Al-doped LiMn2O4 single crystalline nanorods with enhanced elevated-temperature electrochemical performance via a template-engaged method as a cathode material for lithium ion batteries
Dan Zhan*,
Ying Liang,
Ping Cui and
Zuoan Xiao
Department of Chemical Engineering and Food Science, Hubei University of Arts and Science, Xiangyang, 441053, P. R. China. E-mail: dan_zhan@126.com; Fax: +86-710-3592609; Tel: +86-710-3592609
First published on 17th December 2014
Abstract
Al-doped LiMn2O4 single crystalline nanorods have been synthesized using β-MnO2 nanorods as a self-template. The structure and morphology of the as-prepared products were investigated by powder X-ray diffraction (XRD), inductively coupled plasma-atomic emission spectrometry (ICP-AES), scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM). Galvanostatic charge/discharge tests indicate that the Al-doped single crystalline spinel nanorods (corresponding to LiAl0.1Mn1.9O4) deliver a discharge capacity of 107 mA h g−1 at 0.5 C (1 C = 140 mA h g−1) at room temperature, and about 70% of their initial capacity can remain after 500 charge/discharge cycles with a current rate of 3 C at 50 °C. Furthermore, high crystallinity, single-crystalline nature and nanorod morphology can be well retained after 500 electrochemical cycles with a 3 C current rate at 50 °C, revealing the excellent structure stability.
Introduction
Rechargeable lithium ion batteries have attached much interest owing to their promising high-power applications such as in electric vehicles (EVs) and hybrid EVs (HEVs).1 Spinel LiMn2O4 is considered as one of the most prospective cathode materials for lithium ion batteries due to its advantages such as abundant manganese resources, environmental friendliness, low cost and facile production.2 However, the large-scale application of LiMn2O4 is confined by capacity decay during the prolonged electrochemical cycles, especially at an elevated temperature, which is mainly caused by Jahn–Teller effect3 and Mn dissolution.4 To overcome these drawbacks, various approaches have been employed such as bulk doping5,6 and surface modification,7–9 which can effectively alleviate the dissolution of manganese and improve the cycle performance. For bulk doping, some inert metal cations such as Co,10 Cr,11 Ni12 which have near radii size with manganese ion were chosen to partly substitute Mn3+. These guest metal ions incorporate with oxide ions to form stronger chemical bond and then reinforce lattice energy so as to alleviate the dissolution of Mn3+ ions. On the other hand, the substitution of these guest ions for Mn3+ can suppress the Jahn–Teller distortion and enhance the structure stability. Among those dopants, Al has been considered as the most favorable dopant since it is abundant, stable and inexpensive. Moreover, the radii of Al3+ (0.53 Å) is close to that of Mn3+ (0.66 Å),13 the bond energy of Al–O (501.9 ± 10.6 kJ mol−1) is stronger than that of Mn–O (362 ± 25 kJ mol−1).14 Hence, Al3+ ions entered into the lattice of LiMn2O4 to strengthen the stability, so as to enhance the electrochemical performance, especially for cycle performance at an elevated temperature.15,16On the other hand, because the nanometric frame can shorten the Li+ diffusion distances and enlarge the electrode/electrolyte interface, which can facilitate fast Li+ insertion/deinsertion processes, nano-sized electrode materials have been suggested as electrode materials. Up till now, LiMn2O4 with various nano-structured morphologies such as nanoparticles,15 nanorods/nanowires,17,18 nanotubes,19 and even nanochains,20 have been fabricated and used as cathode materials to improve the electrochemical performance. Among various morphologies mentioned above, one-dimensional (1D) nanowires or nanorods not only have large surface-to-volume ratio, but also provide efficient electron transportation pathway. However, nanorods with cubic spinel structure such as LiMn2O4 are difficult to synthesize because the cubic crystals usually cannot grow in a one-dimensional direction. Accordingly, LiMn2O4 nanorods usually were synthesized by regulating the nanorod morphology of the precursor such as α-MnO2,21 β-MnO2,22 which show excellent electrochemical performance.
