Solid phase extraction and ultra performance liquid chromatography-tandem mass spectrometric identification of carcinogenic/mutagenic heterocyclic amines in cooked camel meat
Mohammad Rizwan Khan*,
Mu Naushad,
Zeid Abdullah Alothman,
Ibrahim Hotan Alsohaimi and
Mohammad Saad Algamdi
Advanced Materials Research Chair, Department of Chemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Kingdom of Saudi Arabia. E-mail: mrkhan@ksu.edu.sa; Fax: +966 114675992; Tel: +966 114674198
First published on 21st November 2014
Abstract
In the present study, three kinds of camel (Mjahim, Mgatir and Humor) from Saudi Arabia have been studied for heterocyclic amines (HAs) contents in their cooked meat. The camel meats were cooked using common cooking practices, such as frying, griddling, stewing and barbequing, under controlled temperature. The investigated HAs were IQ, MeIQ, MeIQx, 4,8-DiMeIQx and PhIP. An analytical method based solid-phase extraction and ultra performance liquid chromatography-tandem mass spectrometry was used for the analysis of HAs in cooked samples. The level of IQ and MeIQ were found either below the limit of quantification or not detected in all of the analyzed samples. The fried samples produced MeIQx, 4,8-DiMeIQx and PhIP, between 2.13 ng g−1 and 5.89 ng g−1, whereas the griddled and barbequed samples generated MeIQx, 4,8-DiMeIQx and PhIP ranging from 0.93 to 4.34 ng g−1. The stewing method applied to meat samples generated only PhIP at concentrations between 0.4 ng g−1 and 0.65 ng g−1, while MeIQx and 4,8-DiMeIQx were found to be below the limit of quantification. The low levels of HAs in stewing method might be explained from the fact that the samples were not directly in contact with the cooking pot or blaze, which influences the formation of HAs. These outcomes suggest that camel meat is a significant dietary source of HAs and can be used in epidemiological studies to approximate HA exposure from dietary questionnaires.
1. Introduction
The occurrence of carcinogenic compounds, such as HAs, in foods was first reported in 1939 by a Swedish scientist, E.M.P. Widmark.1 These findings initiated the study of HAs, and since then HAs have been isolated from various proteinaceous cooked foods such as meat and fish.2–4 Some of these HAs have been classified as probable or possible human carcinogens by the International Agency for Research on Cancer (IARC).5 Subsequently, the US National Toxicology Program (NTP) has also classified some HAs as reasonably anticipated human carcinogens.6 Intense research related to their formation, metabolism and carcinogenicity has been performed to assess the relevance of HAs to human cancer.7HAs have been classified into two major groups, amino carbolines and aminoimidazoazaarenes (AIAs).8 Amino carbolines, for example Trp-P-1, Trp-P-2, AαC and MeAαC, are produced by the pyrolysis of amino acids via free radical reaction at temperatures above 300 °C.9 Conversely, AIAs, for instance DMIP, IQ, MeIQ, MeIQx, 4,8-DiMeIQx and PhIP, are produced at normal cooking temperatures through the aldol condensation of pyridines and pyrazines, resulting into a Maillard reaction between sugars, free amino acids, creatine and creatinine.10 Harman and norharman are usually referred to as co-mutagens for the reason that they are not mutagenic in the Ames/Salmonella test because they enhance the genotoxicity of mutagenic heterocyclic amines.11 HAs undergo metabolic activation by means of N-hydroxylation of the exocyclic amine group to generate arylnitrenium ion intermediate, which is the critical metabolite implicated in DNA damage and toxicity.12
To date, more than twenty-five HAs have been characterized as potent mutagens in Ames/Salmonella assay.13–15 A lot of these mutagenic HAs have been isolated from various protein-rich foods such as cooked meat and fish.2–4,16,17 A number of HAs have also been detected in environmental constituents such as diesel exhaust and airborne particles,18 river water and mainstream cigarette smoke.19,20 To approximate the ingestions and threats to human health, it is highly essential to measure HAs contents in various meat products cooked in diverse ways. The range and quantity of HAs are determined by the factors involved in the cooking process such as temperature, time, heat transfer and the composition of meat/fish.8 The aim of the present study was to evaluate for the first time the occurrence of HAs in camel meat processed under controlled cooking parameters and to approximate the deviation in HA content in the same type of camel prepared by diverse cooking process. The results obtained from the proposed work will raise awareness in the Saudi population, as well as among the worldwide population about these carcinogenic compounds, and it could be used to estimate the intake of HAs from cooked camel meat.
2. Material and methods
2.1. Chemicals and reagents
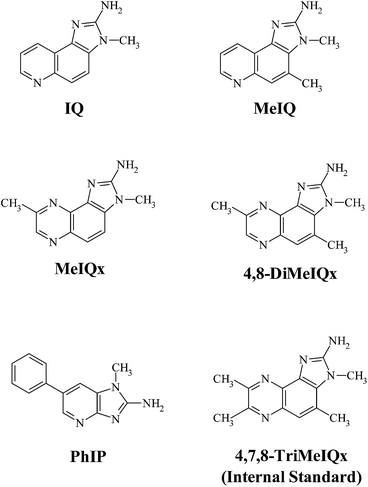
HPLC-grade ethyl acetate, acetonitrile and methanol were obtained from Merck (Darmstadt, Germany). Water was purified through a Milli-Q water purification system (Millipore Corporation, Bedford, USA). Formic acid (98%), ammonium acetate and ammonium formate were purchased from Merck (Darmstad, Germany), and ammonia solution (25%) was supplied from Panreac Quimica (Barcelona, Spain). Sodium hydroxide (NaOH) was obtained from BDH Laboratory Supplies (Poole, UK). All the supplied chemicals were of analytical grade. Hydromatrix bulk material was obtained from Agilent Technologies (Apple Valley, USA), and Extrelut NT20 extraction cartridges were provided by Merck (Darmstad, Germany). Bond Elut propylsulfonyl silica PRS (500 mg) and octadecylsilane C18 (100 mg) cartridges, stopcocks, coupling pieces were obtained from Varian (Harbor City, USA). The sample was bottled in 40 mL amber flasks (Thermo Scientific, Rockwood, USA) provided with screw cap and PTFE seal.The studied HAs are shown in Fig. 1. They are 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx), 2-amino-3,4,7,8-tetramethylimidazo[4,5-f]quinoxaline (4,7,8-TriMeIQx), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), which were purchased from Toronto Research Chemicals (Toronto, Canada). The chemical purity of the studied HAs was more than 99%. 4,7,8-TriMeIQx was used as an internal standard (I.S.). Stock standard solutions of each amine at concentration level of 200 μg g−1 were prepared in methanol and used for further dilutions. Standard mixtures of the investigated HAs at concentration levels between 0.0002 μg g−1 and 1.00 μg g−1 containing 4,7,8-TriMeIQx (0.5 μg g−1) as internal standard were prepared by weight to establish the range of linearity and for the construction of calibration curves in all the systems. All the solutions and the sample purified fractions were passed through a 0.22 μm PTFE filter (Macherey-Nagel Gmbh, Düren, Germany) before being injected into the ultra performance liquid chromatography (UPLC) system.
The camel meat cooking temperatures were measured with type-K insulated-wire probes and monitored with the Normadics TC6 software (Cole-Parmer, Vernon Hills, USA). The cooked food was blended with a Microtron® MB 800 (Kinematica AG, Littau, Switzerland). For sample analysis, the ground cooked meat was mixed with NaOH (1 M) and homogenized with an Ultra-Turrax® T25 digital (IKA®, Staufen, Germany). For the solid-phase extraction procedure and solvent evaporation, Visiprep™ and Visidry™ vacuum manifolds (Supelco, Gland, Switzerland) were used.
2.2. Samples treatment
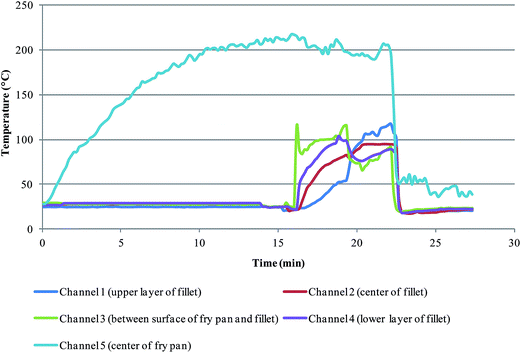
Fresh camel meats of different kinds (Mjahim, Mgatir and Humor) were obtained from a local supermarket, (Riyadh, Saudi Arabia). The meats were prepared into fillets of dimension (6 cm × 6 cm × 1 cm). A total of 6 kg of raw camel meat was thermally processed in different batches using different cooking methods. The fillets were pan-fried using olive oil (Tafel, France) over an electric heater (Nippotec, China), griddled on an electric griddle plate (Palson Rodeo, China), stewed in a Teflon-coated pressure cooker (Prestige, India) over an electric heater and barbequed over electric barbeque grill (Severin, China). During the frying method, five probes were used to monitor the temperature at 2 mm below the meat surface, which were at both the upper and lower sides and of the fillet; in the centre of fillet; between pan and meat, and in centre of pan. Cooking temperature variation has been illustrated in Fig. 2. Probes were calibrated before the cooking procedure by plunging them in boiling Milli-Q water and assigning the measured temperature to be 100 °C. The duration of the cooking process was 4 minutes and the fillets were turned once after 2 minutes the cooking process began. The cooking temperature was recorded every 5 seconds. The cooked meats were allowed to cool to room temperature followed by weighing and finally the weight loss of the cooked samples was calculated. The cooking parameters and the weight loss in cooked meat samples are illustrated in Table 1. The cooked meat samples were then ground using a Microtron MB 800 food blender followed by labeling, bottling and storing at −30 °C until analysis. The weight loss was calculated as the variation between the weight of meat samples before and after the cooking process.| Meat | Type (Arabic) | Raw meat (g) | Cooking method | Cooking temperature (°C) | Cooking time (min/side) | Cooking weight loss (%) |
|---|---|---|---|---|---|---|
| Camel | Mjahim | 300–400 | Frying | 220 | 4 | 47.10 |
| Mgatir | 300–400 | Frying | 220 | 4 | 44.31 | |
| Humor | 300–400 | Frying | 220 | 4 | 45.22 | |
| Camel | Mjahim | 400–500 | Griddling | 210 | 5 | 25.54 |
| Mgatir | 400–500 | Griddling | 210 | 5 | 27.13 | |
| Humor | 400–500 | Griddling | 210 | 5 | 25.97 | |
| Camel | Mjahim | 300–400 | Stewing | 220 | 6 | 15.65 |
| Mgatir | 300–400 | Stewing | 220 | 6 | 14.32 | |
| Humor | 300–400 | Stewing | 220 | 6 | 15.89 | |
| Camel | Mjahim | 300–400 | Barbequing | 210 | 5 | 20.12 |
| Mgatir | 300–400 | Barbequing | 210 | 5 | 19.80 | |
| Humor | 300–400 | Barbequing | 210 | 5 | 19.25 |
2.3. Sample extraction and purification
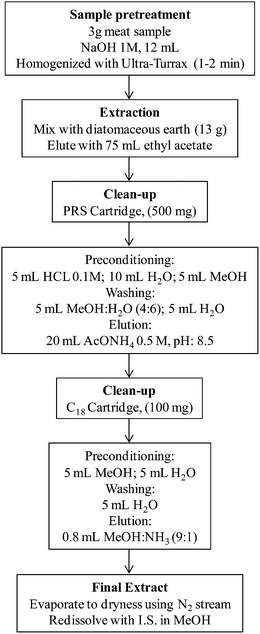
The sample extraction and purification method applied for the analysis of HAs had been formerly developed for cooked meat.21 Briefly, 3 g of ground cooked camel meat was homogenized with NaOH (12 mL, 1 M) using an Ultra-Turrax T25 digital. The alkaline semisolid meat sample was thoroughly mixed with 13 g of diatomaceous earth (refill material) and was loaded into an empty extraction cartridge, which was attached on-line with a Bond Elut PRS (500 mg) cartridge, formerly preconditioned with HCl (5 mL, 0.1 M) followed by Milli-Q water (10 mL) and methanol (5 mL). HAs were extracted from diatomaceous earth and eluted to the Bond Elut PRS (500 mg) cartridge by means of ethyl acetate (75 mL) as an extraction solvent. The PRS cartridge was vacuum dried using Visiprep and sequentially washed with a mixture of methanol–water (15 mL, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v) and Milli-Q water (5 mL). Subsequently, the PRS cartridge was coupled to a Bond Elut C18 cartridge, which was previously preconditioned with methanol (5 mL) and Milli-Q water (5 mL), and HAs were desorbed with ammonium acetate (20 mL, 0.5 M) at pH 8.5. Finally, a C18 cartridge was rinsed with Milli-Q water (5 mL) and dried out under slight vacuum, and the HAs were eluted to eppendorf (1.5 mL) using a mixture of methanol–ammonia (800 μL, 9
6, v/v) and Milli-Q water (5 mL). Subsequently, the PRS cartridge was coupled to a Bond Elut C18 cartridge, which was previously preconditioned with methanol (5 mL) and Milli-Q water (5 mL), and HAs were desorbed with ammonium acetate (20 mL, 0.5 M) at pH 8.5. Finally, a C18 cartridge was rinsed with Milli-Q water (5 mL) and dried out under slight vacuum, and the HAs were eluted to eppendorf (1.5 mL) using a mixture of methanol–ammonia (800 μL, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The solvent was gently evaporated under a stream of nitrogen and the filtrate was reconstituted with methanolic solution (100 μL) containing I.S. (4,7,8-TriMeIQx). The flowchart for the sample preparation is demonstrated in Fig. 3.
1, v/v). The solvent was gently evaporated under a stream of nitrogen and the filtrate was reconstituted with methanolic solution (100 μL) containing I.S. (4,7,8-TriMeIQx). The flowchart for the sample preparation is demonstrated in Fig. 3.
Quantification and estimation of recoveries relating to HAs were carried out by the standard addition method using two non-spiked samples and three spiked levels. Spiked samples were prepared by adding calculated volume of a methanolic solution of five HAs equivalent to the three spiking levels (50, 100 and 500%) before homogenization with diatomaceous earth. External calibration curves for five HAs were constructed and used for recovery calculations. Recovery rates of each HAs were measured from the slope of the linear regression obtained between the added analyte concentration and the calculated analyte concentration.
2.4. Instrumentation
The characterization of raw and cooked camel meat was performed in terms of the free amino acids by a Biochrome 20 amino acid analyzer (Cambridge, UK) using ion-exchange chromatography with a column of polystyrene–divinylbenzene stationary phase with sulphonate groups (Pharmacia LKB Biotechnology, Biochrome, Cambridge, UK).22 The content of ashes, fat, moisture (based on gravimetric methods) and total nitrogen (by the Kjeldahl method) were determined according to the AOAC procedures.23–25 Analyses were carried out in triplicate. Density was calculated by measuring the volume and weight of the material at room temperature.The analysis of HAs was carried out on an Acquity® ultra performance liquid chromatography (UPLC) system equipped with a quaternary pump (Waters, Milford, USA). The reversed-phase analytical column used was an Acquity® BEH C18 column (50 mm × 2.1 mm id, 1.7 μm particle size) (Waters, Milford, USA). The most favorable separation was attained with a binary mobile phase at a flow rate of 1 mL min−1. Solvent A: acetonitrile and solvent B: 30 mM formic acid/ammonium formate (pH 4.75) were used. The gradient elution program was as follows: 0–0.1 min, 5% A; 0.1–1.5 min, 5–30% A; 1.5–1.8 min, 30–60% A; 1.8–2.4 min, 60% A; 2.4–2.5 min, return to its initial conditions; 2.5–3 min, equilibration of the column. The sample injection volume was 10 μL.26 The analytical column was rinsed with water–methanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) for 5 min after every 10 injections.
50, v/v) for 5 min after every 10 injections.
The UPLC system was coupled to a Quattro Premier triple quadrupole mass spectrometer (Micromass, Milford, USA) using the electrospray ionization (ESI) source in the form of Z-spray. For HAs, the mass spectrometer apparatus was operated in the positive mode and the data was acquired in multiple reaction monitoring (MRM) mode. The source operational parameters were as follows: capillary voltage, 3.0 kV; cone voltage, 40 V; desolvation temperature, 400 °C; source temperature, 100 °C; cone gas flow rate, 49 L h−1; desolvation gas flow rate, 804 L h−1. Nitrogen gas of high purity (99.99%), generated using a nitrogen generator model NM30LA (Peak Scientific, Inchinnan, UK), and argon were used as the cone and collision gases, respectively. An Oerlikon rotary pump, model SOGEVACSV40 BI (Cedex, France) was used to provide the primary vacuum to the MS system. The MRM parameters applied with the MS/MS system have been presented in Table 2.26 Data acquisition was carried out by the MassLynx V4.1 software (Waters, Milford, USA).
| HAs | Precursor ion [M + H]+ m/z | Quantification | Confirmation | ||
|---|---|---|---|---|---|
| Product ion (m/z) | Collision energy (eV) | Product ion (m/z) | Collision energy (eV) | ||
| a Dwell time was 0.025 s in all the cases. | |||||
| IQ | 199 | 184 | 30 | 157 | 35 |
| MeIQ | 213 | 198 | 25 | 197 | 30 |
| MeIQx | 214 | 199 | 30 | 131 | 25 |
| 4,8-DiMeIQx | 228 | 213 | 30 | 187 | 25 |
| 4,7,8-TriMeIQx | 242 | 227 | 25 | 201 | 30 |
| PhIP | 225 | 210 | 25 | 183 | 30 |
3. Results and discussion
3.1. Preparation and characterization of camel meat
The contents of HAs produced during cooking depend on the type of meat and cooking methods. In this study, three different kinds of camel meat were thermally processed in ways to represent most common cooking practices, such as frying, griddling, stewing and barbequing, under controlled temperature, which have been analyzed for five heterocyclic amines. As an example, Fig. 2 demonstrates the temperature variation at four sites of fillets and pan temperature in one of the cooking processes (Frying). The total raw camel meat of 6 kg was cooked in a number of batches using diverse cooking methods (Frying, Griddling, Stewing and Barbequing). In all the cases, the cooking treatment was within the range as demonstrated in Fig. 2. Obtaining temperature profiles for the duration of cooking is very essential, when studying Maillard reaction products, for instance HAs, and this has been carried out in previous studies.27,28 The camel meat has been thermally processed under moderate conditions; no charring was observed.To characterize the camel meat, a number of parameters, such as moisture, ash, fat, protein and free amino acid contents, were studied in both the raw as well as cooked samples. The percentage varied from raw to cooked meat, moisture (74.06% to 3.68%), ashes (1.03% to 2.92%), fat (3.56% to 3.87%), protein (22.72% to 22.56%) and free amino acids (28.83% to 24.21%), (Table 3). The obtained values are considerably similar to beef and fish meat composition29 and also to the previously studied camel meat.30 The amount of HAs in the cooked meat samples are influenced by the concentrations of amino acids. Nevertheless, certain amino acids play a role in the formation of specific HAs. Few amino acids, for instance histidine, exhibit a chemoprotective effect against the formation of a number of heterocyclic amine such as PhIP.3
| Parameters | Raw meat, % value ± sc (n = 3) | Cooked meat, % value ± s (n = 3) |
|---|---|---|
| a Value based on 50 g raw weight.b Protein = NX6.25.c Standard deviation. | ||
| Moisture (%) | 74.06 ± 1.1 | 3.68 ± 2.2 |
| Ashes (%) | 1.03 ± 3.2 | 2.92 ± 2.5 |
| Fat (%) | 3.56 ± 2.1 | 3.87 ± 2.1 |
| Proteinb (%) | 22.72 ± 1.4 | 22.56 ± 1.4 |
| Free amino acids (mg g−1) | 28.83 ± 1.2 | 24.21 ± 1.3 |
3.2. Detection and quantification of HAs
Eating thermally processed meat and fish has been epidemiologically associated to food borne outbreaks. Thus, the detection and quantification of HAs in camel meat is toxicologically important. To our knowledge, this is the first report of HA levels in camel meat that is frequently consumed in the Saudi Arabia and prepared by household cooking methods under controlled temperature. We studied the occurrence of five HAs, namely, IQ, MeIQ, MeIQx, 4,8-MeIQx and PhIP, that can be found in cooked meat and fish products and have been usually investigated and reported in numerous literatures.2,3,21,31 The results of the determination of HAs in cooked camel meat are presented in Table 4, where it can be observed that PhIP was the most frequently detected HAs in all the samples and was found at the maximum concentration from 0.40 ng g−1 to 5.89 ng g−1, followed by MeIQx (below the limit of quantification to 2.63 ng g−1) and 4,8-DiMeIQx (below the limit of quantification to 2.42 ng g−1); neither IQ nor MeIQ was detected in any of the analyzed sample. The detection limits and recovery rates are displayed in Table 5. The detection limits and recovery rates estimated for IQ, MeIQ, MeIQx, 4,8-MeIQx and PhIP range from 0.01 to 0.03 ng g−1 and from 55 to 67%, respectively. From the obtained results, it was found that these amines were extracted more efficiently from camel meat than from beef samples studied in an earlier work using the similar SPE clean-up procedure, except for that dichloromethane was used as a substitute of ethyl acetate as extraction solvent.21 The enhancement in the recovery rates can be due to the little diverse composition of camel meat from beef and due to the extraction solvent. To demonstrate the results, the chromatograms of HAs detected in fried camel (Mjahim) sample determined by UPLC-MS/MS in MRM acquisition mode are displayed in Fig. 4; the structures and transitions between precursor and product ions are also specified in each window. Good sensitivity and selectivity were achieved and were found to be in accordance with the previous studies.22,26| Sample | IQ | MeIQ | MeIQx | 4,8-DiMeIQx | PhIP |
|---|---|---|---|---|---|
| a Standard deviation obtained from standard addition calibration curve.b Amount detected below the limit of quantification (signal-to-noise ratio of 10).c nd: not detected. | |||||
| Camel, Mjahim (Fried) | nqb | nq | 2.63 ± 0.15 | 2.42 ± 0.13 | 5.89 ± 0.18 |
| Camel, Mgatir (Fried) | nq | nq | 2.24 ± 0.14 | 2.14 ± 0.14 | 5.36 ± 0.17 |
| Camel, Humor (Fried) | nq | nq | 2.33 ± 0.15 | 2.13 ± 0.14 | 5.63 ± 0.17 |
| Camel, Mjahim (Griddled) | nd | nd | 1.67 ± 0.12 | 1.01 ± 0.12 | 3.92 ± 0.14 |
| Camel, Mgatir (Griddled) | nd | nd | 1.39 ± 0.12 | 0.93 ± 0.11 | 3.67 ± 0.13 |
| Camel, Humor (Griddled) | nd | nd | 1.54 ± 0.12 | 0.98 ± 0.11 | 3.85 ± 0.14 |
| Camel, Mjahim (Stewed) | nd | nd | nq | nq | 0.65 ± 0.002 |
| Camel, Mgatir (Stewed) | nd | nd | nq | nq | 0.40 ± 0.001 |
| Camel, Humor (Stewed) | nd | nd | nq | nq | 0.49 ± 0.001 |
| Camel, Mjahim (Barbequed) | nd | nd | 2.01 ± 0.12 | 1.67 ± 0.12 | 4.34 ± 0.14 |
| Camel, Mgatir (Barbequed) | nd | nd | 1.85 ± 0.12 | 1.55 ± 0.11 | 3.88 ± 0.13 |
| Camel, Humor (Barbequed) | nd | nd | 1.96 ± 0.12 | 1.59 ± 0.11 | 4.14 ± 0.14 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
| Sample | IQ | MeIQ | MeIQx | 4,8-DiMeIQx | PhIP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOD, ng g−1 | R, % | LOD, ng g−1 | R, % | LOD, ng g−1 | R, % | LOD, ng g−1 | R, % | LOD, ng g−1 | R, % | |
| Camel, Mjahim (Fried) | 0.03 | 65 | 0.02 | 59 | 0.02 | 56 | 0.01 | 56 | 0.02 | 62 |
| Camel, Mgatir (Fried) | 0.02 | 65 | 0.02 | 58 | 0.03 | 55 | 0.02 | 58 | 0.01 | 61 |
| Camel, Humor (Fried) | 0.03 | 64 | 0.03 | 59 | 0.02 | 56 | 0.02 | 57 | 0.02 | 61 |
| Camel, Mjahim (Griddled) | 0.02 | 66 | 0.03 | 60 | 0.01 | 56 | 0.01 | 58 | 0.02 | 57 |
| Camel, Mgatir (Griddled) | 0.02 | 66 | 0.02 | 61 | 0.02 | 57 | 0.02 | 56 | 0.01 | 57 |
| Camel, Humor (Griddled) | 0.02 | 67 | 0.03 | 60 | 0.01 | 57 | 0.01 | 58 | 0.01 | 58 |
| Camel, Mjahim (Stewed) | 0.02 | 67 | 0.01 | 57 | 0.01 | 61 | 0.02 | 58 | 0.01 | 61 |
| Camel, Mgatir (Stewed) | 0.02 | 65 | 0.02 | 58 | 0.01 | 62 | 0.01 | 57 | 0.01 | 63 |
| Camel, Humor (Stewed) | 0.02 | 65 | 0.02 | 60 | 0.02 | 61 | 0.02 | 58 | 0.01 | 63 |
| Camel, Mjahim (Barbequed) | 0.03 | 66 | 0.03 | 61 | 0.03 | 61 | 0.03 | 59 | 0.02 | 65 |
| Camel, Mgatir (Barbequed) | 0.03 | 64 | 0.02 | 59 | 0.02 | 60 | 0.03 | 58 | 0.02 | 64 |
| Camel, Humor (Barbequed) | 0.02 | 64 | 0.03 | 59 | 0.03 | 58 | 0.02 | 57 | 0.01 | 65 |
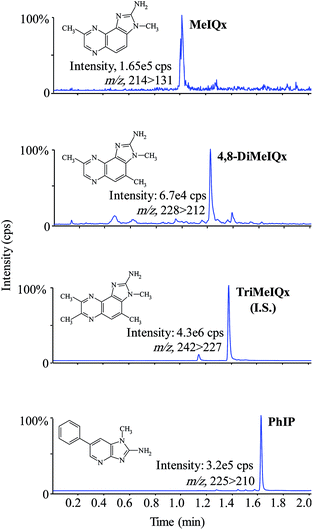 | ||
| Fig. 4 UPLC-MS/MS chromatogram of HAs in fried camel (Mjahim). The transitions between precursor and product ions are designated in each window. | ||
According to the outcomes, IQ and MeIQ have not been found very often in cooked meats, the reason for this fact can possibly be that elevated thermal treatment are needed for their formation.10 Conversely, MeIQx, and 4,8-DiMeIQx are generally formed at concentrations greater than 0.1 ng g−1 at cooking temperatures of approximately 210 °C,31 while the level of these HAs detected in camel meat were up to 2.63 ng g−1. However, the cooking parameters applied in the present work are hard to evaluate as compared to the earlier studies. The amounts of PhIP found in the cooked camel meat were lower compared to the concentrations quantified in the foodstuffs reported in the published works, which ranges up to 121 ng g−1, which is usually found at the highest levels in swordfish, followed by poultry meat,3,31 and the lowest concentrations are found in offal products.2 In fact, PhIP has been designated as one of the heterocyclic amines with the most important contribution on a daily basis ingestion of HAs.32,33
The amounts of HAs are greatly affected by the heat transference during the cooking process.34 In stewing method, the meat is less in contact with the heating surface and frequent boiling affects the heat transfer.35 Thus, in the present study, the stewing method produced low cooking weight loss of up to 15.89% and only one type of HAs (PhIP) was detected up to 0.65 ng g−1. The other HAs, such as IQ, MeIQ, MeIQx and 4,8-DiMeIQx, were either not detected or found to be below the limit of quantification (Table 4). On the contrary, it can be seen in Table 4 that the meat cooked by frying, griddling and barbequing methods, comparatively provided more heat transference. Therefore, they resulted in high cooking weight loss of up to 47.10% and high amounts of HAs up to 5.89 ng g−1. These data indicated that the cooking method having a high cooking weight loss influenced the level of HAs formation. Furthermore, it is also clear that the amounts of HAs in such types of camel meat were found to be in comparable range, cooked by same method. It is also revealed that the meat compositions values of the studied camels were similar.
4. Conclusions
HAs have been analyzed in three types of camels (Mjahim, Mgatir and Humor) from Saudi Arabia. The meats were cooked using ordinary cooking practices, for instance frying, griddling, stewing and barbequing. The fried samples showed high cooking weight loss and produced higher level of heterocyclic amines. In contrast, the stewed samples demonstrated low cooking weight loss and produced only one type of HAs (PhIP), which was also at a very low concentration. Thus, using stewing method, the amount of HAs can be kept low. From the obtained results, it has been concluded that the cooking weight loss played a vital role on the formation of heterocyclic amines in such type of meat samples. The data obtained from the present study could be used to evaluate the human intake of HAs in Saudi Arabia, and it signifies that a simple cooking method (stewing) reduces the threat of exposure to HAs, and therefore to obtain better food quality and protection.Acknowledgements
This work was supported by NSTIP strategic technologies program number (12-AGR2594-02) in the Kingdom of Saudi Arabia.References
- E. M. P. Widmark, Nature, 1939, 143, 984 CrossRef.
- M. R. Khan, L. M. Bertus, R. Busquets and L. Puignou, Food Chem., 2009, 112, 838–843 CrossRef CAS PubMed.
- M. R. Khan, R. Busquets, J. Saurina, S. Hernández and L. Puignou, Chem. Res. Toxicol., 2013, 26, 1014–1022 CrossRef CAS PubMed.
- T. Sugimura, K. Wakabayashi, M. Nagao and H. Esumi, A new class of carcinogens: heterocyclic amines in cooked food, in Food, Nutrition and Chemical Toxicity, ed. D.V. Parke, C. Ioannides, R. Walker and G. Smith, Nishimura Ltd., 1993, pp. 259–276 Search PubMed.
- IARC, Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines, and Mycotoxins, IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, International Agency for Research on Cancer, Lyon, France, vol. 56, 1993, pp. 165–242 Search PubMed.
- NTP, Report on Carcinogens, US Department of Health and Human Services, Public Health Services, Washington DC, USA, 11th edn, 2004 Search PubMed.
- T. Sugimura, Nutrition and dietary carcinogens, Carcinogenesis, 2000, 21, 387–395 CrossRef CAS PubMed.
- M. Jagerstad, K. Skog, P. Arvıdsson and A. Solyakov, Z. Lebensm.-Unters. -Forsch. A, 1998, 207, 419–427 CrossRef.
- M. Jagerstad, A. Laser-Reutersward, R. Olsson, S. Grivas and T. Nyhammar, Food Chem., 1983, 12, 255–264 CrossRef.
- P. Arvidsson, M. A. J. S. Vanboekel, K. Skog and M. Jagerstad, J. Food Sci., 1997, 62, 911–916 CrossRef CAS PubMed.
- T. Sugimura, M. Nagao and K. Wakabayashi, Adv. Exp. Med. Biol., 1982, 136B, 1011–1025 CAS.
- R. J. Turesky and L. Le Marchand, Chem. Res. Toxicol., 2011, 24, 1169–1214 CrossRef CAS PubMed.
- T. Sugimura, Multistep carcinogenesis: A 1992 perspective. Sci., 1992, vol. 258, pp. 603–607 Search PubMed.
- J. S. Felton, M. G. Knize, N. H. Shen, P. R. Lewis, B. D. Andresen, J. Happe and F. T. Hatch, Carcinogenesis, 1986, 7, 1081–1086 CrossRef CAS PubMed.
- G. Becher, M. G. Knize, I. F. Nes and J. S. Felton, Carcinogenesis, 1988, 9, 247–253 CrossRef CAS PubMed.
- U. Rahman, A. Sahar, M. I. Khan and M. Nadeem, LWT--Food Sci. Technol., 2014, 59, 229–233 CrossRef CAS PubMed.
- K. Skog and M. Jagerstad, Modifying cooking conditions and ingredients to reduce the formation of heterocyclic amines, in Acrylamide and other hazardous compounds in heat-treated foods, ed. K. Skog and J. Alexander, Woodhead Publishing Ltd, Cambridge, 2006, pp. 407–424 Search PubMed.
- S. Manabe, N. Kurihara, T. Shibutani, O. Wada, A. Ueki and H. Suzuki, Carcinogenesis, 1993, 14, 903–906 CrossRef CAS PubMed.
- Y. Ono, I. Somiya and Y. Oda, Water Res., 2000, 34, 890–894 CrossRef CAS.
- T. A. Saski, J. M. Wilkins, J. B. Forehand and S. C. Moldoveanu, Anal. Lett., 2001, 34, 1749–1761 CrossRef PubMed.
- F. Toribio, L. Puignou and M. T. Galceran, J. Chromatogr. A, 1999, 836, 223–233 CrossRef CAS.
- D. H. Spackman, W. D. Stein and S. Moore, Anal. Chem., 1958, 30, 1185–1190 CrossRef.
- Official Methods of Analysis of AOAC International, 17th ed., vol. II, p. 39.1.08.
- Official Methods of Analysis of AOAC International, 17th ed., vol. II, p. 39.1.09.
- Official Methods of Analysis of AOAC International, 17th ed., vol. II, p. 39.1.15.
- E. Barcelo-Barrachina, E. Moyano, M. T. Galceran, J. L. Lliberia, B. Bago and M. A. Cortes, J. Chromatogr. A, 2006, 1125, 195–203 CrossRef CAS PubMed.
- E. Persson, I. Sjöholm and K. Skog, Eur. Food Res. Technol., 2002, 214, 455–459 CrossRef CAS PubMed.
- E. Persson, G. Graziani, R. Ferracane, V. Fogliano and K. Skog, Food Chem. Toxicol., 2003, 41, 1587–1597 CrossRef CAS.
- K. Puangsombat, P. Gadgil, T. A. Houser, M. C. Hunt and J. S. Smith, Meat Sci., 2012, 90, 739–746 CrossRef CAS PubMed.
- S. A. Babiker and O. K. Yousif, Meat Sci., 1990, 27, 283–287 CrossRef CAS.
- R. Busquets, M. Bordas, F. Toribio, L. Puignou and M. T. Galceran, J. Chromatogr. B, 2004, 802, 79–86 CrossRef CAS PubMed.
- M. Kobayashi, T. Hanaoka, S. Nishioka, H. Kataoka and S. Tsugane, Mutat. Res., 2002, 506–507, 233–241 CrossRef CAS.
- G. A. Keating and K. T. Bogen, J. Chromatogr. B, 2004, 802, 127–133 CrossRef CAS PubMed.
- K. Skog, K. Augustsson, G. Steineck, M. Stenberg and M. Jagerstad, Food Chem. Toxicol., 1997, 35, 555–565 CrossRef CAS.
- C. P. Salmon, M. G. Knize, F. N. Panteleakos, R. W. Wu, D. O. Nelson and J. S. Felton, J. Natl. Cancer Inst., 2000, 92, 1773–1778 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |