the N-doped catalyst at different temperature. However, no obvious desorption of HCl for AC was found. Furthermore, N-doped AC revealed more quantity of adsorption to HCl with increasing pyrolysis temperature from 773 K to 1273 K, which corresponded to the order of catalytic activity in Fig. 4. Therefore, the relatively strong bonding between HCl and N-doped AC and HCl easily activated with the higher desorption temperature.11 Fig. 7b shows that a considerable amount of C2H2 desorbs from PANI-AC500 after doping into the carbon matrix with nitrogen, and the desorption of C2H2 becomes much more evident over PANI-AC900. The desorption covers the temperature range of 401.4 K to 445.8 K, indicating that N-doped AC activates C2H2 immediately. Furthermore, the adsorption amount of C2H2 decreases in the following order: PANI-AC900 > PANI-AC800 > PANI-AC700 > PANI-AC600 > PANI-AC500 > AC, which is consistent with their activity sequence exhibited in acetylene hydrochlorination reaction.
On the basis of the above experiments, nitrogen species, particularly pyrrolic N, play more crucial roles to improve the catalytic performance. Graphitic nitrogen is also involved in acetylene hydrochlorination reaction. The correlating result obtained was highly consistent with that in the previous work. Bao et al. pointed out that the carbon atoms bonded with pyrrolic nitrogen atoms were the active site in acetylene hydrochlorination reaction based on the experiment studied and the theoretical calculations. Pyrrolic N induced not only localized electronic states below the Fermi level on the carbon sites but also an electronic state that possesses a higher energy level and density. Both benefit from the adsorption of acetylene.12 The relationship between graphitic nitrogen and activity performance has long been a topic of debate. Su et al. reported that the most stable graphitic N structure was formed through ring condensation by further temperature increase.21 Oshima et al. found that N-CNTs with a comparably larger amount of graphitic nitrogen exhibit a higher ORR electrocatalytic activity than those containing a large amount of pyridinic nitrogen.22 However, Ma and coworkers reported that graphitic nitrogen did not directly participate in the activation of substrates but instead changed the electronic structure of the adjacent carbon atoms, exhibiting a metal-like band electronic phenomenon.23 Although it has been reported in ORR reaction, the mechanism of the ORR reaction is similar to that of acetylene hydrochlorination. C2H2 is the electron donor that adsorbs onto the catalytic active site for acetylene hydrochlorination, which is similar to O2 adsorption in the ORR process.11 The graphitic nitrogen might enhance catalytic activity better than carbon materials in acetylene hydrochlorination. In this study, we revealed the possible nitrogen doping mechanism for graphitic nitrogen. The higher nitrogen concentration is likely due to a stronger interaction of PANI with the functionalized AC, which leads to all kinds of species. These nitrogen species would transform gradually. The most stable graphitic N structure was formed through ring condensation by further temperature increase and then changed the electronic structure of the adjacent carbon atoms to adsorb HCl and C2H2 easily. However, we need to do more work to explore the mechanism of graphitic nitrogen interaction with acetylene hydrochlorination reaction by experiment and theoretical simulations. Therefore, efficient techniques are significant to control synthesis with a single-type species in carbon materials to explain the catalytic performance of different nitrogen sites. Ultimately, the catalyst can be further optimized for practical applications.
4. Conclusions
In summary, N-doped AC was used as a metal-free catalyst for acetylene hydrochlorination. The obtained PANI-AC displayed preferable catalytic activity. The catalytic performance of PANI-AC was closely linked with pyrrolic N. Graphitic N was also involved in the catalytic performance. Both HCl and C2H2 can be adsorbed and desorbed compatibly and make N-doped carbon catalysts as a metal-free substitute for toxic HgCl2. This method may eventually lead to a green, sustainable process for the PVC industry in the future.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program, 2012CB720302), National Natural Science Funds of China (NSFC, 21366027), the Doctor Foundation of Bingtuan (2013BB010), and Foundation of young scientist in Shihezi University (2013ZRKXJQ03).References
- Y. Pu, J. Zhang, L. Yu, Y. Jin and W. Li, Appl. Catal., A, 2014, 488, 28–36 CrossRef CAS PubMed.
- K. Shinoda, Chem. Lett., 1975, 4, 219–220 CrossRef.
- A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed.
- J. Ma, S. Wang and B. Shen, React. Kinet., Mech. Catal., 2013, 110, 177–186 CrossRef CAS.
- H. Zhang, B. Dai, X. Wang, L. Xu and M. Zhu, J. Ind. Eng. Chem., 2012, 18, 49–54 CrossRef CAS PubMed.
- H. Zhang, B. Dai, X. Wang, W. Li, Y. Han, J. Gu and J. Zhang, Green Chem., 2013, 15, 829–836 RSC.
- C. Huang, M. Zhu, L. Kang, X. Li and B. Dai, Chem. Eng. J., 2014, 242, 69–75 CrossRef CAS PubMed.
- J. Zhao, J. Xu, J. Xu, J. Ni, T. Zhang, X. Xu and X. Li, ChemPlusChem, 2014, 1–7 Search PubMed.
- D. Su, J. Zhang, B. Frank, A. Thomas, X. Wang, J. Paraknowitsch and R. Schlögl, ChemSusChem, 2010, 3, 169–180 CrossRef CAS PubMed.
- K. Zhou, B. Li, Q. Zhang, J. Huang, G. Tian, J. Jia, M. Zhao, G. Luo, D. Su and F. Wei, ChemSusChem, 2014, 7, 723–728 CrossRef CAS PubMed.
- X. Li, Y. Wang, L. Kang, M. Zhu and B. Dai, J. Catal., 2014, 311, 288–294 CrossRef CAS PubMed.
- X. Li, X. Pan, L. Yu, P. Ren, X. Wu, L. Sun, F. Jiao and X. Bao, Nat. Commun., 2014, 5, 3688 CAS.
- D. Xie, Q. Jiang, G. Fu, Y. Ding, X. Kang, W. Cao and Y. Zhao, Rare Met., 2011, 30, 94–97 CrossRef CAS PubMed.
- M. Borghei, P. Kanninen, M. Lundahl, T. Susi, J. Sainio, I. Anoshkin, A. Nasibulin, T. Kallio, K. Tammeveski and E. Kauppinen, Appl. Catal., B, 2014, 158, 233–241 CrossRef PubMed.
- D. Srivastava, T. Susi, M. Borghei and L. Kari, RSC Adv., 2014, 4, 15225–15235 RSC.
- G. Wu, K. L. More, C. M. Johnston and P. Zelenay, Science, 2011, 332, 443–447 CrossRef CAS PubMed.
- E. N. Konyushenko, J. Stejskal, M. Trchová, J. Hradil, J. Kovářová, J. Prokeš, M. Cieslar, J.-Y. Hwang, K.-H. Chen and I. Sapurina, Polymer, 2006, 47, 5715–5723 CrossRef CAS PubMed.
- G. Wu, M. Nelson, S. Ma, H. Meng, G. Cui and P. K. Shen, Carbon, 2011, 49, 3972–3982 CrossRef CAS PubMed.
- S. Vohra, M. Kumar, S. K. Mittal and M. Singla, J. Mater. Sci.: Mater. Electron., 2013, 24, 1354–1360 CrossRef CAS.
- M. Kumar, A. Pathak, M. Singh and M. Singla, Thin Solid Films, 2010, 519, 1445–1451 CrossRef CAS PubMed.
- R. Arrigo, M. Hävecker, R. Schlögl and D. S. Su, Chem. Commun., 2008, 40, 4891–4893 RSC.
- H. Niwa, K. Horiba, Y. Harada, M. Oshima, T. Ikeda, K. Terakura, J.-i. Ozaki and S. Miyata, J. Power Sources, 2009, 187, 93–97 CrossRef CAS PubMed.
- Y. Gao, G. Hu, J. Zhong, Z. Shi, Y. Zhu, D. S. Su, J. Wang, X. Bao and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 2109–2113 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13862g |
| This journal is © The Royal Society of Chemistry 2015 |