In this work, Al-doped single crystalline LiMn2O4 nanorods were synthesized based on our previous work. The electrochemical performance of the as-synthesized Al-doped single-crystalline LiMn2O4 nanorods was studied by galvanostatic charge/discharge cycling. The structure and morphology of the as-synthesized nanorods after prolonged electrochemical cycle at an elevated temperature was also investigated which confirmed the stability of Al-doped single-crystalline LiMn2O4 nanorods as cathode materials for rechargeable Li-ion battery, and hence explained the excellent electrochemical cycle performance of the as-synthesized Al-doped single-crystalline LiMn2O4 nanorods. This kind of materials would be favorable for high-performance lithium ion battery in view of the combined merits of both doping and 1D single crystalline nanorod architecture.
Experimental
Material preparation
β-MnO2 nanorod and pristine LiMn2O4 nanorod (LMO-NRS) were synthesized similar to our previous work.23 All reagents used were of analytical grade without further purification. The Al-doped LiMn2O4 nanorods were prepared as follows: firstly, the as-prepared β-MnO2 nanorod, Al(NO3)3·9H2O and LiOH·H2O were mixed at different molar ratio of LiAlxMn2−xO4 (x = 0.05, 0.10, 0.15) and dispersed into 5 ml methanol to form a thick slurry, which was further ground for several hours, and then dried at room temperature. The obtained powder was calcined in tube furnace at 700 °C for 10 h in air to obtain the Al-doped LiMn2O4 nanorods (marked as LMO-5, 10, 15, respectively).Material characterization
The phase identification and structure analyses of these samples were carried out by using a Bruker D8 advance X-ray diffractometer (XRD) with CuKα radiation (λ = 0.15418 nm). The chemical compositions of the products were measured by using IRIS Intrepid II XSP inductively coupled plasma-atomic emission spectrometry (ICP-AES). The morphology and size of these samples were observed on a Quanta 200 scanning electron microscope (SEM) and a JEM 2100 transmission electron microscope (TEM) and high-resolution transmission electron microscope (HRTEM).Cell assembly and electrochemical measurement
The electrodes were fabricated by roll-pressing a mixed paste of the active material, carbon black and PTFE with a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 into ca. 0.1 mm thick film and then pressing the electrode film onto a stainless mesh. The electrolyte was 1.0 M LiPF6 solved in EC/DMC (1
5 into ca. 0.1 mm thick film and then pressing the electrode film onto a stainless mesh. The electrolyte was 1.0 M LiPF6 solved in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume). The electrochemical measurement was performed by using CR2016 coin-type cells assembled in an argon-filled glove box (Mikrouna Super 1220/750) with lithium metal as the negative electrode. A Celgard 2300 microporous membrane was used as the separator. The cyclic performance was performed at different current rate (1 C = 148 mA g−1) between 3.5 V and 4.3 V vs. Li+/Li on a battery tester (LAND, CT2001A, China).
1 in volume). The electrochemical measurement was performed by using CR2016 coin-type cells assembled in an argon-filled glove box (Mikrouna Super 1220/750) with lithium metal as the negative electrode. A Celgard 2300 microporous membrane was used as the separator. The cyclic performance was performed at different current rate (1 C = 148 mA g−1) between 3.5 V and 4.3 V vs. Li+/Li on a battery tester (LAND, CT2001A, China).
Results and discussion
Crystal phase and composition analysis of Al-doped LiMn2O4 nanorods
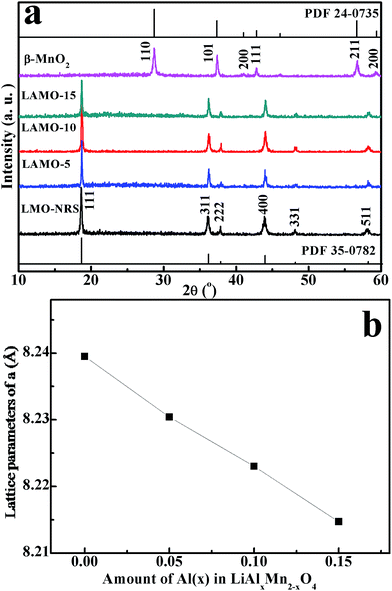
Fig. 1a depicts the XRD pattern of β-MnO2 precursor and Al-doped LiMn2O4 series of products obtained at 700 °C for 10 h. It can be seen that all the LiMn2O4 products shows the well-defined XRD pattern of cubic phase (JCPDS no. 35-0782) with Fd3m space group, and no diffraction peaks of β-MnO2 or other else can be detected, suggesting the high purity of these products. Even for LAMO-15 with the most Al-doping amount, no (220) diffraction peak was observed at 2θ = 31° due to the diffraction of the tetrahedral sites (8a) for a spinel structure, indicating that tetrahedral sites (8a) are only occupied by Li atoms that give undetectable (220) signals due to the very low X-ray scattering ability.16 Therefore, the Al3+ ions could be deemed to incorporate into the octahedral 16d sites to partially substitute manganese ions.The experimental XRD data was refined by Rietveld method on the base of a cubic spinel phase with a space group Fd3m. It was found that the refined value of lattice parameters a decreases linearly with increasing Al-doping content shown in Fig. 1b. As known, the radii of Al3+ (0.53 Å) is smaller than Mn3+ (0.60 Å),13 and the bond energy of Al–O (501.9 ± 10.6 kJ mol−1) is stronger than Mn–O (362 ± 25 kJ mol−1).14 As a result of Mn3+ in the lattice of LiMn2O4 replaced partly by Al3+, the cell volume diminished and then the crystal stability enhanced.
Table 1 shows the ICP-AES analysis result of Al-doped products. It can be seen that the actual Al-doping amount is consistent with the charge ratio. Furthermore, the ratio of Li/Al/Mn reveals the formation of a lithium-rich Al-doped spinel lithium manganese oxide phase, also suggesting that some manganese sites in the spinel lattice is occupied by lithium.19
| Content | LAMO-5 | LAMO-10 | LAMO-15 |
|---|---|---|---|
Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn Mn |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.85 1.85 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10 0.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.76 1.76 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.14 0.14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.70 1.70 |
Microstructure analysis of Al-doped LiMn2O4 nanorods
SEM image shows the rodlike morphology of LAMO-10 sample in Fig. 2a. It is observed clearly that the rods have the diameter of about 100 nm and the length of several hundred nm. The TEM image (the inset of Fig. 2a) depicts the smooth edge and good crystallinity, and the size dimension is in accordance with the observation from SEM image. The clear lattice fringes and the well-defined spots in FFT pattern reveal the single-crystal characteristics and high crystallinity of LAMO-10 nanorods. Two set of d-spacing were measured to be 0.47 and 0.41 nm, which corresponds to (111) and (002) crystal face for cubic LiMn2O4 (JCPDS no. 35-0782), respectively. This suggests that the Al-doping product with high purity still retain spinel structure.22 The above results imply that the nanorods can grow preferentially along the [110] direction.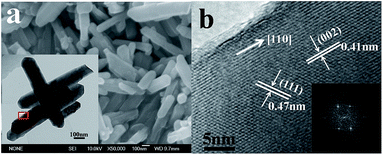 | ||
| Fig. 2 SEM image (a), TEM image (inset of a) and HRTEM image corresponding to the rectangle region in the inset of a (b) of LAMO-10. | ||
The whole conversion from tetragonal to cubic might involve a minimal reorganization of structure in the parent solid, and leads to the formation of more stable spinel [Mn2O4] framework with a higher symmetry than that of the original template, which is similar to the model reported by David et al.24 Importantly, both nanorod morphology and highly crystallinity of the precursor could be maintained with high fidelity in the final product, which is confirmed in our previous work.23 Some researchers take it as a self-sacrificial template or template-engaged mechanism.19 The templates are engaged in chemical reactions as precursor and could be completely converted into the target product without additional post processing. In the present case, the β-MnO2 nanorods can act as self-sacrificial template to produce LiMn2O4 nanorods although further work is needed to clarify the relation between the morphology and the phase restructuring during the lithiation reaction.
Electrochemical performance of Al-doped LiMn2O4 nanorods
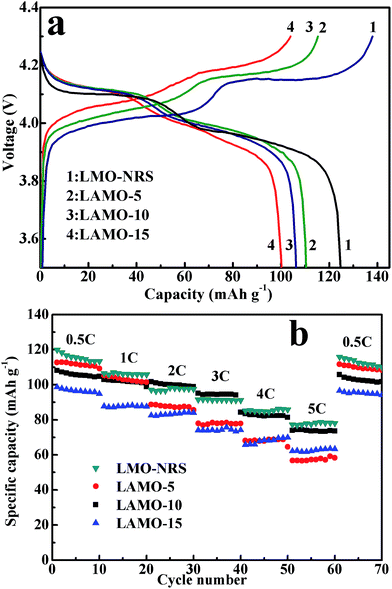
Fig. 3a shows the first charge/discharge profiles of the four samples with different Al-doping amount at 0.5 C (1 C = 148 mA h g−1) at room temperature. As seen, the discharge capacity of LMO-NRS, LAMO-5, 10, 15 is 125, 112, 107, 101 mA h g−1, respectively. The gradual decreasing of the discharge capacity with the increasing Al-doping amount is obviously owing to the increasing substitution of Mn ions by electrochemically inert Al ions.However, these Al-doped LiMn2O4 samples exhibits the first coulombic efficiency of more than 95%, higher than those undoped LiMn2O4 (90%). This suggests that the Al-doped LiMn2O4 samples have higher crystal stability and resulting in higher Li+ insertion/deinsertion reversibility,25 since substitution of stronger Al–O bond for Mn–O bond in crystal lattice enhances the crystal stability.
Meanwhile, it is observed that the discharge voltage plateau gradually drops and the charge voltage plateau rises gradually with the increasing Al-doping amount, although all the charge/discharge curves exhibits the similar shape. Generally, more difficult insertion/deinsertion of lithium ion in electrode material leads to higher charge plateau and lower discharge plateau in charge/discharge curves. As mentioned above, the substitution of stronger Al–O bond for Mn–O bond in crystal lattice also causes the shrinkage of the unit cell as shown in Fig. 1b, so as to need expending more energy for lithium ions extraction or insertion. In addition, it is also observed that two plateaus in each charge/discharge curve are becoming less distinct with the Al-doped amount, which corresponds to the two-phase transformation of λ-MnO2/Li0.5Mn2O4 and Li0.5Mn2O4/LiMn2O4, respectively.3 Since the voltage plateau reflects the insertion/deinsertion process of lithium ion in cathode materials, it is inferred that Al-doping in LiMn2O4 material influence the insertion/deinsertion process. Al3+ ions are commonly considered to incorporate into the octahedral 16d site to partially substitute manganese ions. Nonetheless, if the Al-doping amount is too much, some Al3+ may enter into LiO4 tetrahedral occupying the position originally belong to Li, and Li is pushed to the octahedral position. As a result, the insertion and extraction of lithium ion is hindered by this lattice dislocation. Accordingly, Al-doping amount should be moderate to maintain the capacity.
Fig. 3b shows the rate performance of the products (LMO-NRS, LAMO-5, 10, 15) at current rates ranging from 0.5 C to 5 C. As can be seen, the discharge capacities of all products decrease gradually with the increasing current density, indicating the diffusion-controlled kinetics process for the electrode reactions of LiMn2O4.18 During the first 10 cycles at 0.5 C current rate, the discharge capacity drops with the increasing Al-doping amount, agreeing with the observation from Fig. 3a, and this trend continues to the 20th cycle. However, from the 3rd 10 cycles at 2 C current rate to the 4th 10 cycles at 3 C, the discharge capacity of LAMO-10 is higher than that of LMO-NRS. This is due to that LAMO-10 has the lower initial capacity derived from Al-doping, as well as lower capacity decay rate during electrochemical cycle than LMO-NRS. As a result, LMO-NRS with higher initial capacity shows lower capacity than LAMO-10 after cycle 20 cycles. Therefore, it is easy to understand that LAMO-15 shows nearly similar capacity with LAMO-5 during the 40–50 cycles. It is noted that when the current rate go back to 0.5 C, the discharge capacity can be recovered to around the beginning level, indicating a good reversibility of the products upon cycling. On the whole, the cathode material cycle performance is improved at the cost of the capacity reduction. Considering the influence of the two factors, Al-doping amount is neither too much nor too little to keep excellent electrochemical performance of LiMn2O4 cathode material. From the above analysis, 10% is the optimum Al-doping amount corresponding to LAMO-10 product.
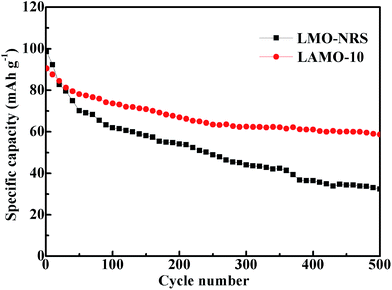
As one of cathode materials for lithium ion battery, the application of spinel LiMn2O4 is confined by its poor electrochemical performance at elevated temperature. To investigate the cycle performance at elevated temperature of Al-doped LiMn2O4 material, LAMO-10 and LMO-NRS were electrochemical cycled with 3 C current rate at 50 °C, respectively. As shown in Fig. 4, LAMO-10 exhibits lower initial discharge capacity (about 90 mA h g−1), but it still retain the discharge capacity of about 62 mA h g−1 after 500 electrochemical cycles, equivalent to the capacity decay rate of 30%. While the discharge capacity of LMO-NRS with no Al-doping only keep 32 mA h g−1 from the initial capacity of 98 mA h g−1 after 500 cycles, about the capacity decay rate of 67%. The slope of cycle performance curve for LMO-NRS is obviously higher than that for LAMO-10 observed from Fig. 4, suggesting higher fading rate of discharge capacity. As known, the dissolution rate of Mn in LiMn2O4 was accelerated at elevated temperature during the electrochemical cycle, and the dissolution is intensified by two phase structure transformation in 4.1 V region. In addition, the decomposition of electrolyte at elevated temperature also results capacity fading. The structure aberration resulting from Jahn–Teller effect can be restrained effectively by the addition of inert Al3+ in LiMn2O4, and then the capacity fading slows down. Accordingly, the cycle performance of LAMO-10 at elevated temperature was enhanced effectively, compared with LMO-NRS.
Structure stability after electrochemical cycles of Al-doped LiMn2O4 nanorods
Al-doped LiMn2O4 nanorods may goes through structure collapse after prolonged charge/discharge cycling, such as becoming amorphous or another phase. To investigate the stability of the structure and morphology for the nanorods, XRD, TEM and HRTEM were employed to probe the structure and morphology variation of the nanorods after 500 cycles at 3 C at 50 °C.Fig. 5 shows the XRD pattern of LAMO-10 and LMO-NRS after 500 charge/discharge cycles at 3 C current rate at 50 °C. As shown, the main diffraction peaks of both LMO-NRS and LAMO-10 still be kept, ascribed to spinel LiMn2O4 (JCPDS 24-0735), indicating LMO-NRS and LAMO-10 can still hold the spinel structure even after 500 charge/discharge cycles. The obvious signal noises in the XRD patterns of the cycled LMO-NRS and LAMO-10 (Fig. 5b and c) as compared to Fig. 5a can be ascribed to the addition of PTFE and carbon black during the electrode preparation process as shown in the experimental section. However, especially for LMO-NRS, the crystallinity becomes obviously weaker as compared to that before cycled, which might stem from the Mn dissolution and poor crystal integrity after prolonged electrochemical cycles. For LAMO-10, even after 500 cycles at 3 C at 50 °C, the structure still retain high crystallinity as well as spinel crystal phase, suggesting its strong crystal stability derived from Al-doping.
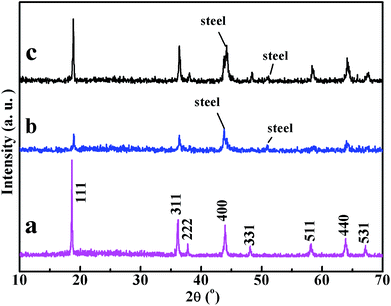 | ||
| Fig. 5 XRD pattern of LMO-NRS (a) before cycled; after (b) 500 charge/discharge cycles at 3 C current rate at 50 °C; (c) of LAMO-10 after 500 charge/discharge cycles at 3 C current rate at 50 °C. | ||
It was found that most cycled Al-doped LiMn2O4 nanorods can sustain rod-like morphology without obvious change from TEM image in Fig. 6a (the blurry and irregular substance was considered as carbon black and PTFE which was added during the preparation of electrode26). Moreover, three sets of clear lattice with d-spacing of 0.48, 0.24 and 0.28 nm in HRTEM image (Fig. 6b) can be indexed to (1-11), (13-1) and (220) crystal planes of spinel LiMn2O4, respectively. The distinct crystal planes and the well-defined spots in the inserted Fourier transform (FFT) pattern indicate the structure and morphology for the cycled Al-doped LiMn2O4 nanorods still retain single crystal structure with high crystallinity, in accordance with the XRD analysis in Fig. 5. The above results reveal that the structure and morphology for Al-doped LiMn2O4 nanorods is very stable even after prolonged electrochemical cycles with high current rate at an elevated temperature, which is significant for a cathode material with enhanced performance such as high capacity, high-rate capability and excellent cycling performance for Li ion battery in high-power application.
Conclusion
In conclusion, Al-doped LiMn2O4 nanorods with highly crystallinity were synthesized successfully via a template-engaged method using pre-synthesized β-MnO2 nanorods as self-templates. When applied as cathode materials for lithium ion battery, Al-doped LiMn2O4 nanorods presented superior electrochemical performance especially at an elevated temperature. Considering the enhanced cycle stability at cost of the reduction of capacity, LAMO-10 with the Al-doping amount of 10% was deemed to be appropriate, which shows capacity retention ratio of 70% with 3 C current rate at 50 °C after 500 discharge/charge cycles, much higher than that (33%) for LMO-NRS without Al-doping. Moreover, these Al-doped LiMn2O4 nanorods maintain the spinel structure and the nanorod morphology with highly crystallinity after 500 electrochemical cycles at 50 °C. The excellent electrochemical cycle performance is contributed to the nanorod morphology and the structure stability derived from inert Al-doping. It is suggested that the single-crystalline Al-doped LiMn2O4 nanorods could be a promising cathode material for high-power lithium-ion battery in view of the remarkably improved performance and moderate preparation method.Acknowledgements
This work was supported by the Scientific Research and Development Project of Xiangyang City (20270268040), the Natural Science Foundation of Hubei Province (2014CFC1153).Notes and references
- A. Yoshino, Angew. Chem., Int. Ed., 2012, 51, 5798–5800 CrossRef CAS PubMed.
- K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691–714 RSC.
- Y. Xia and M. Yoshio, J. Electrochem. Soc., 1996, 143, 825–833 CrossRef CAS PubMed.
- Y. Xia, Y. Zhou and M. Yoshio, J. Electrochem. Soc., 1997, 144, 2593–2600 CrossRef CAS PubMed.
- W. R. D. Han, W. Kim, J. Eom and H. Kwon, J. Phys. Chem. C, 2013, 117, 4913–4919 Search PubMed.
- D. Capsoni, M. Bini, G. Chiodelli, V. Massarotti, C. B. Azzoni, M. C. Mozzati and A. Comin, Phys. Chem. Chem. Phys., 2001, 3, 2162–2166 RSC.
- L. Xiong, Y. Xu, T. Tao, J. Song and J. B. Goodenough, J. Mater. Chem., 2012, 22, 24563 RSC.
- S. Lee, Y. Cho, H.-K. Song, K. T. Lee and J. Cho, Angew. Chem., Int. Ed., 2012, 51, 8748–8752 CrossRef CAS PubMed.
- W.-K. Kim, D.-W. Han, W.-H. Ryu, S.-J. Lim and H.-S. Kwon, Electrochim. Acta, 2012, 71, 17–21 CrossRef CAS PubMed.
- S. Mandal, R. M. Rojas, J. M. Amarilla, P. Calle, N. V. Kosova, V. F. Anufrienko and J. M. Rojo, Chem. Mater., 2002, 14, 1598–1605 CrossRef CAS.
- W. Xu, A. Yuan, L. Tian and Y. Wang, J. Appl. Electrochem., 2011, 41, 453–460 CrossRef CAS.
- Y. Xu, G. Chen, E. Fu, M. Zhou, M. Dunwell, L. Fei, S. Deng, P. Andersen, Y. Wang, Q. Jia and H. Luo, RSC Adv., 2013, 3, 18441–18445 RSC.
- Y.-S. Lee, N. Kumada and M. Yoshio, J. Power Sources, 2001, 96, 376–384 CrossRef CAS.
- W.-J. Zhou, S.-J. Bao, B.-L. He, Y.-Y. Liang and H.-L. Li, Electrochim. Acta, 2006, 51, 4701–4708 CrossRef CAS PubMed.
- Y. L. Ding, J. Xie, G. S. Cao, T. J. Zhu, H. M. Yu and X. B. Zhao, J. Phys. Chem. C, 2011, 115, 9821–9825 CAS.
- L. Xiao, Y. Zhao, Y. Yang, Y. Cao, X. Ai and H. Yang, Electrochim. Acta, 2008, 54, 545–550 CrossRef CAS PubMed.
- E. Hosono, T. Kudo, I. Honma, H. Matsuda and H. Zhou, Nano Lett., 2009, 9, 1045–1051 CrossRef CAS PubMed.
- D. K. Kim, P. Muralidharan, H.-W. Lee, R. Ruffo, Y. Yang, C. K. Chan, H. Peng, R. A. Huggins and Y. Cui, Nano Lett., 2008, 8, 3948–3952 CrossRef CAS PubMed.
- Y. L. Ding, J. A. Xie, G. S. Cao, T. J. Zhu, H. M. Yu and X. B. Zhao, Adv. Funct. Mater., 2011, 21, 348–355 CrossRef CAS.
- W. Tang, X. J. Wang, Y. Y. Hou, L. L. Li, H. Sun, Y. S. Zhu, Y. Bai, Y. P. Wu, K. Zhu and T. van Ree, J. Power Sources, 2012, 198, 308–311 CrossRef CAS PubMed.
- J.-Y. Luo, H.-M. Xiong and Y.-Y. Xia, J. Phys. Chem. C, 2008, 112, 12051–12057 CAS.
- Y. Yang, C. Xie, R. Ruffo, H. Peng, D. K. Kim and Y. Cui, Nano Lett., 2009, 9, 4109–4114 CrossRef CAS PubMed.
- D. Zhan, Q. Zhang, X. Hu, G. Zhu and T. Peng, Solid State Ionics, 2013, 239, 8–14 CrossRef CAS PubMed.
- W. I. F. David, M. M. Thackeray, P. G. Bruce and J. B. Goodenough, Mater. Res. Bull., 1984, 19, 99–106 CrossRef CAS.
- R. Thirunakaran, R. Ravikumar, S. Gopukumar and A. Sivashanmugam, J. Alloys Compd., 2013, 556, 266–273 CrossRef CAS PubMed.
- B. Zhang, C. Lai, Z. Zhou and X. P. Gao, Electrochim. Acta, 2009, 54, 3708–3713 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |