Effect of Sr substituted La2−xSrxNiO4+δ (x = 0, 0.2, 0.4, 0.6, and 0.8) on oxygen stoichiometry and oxygen transport properties
T. Inprasita,
S. Wongkasemjitab,
S. J. Skinnerc,
M. Burrielcd and
P. Limthongkul*e
aThe Petroleum and Petrochemical College, Chulalongkorn University, Bangkok, 10130, Thailand
bCenter of Excellence for Petroleum, Petrochemicals and Advanced Materials, Chulalongkorn University, Bangkok 10330, Thailand
cDepartment of Materials, Imperial College London, Exhibition Road, London SW7 2AZ, UK
dCatalonia Institute for Energy Research (IREC), Department of Advanced Materials for Energy, Jardins de les Dones de Negre 1, 2nd floor, 08930-Sant Adriá del Besòs, Barcelona, Spain
eNational Metal and Materials Technology Center, National Science and Development Agency, 114 Thailand Science Park, Phahonyothin Road, Khlong Nueng, Khlong Luang, Pathumthani 12120, Thailand. E-mail: pimpal@mtec.or.th
First published on 28th November 2014
Abstract
Stoichiometry and oxygen diffusion properties of La2−xSrxNiO4±δ with x = 0.2, 0.4, 0.6, and 0.8 prepared via a sol–gel method were investigated in this study. Iodometric titration and thermogravimetric analysis were used to determine the oxygen non-stoichiometry. Over the entire compositional range, the samples exhibit oxygen hyperstoichiometry with the minimum value δ = 0.14 at x = 0.4. Mixed effects of reduction of oxygen excess and increasing valence of Ni were found to serve as charge compensation mechanisms; the former dominated at a low level of substitution, x < 0.4, while the latter dominated at higher levels of Sr (0.4 < x < 0.8). The highest oxygen diffusion coefficient was found for the minimum amount of Sr substitution, x = 0.2, continuously decreasing with x until x = 0.6. An unusual increase in D* was observed when the Sr content increased up to x = 0.8.
1 Introduction
Materials with mixed ionic and electronic conducting properties (MIECs) have attracted much attention for electrochemical applications, such as SOFCs, high temperature steam electrolysis, sensors and ceramic membranes.1,2 Most studies concerned with these mixed conducting materials, especially for SOFC cathodes, have focused on perovskite-type oxides such as lanthanum manganite, lanthanum cobaltite and lanthanum nickelate.3,4 With SOFC development directed towards operation in the intermediate temperature range (400–600 °C), these materials do not satisfy all of the technological requirements, especially mechanical compatibility with other cell components and conductivity.3 Materials with the Ruddlesden–Popper type structure, A2BO4, have attracted much interest recently due to their mixed conducting properties while maintaining high electrical conductivity in the target temperature range.5–8 The Ruddlesden–Popper structure with the general formula An+1MnO3n+1 (n = 1,2,3,…) is a structure that comprises ABO3 perovskite layers, alternating with AO rock-salt layers, stacked along the c axis. The relatively open structural framework afforded by the rock-salt intergrowth allows the accommodation of hyperstoichiometric oxide-ions in the rock-salt layer as interstitials. Therefore, these oxygen excess materials caught a lot of interest as cathodes for SOFC applications due to their potentially high oxygen transport property. La2NiO4+δ and related materials exhibiting Ruddlesden–Popper structure have been proposed as candidates for energy related electrochemical applications because of their excellent transport and electrocatalytic properties.2,7–10 Moreover, materials in this group exhibit coefficient of thermal expansion (CTE) values in the range of 12.4–13.6 × 10−6 K−1, close to the common SOFC electrolyte materials such as the ceria-based electrolytes (13.2 × 10−6 K−1).4,11 The La2NiO4+δ structure is made up of sheets of (NiO6) corner sharing octahedra, interleaved with La2O2 layers in which the additional oxygen could be localized. The compound is therefore able to accept oxygen overstoichiometry that leads to potentially high oxygen diffusivity compared to that of the common cathode materials.1,2,7,9,12 However, due to insufficient electrical conductivity in La2NiO4+δ, 10–70 S cm−8, attempts to improve such properties have been conducted through substitution of the A site with alkaline-earth ions. Ca and Sr are the most popular dopants due to their compatible ionic radii. However, Ca has been found to have little effect on electrical conductivity, while the replacement of La by Sr leads to a much larger improvement.5,6,10,13Most efforts on La2−xSrxNiO4+δ (LSNO) have been devoted to understanding structural stability, electrical and thermal behavior of La2−xSrxNiO4+δ compounds with only a few investigations of the transport of conducting species in the system.5,6,8,11,13–16 Zhao et al. studied the lanthanum deficient compound, La2−xNiO4+δ (x = 0, 0.05).17 They found that the D* and k* values for La1.95NiO4.13 were higher than that of the stoichiometric compound. Kharton et al. investigated factors affecting ionic transport in oxygen-hyperstoichiometric phases of A2BO4 structures such as the lanthanum nickelates and cuprates.18 They found that decreasing radii of the rare-earth cations in the A-sublattice of both cuprates and nickelates led to a dramatic decrease in ionic transport, similar to that observed with perovskite oxides.18 Skinner and Kilner studied oxygen transport of La2−xSrxNiO4+δ (x = 0, 0.1) and found that oxygen diffusivity of La1.9Sr0.1NiO4+δ was higher than that of LaxSr1−xCoyFe1−yO3−δ, particularly at lower temperatures, but lower than that of LaCoO3. However, La1.9Sr0.1NiO4+δ appears to be more stable than either of these two materials in terms of thermal expansion behavior at high temperatures.19
A few studies have shown that higher Sr substitution (x > 0.1) could improve the mixed ionic-electronic conductivity (MIEC) of these materials. For example, Ishikawa et al. reported that the electron density in LaSrNiO4 was almost an order of magnitude larger than that of La2NiO4.20 We also have seen in our previous work that 0.8 mol of Sr substitution in La2−xSrxNiO4+δ exhibited superior conductivity while maintaining similar thermal expansion coefficient compared to that of the sample with no or lower Sr substitution amount.11 However, there are still very few reports on the ionic transport in Sr substituted La2NiO4+δ, particularly with Sr substitution content at x > 0.1. In this work, we, therefore, aim to extend the knowledge of oxygen transport in LSNO to a higher level of Sr substitution and to evaluate these materials as potential SOFC/SOEC electrodes, and correlate transport properties with oxygen reduction capability. Effects of Sr substitution in La2−xSrxNiO4+δ (x = 0.2, 0.4, 0.6, and 0.8) on the oxygen stoichiometry, phase and transport properties were investigated in this research.
2 Experimental
2.1 Preparation of La2−xSrxNiO4+δ (x = 0.2, 0.4, 0.6, and 0.8)
La2−xSrxNiO4+δ (x = 0.2, 0.4, 0.6, and 0.8) powders were prepared by a sol–gel method following the process described in our previous work.11 Lanthanum(III) acetate hydrate ((CH3COO)3La·xH2O, 99.9%, Sigma-Aldrich), strontium acetate ((CH3COO)2Sr, 99.995%, Sigma-Aldrich), and nickel acetate ((CH3COO)2Ni·4H2O, 98%, Sigma-Aldrich) were dissolved in deionized water. The mixture was homogeneously stirred to obtain a clear solution before adding ethanolamine (Labscan Co.), which was used as the directing agent. The mixture was stirred continuously for 6 h before being left at room temperature to gel. The gel was then calcined at 1050 °C with a heating rate of 3 °C min−1 for 2 h, resulting in black powders.To obtain high density samples for 18O2 isotope exchange measurements, the synthesized powders were ground, uniaxially pressed at 65 MPa followed by isostatically pressing at 300 MPa to form 13 mm diameter pellets. The pellets were fired in a box furnace at 1400 °C for 5 h with a heating rate of 5 °C min−1 in air. The Archimedes' method was used to measure the density of the sintered samples to ensure that the samples were adequately dense for the diffusion analysis. All samples tested achieved a density greater than 95% of the theoretical density for the material, ensuring only closed porosity was present in the samples.
2.2 Oxygen content
Oxygen content in the La2−xSrxNiO4+δ samples was determined using two methods. The first one is an indirect measurement through a reduction of Ni3+/Ni2+ ions to Ni using iodometric titration. The other is the determination of the amount of oxygen via sample weight loss under a reducing atmosphere through thermogravimetric analysis (TGA). For the analysis using the first method, oxygen overstoichiometry (δ) of the compound was assumed to be directly correlated to the Ni3+ content according to the formulation La2−xSrxNi2+1−τNi3+τO4+τ/2 with δ = τ/2.1,21 Ni3+ content (τ) was determined by iodometry. In the experiment, 0.1 g of sample was dissolved in a 4 ml HCl solution (ACS, 36.5–38.0%, Alfa Aesar) containing 1 g excess potassium iodide (KI, 99.99%, Alfa Aesar). The experiment was performed under flowing nitrogen to prevent oxidation of Ni ions in air. I− anions reduce Ni3+ to Ni2+ forming I2 (eqn (1)). The resulting I2 was then titrated by sodium thiosulfate solution (Na2S2O3, 99.99%, Sigma-Aldrich) using a starch solution (ACS, 1% solution, Alfa Aesar) as the indicator (eqn (2)). All reactions are summarized in eqn (3).| 2Ni3+ + 2I− → 2Ni2+ + I2 | (1) |
| I2 + 2S2O32− → 2I− + S4O62− | (2) |
| Summary: 2Ni3+ + 2S2O32− → 2Ni2+ + S4O62− | (3) |
To confirm the concentration of Na2S2O3, 0.01 M of KIO3 (99.995%, Sigma-Aldrich) in 0.01 M of KI and 1 M of H2SO4 (ACS, 95.0–98.0%, Alfa Aesar) was titrated by Na2S2O3 solution using starch as the indicator as shown in eqn (4).21 Knowing the amount of the reducing agent used, oxygen overstoichiometry, δ, could then be calculated.
| 6S2O32− + IO3− + 6H+ → I− + 3S4O62− + 3H2O | (4) |
To confirm the result from the iodometry, thermogravimetric analysis (TGA) was performed. The TGA (TGA/STDA851e Mettler Toledo) experiment was done under a reducing atmosphere using 5% H2/Ar flowing gas (BIG, Thailand). The analysis was performed from room temperature up to 1000 °C with a heating rate of 5 °C min−1. The La2−xSrxNiO4+δ compound was reduced to La2−xSrxNiO4 in the first stage of the test (eqn (5)). Further exposure to the reducing atmosphere at higher temperatures should result in the decomposition of the multi-cation compounds into La2O3, SrO and Ni metal (eqn (6)). The total reaction involved is shown in eqn (7) in which the amount of oxygen in the starting material could be calculated, and the overstoichiometric amount of oxygen, δ, could then be determined.
| La2−xSrxNiO4+δ + δH2 → La2−xSrxNiO4 + δH2O | (5) |
| La2−xSrxNiO4 + (1 + 0.5x)H2 → (1 − 0.5x)La2O3 + xSrO + Ni + (1 + 0.5x) + δH2O | (6) |
| La2−xSrxNiO4+δ + {(1 + 0.5x)+δ}H2 → (1 − 0.5x)La2O3 + xSrO + Ni + {(1 + 0.5x)+δ}H2O | (7) |
2.3 Oxygen diffusion and surface exchange coefficients
Isotopic oxygen transport and surface exchange coefficients were investigated for the La2−xSrxNiO4+δ (x = 0.2, 0.4, 0.6, and 0.8) pellets. The sintered pellets were ground with SiC paper (1000 and 800 grit), and were then polished with successive grades of diamond suspension of 15, 6, 3, 1 and 0.25 μm. Oxygen isotopic exchange was performed over the temperature range of 550 to 800 °C at a nominal pressure of 200 mbar. The polished samples were annealed at the testing temperature in research grade 16O2 (>99.9995%, BOC, UK) for a period of at least ten times the exchange time with 18O2 (26%, Isotec Inc., USA) to ensure that an equilibrium was established. 18O2 exchange was performed immediately after the equilibration step with the exchange times between 45 and 240 min, depending on the exchange temperature. All samples were immediately quenched to room temperature after the ion exchange experiment. After removing the sample from the exchange tube, the pellets were sectioned using a diamond bladed saw perpendicular to the exchanged surface. The exposed cross-sections were again polished using diamond suspensions, as described above and mounted together (top surfaces in contact) to perform the Secondary Ion Mass Spectrometry (SIMS) analysis. All the exchanged samples were measured by time-of-fight SIMS (ToF-SIMS) on a ToF-SIMS5 machine (ION-TOF GmbH, Germany) equipped with a bismuth LMIG pulsed gun incident at 45°. A 25 kV Bi+ primary ion beam was used to generate the secondary ions using burst alignment mode (eight pulses) for analysis and a Cs+ beam (2 kV) incident at 45° for sputtering. The distribution of the oxygen isotopes (18O and 16O) as well as other characteristic relevant secondary ion species (e.g. LaO−, NiO−, and SrO−) in La2−xSrxNiO4+δ samples with x = 0.2 at 625–800 °C and at x = 0.8 were measured using SIMS imaging mode (image acquisition interspersed with Cs+ sputtering of the surface). However, for the samples with low isotope concentration, La2−xSrxNiO4+δ samples with x = 0.2 (at 500 °C), x = 0.4 and x = 0.6, the secondary ions (18O, 16O LaO−, NiO−, and SrO−) from the samples were measured in the depth profiling mode. Oxygen diffusion profiles were obtained by summing all of the 16O (18O) images together and summing all the columns (or rows) of this image, to obtain a 16O (18O) linescan. Subsequently the number of counts was normalized to obtain the 18O fraction corrected for the background isotope fraction and the isotope fraction of the annealing gas, as described by De Souza et al.22 Tracer diffusion coefficients, D*, and surface exchange coefficients, k*, of each La2−xSrxNiO4+δ composition were determined by fitting the diffusion data to the solution of the diffusion equation for diffusion in a semi-infinite medium with surface limitation as given by Crank, and described in (ref. 22). Phase analysis was performed on all samples. The samples were analyzed using a Philips PW 1700 Series X-ray diffractometer using Bragg–Brentano configuration with Cu Kα source (λ = 1.5418 Å) over 20–80° 2θ range. Full profile fitting and refinement was performed to confirm the crystal structure along with the corresponding lattice parameters using JADE 9 X-ray analysis software (MDI, USA).3 Results and discussion
3.1 Oxygen content analysis
Table 1 shows the amount of oxygen over stoichiometry (δ) and the corresponding valence of Ni of the La2−xSrxNiO4+δ samples obtained from the iodometry and TGA. The δ values calculated from both methods are in good agreement, and are within 15% of each other. As the values obtained from both techniques match well, for simplicity, the oxygen non-stoichiometry values referred to in the discussion section are the values obtained from the iodometric titration analysis. With Sr substitution onto the La site at x = 0.2, the oxygen content was found to equal to δ = 0.14, in good agreement with previous findings by Skinner and Kilner.19 This value is significantly lower than which has been previously reported by these authors for x = 0, where the oxygen excess content was found to be 0.24.19 The corresponding valence of Ni was found to +2.48 at x = 0.2. This result indicates that the compensation of the Sr2+ cation substitution onto the La3+ site was mainly accommodated by the reduction of the excess O2− with a small compensation from the change of Ni valence. A similar result was also reported by Skinner and Kilner who studied samples with 5% Sr substitution for La in La2−xSrxNiO4+δ. They observed a decrease in δ from 0.24 to 0.19 upon the Sr substitution while Ni valence stayed constant at +2.48 in both cases.19With a higher level of Sr substitution, x = 0.4 and 0.6, the δ values were found to change slightly from 0.14 to 0.15 and 0.17, while the average Ni valence was found to increase continuously, from +2.48 to +2.70 and +2.94 for x = 0.2, 0.4 and 0.6, respectively. Such results show that under the conditions used in this work, with the level of Sr substitution at x = 0.4–0.6, the charge compensation for Sr2+ was done through the change of Ni valence from Ni2+ to Ni3+ and La2−xSrxNiO4±δ always possesses oxygen hyperstoichiometry with a minimum δ of approximately 0.14. This differs from the results found by other authors who have reported a constant decrease in the amount of oxygen with increasing Sr substitution.13,23–27 The majority of reports proposed the reason for the continuous decrease of the oxygen content being associated with moving from the reduction in the number of oxygen interstitials to the formation oxygen of vacancies upon higher Sr substitution, with structural instability of the perovskite units if a large amount of Ni3+ were to be formed.26 Aguadero et al. reported the critical composition to be La1.5Sr0.5NiO4+δ where the average Ni valence should not exceed +2.5, therefore with higher Sr substitution, the compound becomes oxygen deficient.13 The discrepancy in the results likely arises from how the compounds were synthesized along with slight differences in the experimental details.
With a higher amount of Sr substituted for La, at x = 0.8, a slight increase in δ was found together with a significant increase in Ni oxidation state. The δ value was determined to be 0.23, and the corresponding Ni valence corresponded to +3.26. A Ni average valence is higher than 3 indicates that Ni4+ was formed for the x = 0.8 composition. Although the +4 oxidation state of Ni is rarely found in La2−xSrxNiO4+δ compounds, this situation has been previously reported by Makhnach et al. and Tang et al. in Sr-rich La2−xSrxNiO4+δ.5,14
3.2 X-ray diffraction analysis
Fig. 1 shows XRD patterns of the sintered La2−xSrxNiO4+δ samples used in the oxygen exchange experiment. Over the whole range of compositions tested, the samples exhibit the Ruddlesden–Popper (A2BO4) structure, with the I4/mmm tetragonal crystal structure, in agreement with previous reports by other authors.13,14,19,24 Minor quantities ranging from 0.2–5.1 wt% of La2O3 and NiO were found as impurities in all samples. At x = 0.2–0.6, the total amount of impurities was found to be decreasing with increasing amount of Sr. This is in agreement with what has been found earlier by Nie et al.28 where Sr substitution for La was found to help stabilized the Ruddlesden–Popper structure through the accommodation of Sr2+ for the possible Ni3+ which could cause structural instability. The amount of the La2−xSrxNiO4+δ phase was found to be 89.9, 94.8 and 99.1 wt% at x = 0.2, 0.4 and 0.6, respectively. With NiO or La2O3 impurity, the δ value in La2−xSrxNiO4+δ for the samples is expected to be slightly lower than the calculated one. This is due to the lower probability of Ni being in the Ni3+ state in the NiO than that in La2−xSrxNiO4+δ. Moreover, La2O3 has been known to exist in the very close to stoichiometric form.29 At the highest level of Sr substitution, x = 0.8, additional peaks besides I4/mmm were observed. The additional peaks arise from the other polymorph of La2−xSrxNiO4+δ with a lower symmetry structure which could be identified as the orthorhombic structure adopting space group Fmmm. The possibility of the coexistence of the tetragonal and orthorhombic phases has been reported previously by Aguadero et al. who reported the coexistence of the two different K2NiF4-phases when the Sr content is high as 0.75 and 1 mol ratio.13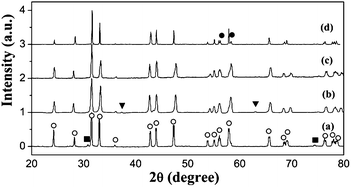 | ||
| Fig. 1 XRD patterns of La2−xSrxNiO4+δ sintered pellets before 18O exchange, (a) x = 0.2, (b) x = 0.4, (c) x = 0.6 and (d) x = 0.8 (○ tetragonal (I4/mmm), ● orthorhombic (Fmmm), ■ La2O3 and ▼ NiO). | ||
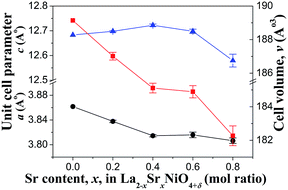
The calculated unit cell parameters and the unit cell volume of the Sr substituted samples are shown in Fig. 2. By increasing the amount of Sr substitution from x = 0 to x = 0.4, the a lattice parameter decreased while the c parameter increased. Such changes in the lattice parameters can be explained by the change in the amount of oxygen excess and the average Ni valence reported in the previous section. With an increase of Sr substitution from x = 0 to 0.4, a slight increase in the amount of oxygen excess could be observed. However, a much more significant increase in the Ni3+ content was found. The higher oxidation state of Ni (Ni2+ to Ni3+) resulted in a decrease in Ni–O bond length thus decreasing the lattice parameter in the perovskite unit, and therefore, a decrease in the lattice parameter in the a, b direction. The slight increase in the c parameter could be explained by the replacement of La3+ by Sr2+(1.27 Å) which has larger ionic radius than La3+(1.20 Å).15 Both effects resulted in a decrease in total cell volume, which is consistent with previous observation by many authors for x = 0 to 0.5.5,13,15,16
At x = 0.6, an opposite trend was observed. The a parameter was found to slightly increase and the c parameter was found to decrease. Such changes when a large amount of Sr was substituted for La in the La2−xSrxNiO4±δ compound were also observed in other reports and could be explained by a Jahn–Teller distortion.5,13,15 However, with x = 0.8, the a parameter did not increase; it decreased instead while the c parameter continued to decrease. This is likely caused by the oxidation of Ni3+ to Ni4+. While the substitution of Sr2+ for La3+ induces an increase in (La,Sr)–O bond length that could lead to an increase in the c lattice parameter, the change of oxidation state from lower to higher charge i.e. Ni3+ to Ni4+ could result in a significant decrease in Ni–O bond length.5 Both effects compete with each other, and in this case, the decrease in Ni–O bond length dominates leading to an overall decrease in the c parameter.
3.3 Oxygen diffusion and surface exchange coefficients
Oxygen isotopic exchange and SIMS analyses were performed on the sintered specimens. LaO−, NiO− and SrO− mapping results showed homogeneous distribution of all species for all samples, except for x = 0.4 in which areas with higher NiO− counts were observed. This result is consistent with the XRD analysis of the x = 0.4 composition where small amounts of NiO were observed (Fig. 1). Table 2 shows the effect of Sr substitution on the oxygen diffusion coefficients (D*) and surface exchange coefficients, k*, at 800 °C. A relatively small change in the surface exchange coefficient was observed across the compositional range studied. This is consistent with previous reports where the surface exchange was found to be more sensitive to parameters influencing the surface state such as synthesis method, and orientation of the crystal.21,30 The oxygen diffusion coefficients, however, were found to be very sensitive to the amount of Sr substitution. Examples of SIMS image and oxygen diffusion profile of La1.8Sr0.2NiO4+δ recorded at 800 °C are shown in Fig. 3.| Temp (°C) | Sr content, x, in La2−xSrxNiO4+δ (mol ratio) | ||||||
|---|---|---|---|---|---|---|---|
| 0 (ref. 19) | 0.1 (ref. 19) | 0.2 | 0.4 | 0.6 | 0.8 | ||
| 550 | D* (cm2 s−1) | — | — | 1.52 × 10−10 | — | — | — |
| k* (cm s−1) | — | — | 1.18 × 10−8 | — | — | — | |
| 625 | D* (cm2 s−1) | — | — | 2.96 × 10−10 | — | — | — |
| k* (cm s−1) | — | — | 3.32 × 10−8 | — | — | — | |
| 700 | D* (cm2 s−1) | 3.38 × 10−8 | 1 × 10−8 | 6.06 × 10−10 | — | — | — |
| k* (cm s−1) | 1.75 × 10−7 | 1.74 × 10−7 | 1.22 × 10−7 | — | — | — | |
| 800 | D* (cm2 s−1) | 1.71 × 10−7 | 1.33 × 10−8 | 1.06 × 10−9 | 1.15 × 10−12 | 3.02 × 10−13 | 2.26 × 10−9 |
| k* (cm s−1) | 2.55 × 10−6 | 6.46 × 10−7 | 2.68 × 10−8 | 5.47 × 10−8 | 2.75 × 10−8 | 7.52 × 10−8 | |
 | ||
| Fig. 3 The (a) normalized 18O SIMS image and (b) 18O concentration of La1.8Sr0.2NiO4+δ after 18O2 exchange at 800 °C for 2 h. | ||
By substituting a small amount of La3+ with Sr2+, i.e. x = 0.2, D* decreased approximately by one order of magnitude with Sr substitution. This trend has previously been observed by other authors.6,9,18,19,22 For example, Skinner and Kilner found that the oxygen diffusion coefficient for 5% Sr substituted La2−xSrxNiO4+δ was lower than in the material with no substitution.19 With the assumption that cation interdiffusion can be neglected coupled with what has been reported earlier that the main transport of oxygen is through interstitialcy mechanism in the oxygen hyper-stoichiometric compound,30 such reduction in the D* value could be explained by a decrease in the amount of oxygen interstitials in the compounds in which δ was reduced.
The La1.8Sr0.2NiO4+δ compound was further evaluated to understand changes in activation energy of the ionic transport in the system compared to that with no substitution and with 5% Sr substitution.19 Additional oxygen exchange experiments were performed for this composition at temperatures between 550–800 °C. The obtained tracer diffusion coefficients and surface exchange coefficients of the La1.8Sr0.2NiO4+δ compound at these temperatures are shown in Fig. 4a and b, respectively. As expected, D* follows the Arrhenius law, increasing with increasing temperature. Since the diffusivity depends on the mobility of oxygen atoms which is strongly affected by temperature, with decreasing temperature, the mobility of oxygen ions also decreases. The activation energy for D* was found to be 0.60 eV (Table 3) close to the values reported by Skinner and Kilner (Ea = 0.57 eV) for La1.8Sr0.2NiO4+δ.19 and lower than that with no Sr substitution (Ea = 0.85 eV). The surface exchange coefficients, k*, for La1.8Sr0.2NiO4+δ were found to also follow the Arrhenius-type behavior for the low temperature range (550–700 °C) with the values similar to those found in the literature for x = 0 and x = 0.2.19,23 However, the k* value do not follow the same trend at 800 °C. The reason for such deviation is not yet conclusive and requires further investigation.
 | ||
| Fig. 4 Arrhenius plots of (a) tracer diffusion coefficients (D*) and (b) surface exchange coefficient (k*) for of La2−xSrxNiO4+δ. | ||
At 800 °C for higher levels of Sr substitution, x = 0.4 to 0.6 the diffusivity values decrease by several orders of magnitude as shown in Fig. 4a and Table 2. At x = 2, D* was found to be 1 × 10−9 cm2 s−1, while it was found that D* was 1 × 10−12 cm2 s−1 for x = 0.4 and 3 × 10−13 cm2 s−1 for x = 0.6. Considering the amount of oxygen excess in the compound which rarely changed between x = 0.2 to 0.6, the decrease in D* is unlikely due to the concentration of the conducting species alone but the change in mobility of the conducting species (oxygen ion).
With higher Sr substitution (x > 0.6), a dramatic increase of approximately 4 orders of magnitude in D* was observed, from 3 × 10−13 cm2 s−1 for x = 0.6 to 2 × 10−9 cm2 s−1, for x = 0.8. Since the orthorhombic phase has a structure that is very similar to the major tetragonal structure31 and from the previous studies by Minervini et al.32 and Cleave et al.33 who have reported similar activation energies for oxygen interstitials in both phases, within the error of the experiments, it could be expected that the oxygen transport property found could be minimally if at all affected by the existence of the orthorhombic phase. Such a large increase in the diffusion coefficient in the sample is likely due to a significant increase in excess oxygen content (δ) which is almost as high as that of the undoped compound (Table 1). However, it is worth noting that the D* value is almost two orders of magnitude lower than that of the undoped composition even with similar oxygen hyperstoichiometric amount. The much lower diffusivity for x = 0.8 is likely due to lower mobility of oxygen ions in the Sr substituted compound compared to that with no Sr substitution. From our previous work, this composition also exhibits the highest electrical conductivity and lowest thermal expansion coefficient over the range of composition studied (x = 0–0.8).11 Although, D* and k* of La1.2Sr0.8NiO4+δ are lower than the values of one of the most promising cathode candidates for IT-SOFCs, La1−xSrxCo1−yFeyO3−δ (LSCF) perovskite (D* ∼ 3 × 10−8 cm2 s−1 and k* ∼ 2 × 10−7 cm s−1),19 the compound exhibits other superior properties. It has higher electrical conductivity at intermediate temperature, as high as 160 S cm−1 at 500 °C, and low thermal expansion coefficient much closer to common IT-SOFC electrolytes, 12.4 × 10−6 °C−1 (400–700 °C) have been previously reported.11
4 Conclusions
Table 4 summarizes the findings of La2−xSrxNiO4+δ (x = 0.2, 0.4, 0.6, and 0.8) synthesized via a sol–gel method. The samples exhibited the I4/mmm tetragonal structure with a minor amount of Fmmm orthorhombic phase for x = 0.8. Trace amount of La2O3 and NiO were found in all samples. Over the entire range of the compositions tested, the samples exhibited oxygen hyperstoichiometry with the minimum δ = 0.14 for x = 0.4. At low levels of Sr substitution (x < 0.2), the lower valence substitution on the A site was accommodated by decreasing the oxygen interstitial content. At higher substitution levels, 0.4 < x < 0.6, the accommodation of Ni with higher valence e.g. Ni3+ to maintain charge neutrality became increasingly important. At x = 0.8, the charge compensation was attributed almost entirely to an increase in Ni oxidation state where the average Ni valence was found to be +3.26. D* was found to be very sensitive to the doping amount while k* changed very little with the amount of doping. At 800 °C, D* decreased with the increasing amount of Sr from 1 × 10−9 cm2 s−1 at x = 0.2 and reached the minimum value of 3 × 10−13 cm2 s−1 at x = 0.6, then increasing again to 2 × 10−9 cm2 s−1 at x = 0.8. The decreasing D* seems to follow the amount of oxygen excess in the compounds up until x = 0.4, then other mechanisms apparently take over.| x, Sr content | Oxygen content analysis | X-ray diffraction analysis | Oxygen transport analysis (800 °C) | ||
|---|---|---|---|---|---|
| δ | Avg Ni valence | Phase | D* (cm2 s−1) | k* (cm s−1) | |
| a T = tetragonal (I4/mmm) phase.b O = orthorhombic (Fmmm) phase. | |||||
| 0.2 | 0.14 ± 0.03 | +2.48 | Ta | 1.06 × 10−9 | 2.68 × 10−8 |
| 0.4 | 0.15 ± 0.01 | +2.70 | Ta | 1.15 × 10−12 | 5.47 × 10−8 |
| 0.6 | 0.17 ± 0.01 | +2.94 | Ta | 3.02 × 10−13 | 2.75 × 10−8 |
| 0.8 | 0.20 ± 0.02 | +3.20 | Ta and Ob | 2.26 × 10−9 | 7.52 × 10−8 |
Acknowledgements
This work was partially funded by the postgraduate education and research programs in Petroleum and Petrochemical Technology (PPT Consortium), Rachadapisake Sompote fund, Chulalongkorn University, the Development and Promotion of Science and Technology, Thailand project (DPST), National Metal and Materials Technology Center (MTEC), Thailand (P-00-30259), a Marie Curie Intra European Fellowship within the seventh European Community Framework Programme (PIEF-GA-2009-252711) and from King Abdullah University of Science & Technology, Saudi Arabia (for M.B). The authors would like to thank Dr Sumittra Charojrochkul from MTEC, Thailand for useful discussion, and staff at the Department of Materials, Imperial College London for their support for the work performed at the Imperial College, London.Notes and references
- E. Boehm, J. M. Bassat, M. C. Steil, P. Dordor, F. Mauvy and J. C. Grenier, Solid State Sci., 2003, 5, 973 CrossRef CAS.
- J. L. Routbort, R. Doshi and M. Krumpelt, Solid State Ionics, 1996, 90, 21 CrossRef CAS.
- R. Chiba, F. Yoshimura and Y. Sakurai, Solid State Ionics, 1999, 124, 281 CrossRef CAS.
- H. Ullmann, N. Trofimenko, F. Tietz, D. Stöver and A. Ahmad-Khanlou, Solid State Ionics, 2000, 138, 79 CrossRef CAS.
- J. P. Tang, R. I. Dass and A. Manthiram, Mater. Res. Bull., 2000, 35, 411 CrossRef CAS.
- Z. Hui, L. Qiang and S. Li-Ping, Sci. China: Chem., 2011, 54, 898 CrossRef.
- M. Greenblatt, Curr. Opin. Solid State Mater. Sci., 1997, 2, 174 CrossRef CAS.
- V. V. Vashook, I. I. Yushkevich, L. V. Kokhanovsky, L. V. Makhnach, S. P. Tolochko, I. F. Kononyuk, H. Ullmann and H. Altenburg, Solid State Ionics, 2009, 119, 23 CrossRef.
- G. Amow and S. J. Skinner, J. Solid State Electrochem., 2006, 10, 538 CrossRef CAS PubMed.
- M. L. Fontaine, C. Laberty-Robert, F. Ansart and P. Tailhades, J. Power Sources, 2006, 156, 33 CrossRef CAS PubMed.
- T. Inprasit, P. Limthongkul and S. Wongkasemjit, J. Electrochem. Soc., 2010, 157, B1726 CrossRef CAS PubMed.
- K. Darcovich, P. S. Whitfield, G. Amow, K. Shinagawa and R. Y. Miyahara, J. Eur. Ceram. Soc., 2005, 25, 2235 CrossRef CAS PubMed.
- A. Aguadero, M. J. Escudero, M. Perez, J. A. Alonso, V. Pomjakushin and L. Daza, Dalton Trans., 2006, 4377 RSC.
- L. V. Makhnach, V. V. Pankova and P. Strobel, Mater. Chem. Phys., 2008, 111, 125 CrossRef CAS PubMed.
- J. Gopalakrishnan, G. Colsmann and B. Reuter, J. Solid State Chem., 1977, 22, 145 CrossRef CAS.
- Y. Takada, R. Kanno, M. Sakano and O. Yamamoto, Mater. Res. Bull., 1990, 25, 293 CrossRef.
- H. Zhao, F. Mauvy, C. Lalanne, J. M. Bassat, S. Fourcade and J. C. Grenier, Solid State Ionics, 2008, 179, 2000 CrossRef CAS PubMed.
- V. V. Kharton, A. P. Viskup, A. V. Kovalevsky, E. N. Naumovich and F. M. B. Marques, Solid State Ionics, 2001, 143, 337 CrossRef CAS.
- S. J. Skinner and J. A. Kilner, Solid State Ionics, 2000, 135, 709 CrossRef CAS.
- K. Ishikawa, S. Kondo, H. Okano, S. Suzuki and Y. Suzuki, Bull. Chem. Soc. Jpn., 1987, 60, 1295 CrossRef CAS.
- E. Boehm, J. M. Bassat, P. Dordor, F. Mauvy, J. C. Grenier and P. Stevens, Solid State Ionics, 2005, 176, 2717 CrossRef CAS PubMed.
- R. A. De Souza, J. Zehnpfenning, M. Martin and J. Maier, Solid State Ionics, 2005, 176, 1465 CrossRef CAS PubMed.
- R. Sayers, R. A. De Souza, J. A. Kilner and S. J. Skinner, Solid State Ionics, 2010, 181, 386 CrossRef CAS PubMed.
- A. Aguadero, J. A. Alonso, M. J. Martinez-Lope, M. T. Fernandez-Diaz, M. J. Escudero and L. Daza, J. Mater. Chem., 2006, 16, 3402 RSC.
- Z. Junjiang and A. Thomas, Appl. Catal., B, 2009, 92, 225 CrossRef PubMed.
- Z. Junjiang, Y. Xiangguang, X. Xuelian and W. Keme, Sci. China, Ser. B, 2007, 50, 41 CrossRef.
- A. Manthiram, J. P. Tang and V. Manivannan, J. Solid State Chem., 1999, 148, 499 CrossRef CAS.
- H. W. Nie, T.-L. Wen, S. R. Wang, Y. S. Wang, U. Guth and V. Vashook, Solid State Ionics, 2006, 177, 1929 CrossRef CAS PubMed.
- J. M. Bassat, P. Odier, A. Villesuzanne, C. Marin and M. Pouchard, Solid State Ionics, 2004, 167, 341 CrossRef CAS PubMed.
- M. Burriel, G. Garcia, J. Santio, J. A. Kilner, R. J. Chater and S. J. Skinner, J. Mater. Chem., 2008, 18(4), 416 RSC.
- C. Frayret, A. Villesuzanne and M. Pouchard, Chem. Mater., 2005, 17, 6538 CrossRef CAS.
- L. Minervini, R. W. Grimes, J. A. Kilner and K. E. Sickafus, J. Mater. Chem., 2000, 10, 2349 RSC.
- A. R. Cleave, J. A. Kilner, S. J. Skinner, S. T. Murphy and R. W. Grimes, Solid State Ionics, 2008, 179, 823 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |