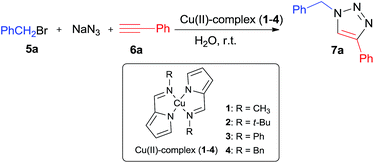
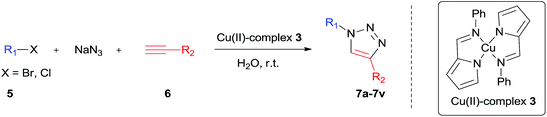2-Pyrrolecarbaldiminato–Cu(II) complex catalyzed three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature†
Changjian Zhou,
Jie Zhang,
Ping Liu,
Jianwei Xie* and
Bin Dai*
School of Chemistry and Chemical Engineering/Key Laboratory for Green Processing of Chemical Engineering of Xinjiang Bingtuan, Shihezi University, Shihezi 832003, China. E-mail: cesxjw@gmail.com; db_tea@shzu.edu.cn; Fax: +86-0993-2057270; Tel: +86-0993-2057213
First published on 17th December 2014
Abstract
2-Pyrrolecarbaldiminato–Cu(II) complexes were established as efficient catalysts for the three-component 1,3-dipolar cycloaddition reaction of benzyl halides and sodium azide with terminal alkynes in water at room temperature, and several regioselective 1,4-disubstituted 1,2,3-triazoles have been synthesized under the reaction conditions in 55–97% yields.
Introduction
1,2,3-Triazoles are prevalent building blocks of several classes of nitrogen-containing heterocyclic compounds and are commonly employed as a powerful tool in many fields of chemistry such as pharmaceuticals, agrochemicals, dyes and materials.1 Consequently, much attention has been given to the development of efficient and practical methods for the synthesis of substituted 1,2,3-triazoles. Of these transformations, Cu(I)-catalyzed alkyne–azide cycloaddition reaction (CuAAC, better known as click chemistry) reported independently in 2002 by Sharpless2 and Meldal,3 was the most versatile protocol for 1,4-disubstituted 1,2,3-triazoles synthesis, which proceeds under mild conditions with high regioselectivity. Since then, CuAAC has been extensively studied and widely used. Traditionally, the standard catalytic system of this method usually consists of Cu(II) salts (such as CuSO4·5H2O) in conjunction with a reducing agent (such as sodium ascorbate), which generates the catalytically active Cu(I) species in situ in the reaction media for CuAAC reaction.2,4 Later, it was discovered that Cu(I) salts could be also used directly for this reaction, but bases and/or ligands were required to stabilize Cu(I) intermediates, protecting it from oxidation and disproportionation, and hamper undesired side-product formation.5 Recently, solid supported Cu(I)6 or Cu(II)7 species as heterogeneous catalytic systems have been prepared and applied for cycloaddition reaction, which have several advantages such as simpler isolation of the reaction products by filtration, as well as recovery and recycling of the catalysts. The protocols directly employed Cu(II) species without deliberate addition of a reducing agent for AAC reaction have been also discussed. The mechanism indicated that the catalytic Cu(I) species were generated in a short induction period via reducing Cu(II) salts by alcoholic solvents8 or sodium azide7c,9 during this procedure.Pyrrolide-imine Schiff base ligands are attractive ligands for coordination to various metal ions, and their metal complexes have been found a number of important applications to various processes.10 For example, 2-pyrrolecarbaldimine-based titanium10a and hafnium10b catalysts were reported for olefin polymerization, while its copper complexes have been used as CVD/ALD (chemical vapor deposition/atomic layer deposition) precursors10c and as oxidation catalyst for benzylic alcohols in aqueous solutions.10d Recently, we found that pyrrolecarbaldiminato–Cu complexes could efficiently catalyze C–N coupling reaction.11 In order to further explore the scope of these complexes to other types of organic reactions, we report herein 2-pyrrolecarbaldiminato–Cu(II) complexes as novel and high active catalysts for alkyne–azide cycloaddition reaction.
Initially, the 1,3-dipolar cycloaddition reaction between benzyl bromide, sodium azide and phenylacetylene was selected as a model reaction to investigate the catalytic activity of four different 2-pyrrolecarbaldiminato–Cu(II) complexes, which synthesized by a simple condensation reaction between pyrrole-2-carbaldehyde and corresponding amines in the presence of Cu(OAc)2 in a one-pot synthesis,11 in neat water at room temperature. According to Table 1, the four Cu(II)-complexes with 10 mol% loading, as expected, all showed high catalytic activities to give the 1,4-disubstituted 1,2,3-triazoles in the yields of 78–95%, and Cu(II)-complexes 3 was found to be the best one (entries 1–4). When the amount of the catalyst 3 was reduced from 10 mol% to 5 mol%, excellent yield of triazole was also obtained only with prolonging the reaction time to 13 h (entry 5). To our surprise, the same results were observed when the catalyst loading decreased to 2.5 mol% and 1.0 mol% (entries 6 and 7). However, it was found that a further decrease of the loading of catalyst 3 to 0.5 mol% or 0.25 mol% led to lower yield and longer reaction time (entries 8 and 9). Control experiment conducted in the absence of Cu(II)-complex from the reaction mixture resulted in no product (entry 10). Therefore, the optimal conditions for 1,4-substituted 1,2,3-triazoles synthesis involves the use of 1 mol% Cu(II)-complexes 3 as the catalyst, H2O as the solvent at room temperature.
| Entry | Catalyst (mol%) | Time | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: benzyl bromide (0.5 mmol), phenylacetylene (0.6 mmol), NaN3 (0.6 mmol), catalyst, r.t.b Isolated yield. | |||
| 1 | 1 (10) | 8 | 78 |
| 2 | 2 (10) | 8 | 83 |
| 3 | 3 (10) | 8 | 95 |
| 4 | 4 (10) | 8 | 87 |
| 5 | 3 (5) | 13 | 98 |
| 6 | 3 (2.5) | 13 | 97 |
| 7 | 3 (1.0) | 13 | 97 |
| 8 | 3 (0.5) | 20 | 91 |
| 9 | 3 (0.25) | 20 | 89 |
| 10 | — | 20 | 0 |
With the optimal conditions in hand, the scope of the Cu(II)-complex 3 catalyzed one-pot cycloaddition reaction was explored for variety of benzyl halide and alkynes. The results are illustrated in Table 2. In general, most of electron-rich, electron-neutral and electron-poor aryl acetylenes reacted with benzyl bromide and NaN3 smoothly to provide the target products in good to excellent yields within 24 h (entries 1 and 3–11). Conversely, the use of alkyl acetylenes gave relatively lower yields (entries 12–16). For benzyl halide, we found that the electron effect of substituents was more obvious than the aryl acetylenes. The substrates containing electron-withdrawing groups seemed to be less reactive than the ones containing electron-neutral and electron-donating groups (entries 17–21). Reaction of benzyl chloride needs longer reaction time due to the lower activity (entries 1 vs. 2). Notably, the o-methylbenzyl bromide reacted also effectively under the standard conditions, which indicated that steric hindrance has no significant effect on the reaction (entry 22). To our delight, the reaction of benzyl bromide, NaN3 and acetylene gas yield 1-benzyl-1H-1,2,3-trizole in 67% yield, opening the possibility of using acetylene gas as an efficient source for 1,2,3-trizoles synthesis (entry 23).
| Entry | Benzyl halide | Alkyne | Product | Time (h) | Yieldb (%) | |
|---|---|---|---|---|---|---|
| a Reaction conditions: Cu(II)-complex 3 (0.005 mmol), benzyl halide (0.5 mmol), NaN3 (0.6 mmol), alkyne (0.6 mmol), water (1 mL), r.t.b Isolated yield.c Reaction temperature: 60 °C.d Under C2H2 balloon. | ||||||
| 1 |  |
 |
 |
7a | 13 | 97 |
| 2 |  |
 |
 |
7a | 24 | 90 |
| 3 |  |
 |
 |
7b | 10 | 97 |
| 4 |  |
 |
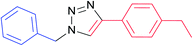 |
7c | 24 | 91 |
| 5 |  |
 |
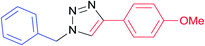 |
7d | 12 | 91 |
| 6 |  |
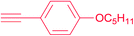 |
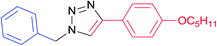 |
7e | 24 | 87 |
| 7 |  |
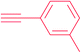 |
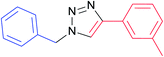 |
7f | 12 | 91 |
| 8 |  |
 |
 |
7g | 10 | 97 |
| 9 |  |
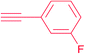 |
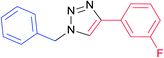 |
7h | 12 | 87 |
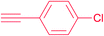
| 10 |  |
 |
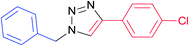 |
7i | 24 | 92 |
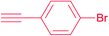
| 11 |  |
 |
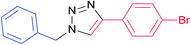 |
7j | 24 | 89 |
| 12 |  |
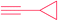 |
 |
7k | 24 | 60 |
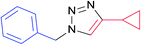
| 13 |  |
 |
 |
7l | 24 | 77 |
| 14 |  |
 |
 |
7m | 24 | 75 |
| 15 |  |
 |
 |
7n | 12 | 96c |
| 16 |  |
 |
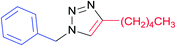 |
7o | 12 | 68c |
| 17 |  |
 |
 |
7p | 14 | 80 |
| 18 | 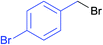 |
 |
 |
7q | 24 | 71 |
| 19 |  |
 |
 |
7r | 30 | 74 |
| 20 |  |
 |
 |
7s | 13 | 90c |
| 21 |  |
 |
 |
7t | 30 | 79 |
| 22 |  |
 |
 |
7u | 17 | 95 |
| 23 |  |
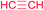 |
 |
7v | 24 | 67c,d |
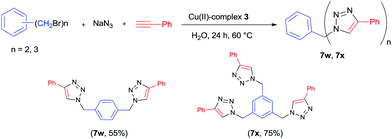
The Cu(II)-complexes 3 catalyzed three-component cyclo-addition reaction was extended to di- and tri-halides in water at 60 °C, and the reactions proceeded without any difficult to give moderate to good yields of the corresponding products (Fig. 1).
Conclusions
In conclusion, we have established for the first time 2-pyrrolecarbaldiminato–Cu(II) complexes as novel and high efficient catalyst for one-pot three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature. A variety of benzyl halides and alkyl/aryl acetylenes even acetylene gas could be reacted well to afford the desired products in high yields under the optimal conditions. In addition, the low catalyst loading (1 mol%), ambient reaction conditions, high regioselectivity presented herein, are the salient features of this protocol, which made the operation much more practical.Experimental
General information
Unless otherwise stated, all reagents were purchased from Adamas-beta and used without further purification. Column chromatography was performed with silica gel (200–300 mesh), purchased from Qingdao Haiyang Chemical Co. Ltd. Thin-layer chromatography was carried out with Merck silica gel GF254 plates and visualized by exposure to UV light (254 nm). All 1,4-disubstituted 1,2,3-triazoles are characterized by 1H NMR and 13C NMR, which were compared with the previously reported data. 1H NMR spectra and 13C NMR spectra were recorded at room temperature on a Varian Inova-400 instrument at 400 MHz and 100 MHz, respectively.General procedure for the synthesis of 1,2,3-triazoles 7a–7v
A 25 mL Schlenk tube was charged with Cu(II)-complex 3 (0.005 mmol), benzyl halides (0.5 mmol), NaN3 (0.6 mmol), alkynes (0.6 mmol) and water (1 mL). The mixture was stirred at room temperature and monitored by TLC until the benzyl halides being consumed. The reaction mixture was then extracted with ethyl acetate (3 × 10 mL). The combined organic phases was washed with water and brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by flash column chromatograph on silica gel (ethyl acetate/petroleum ether as the eluent) to provide the target products.General procedure for the synthesis of di-/tri-triazoles 7w and 7x
A 25 mL Schlenk tube was charged with Cu(II)-complex 3 (0.0025 mmol for dihalides and 0.00375 mmol for trihalides), benzyl halides (0.125 mmol), NaN3 (0.30 mmol for dihalides and 0.45 mmol for trihalides), phenylacetylene (0.6 mmol for dihalides and 0.9 mmol for trihalides) and water (1 mL). The mixture was stirred at 60 °C and monitored by TLC until the benzyl halides being consumed. The reaction mixture was then extracted with ethyl acetate (3 × 10 mL). The combined organic phases was washed with water and brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by flash column chromatograph on silica gel (ethyl acetate/petroleum ether as the eluent) to provide the target products.Acknowledgements
We thank the National Basic Research Program of China (973 Program, no. 2012CB722603) and the NSFC (no. 21103114) for their financial support.Notes and references
- For some recently selected reviews, see: (a) W. Xi, T. F. Scott, C. J. Kloxin and C. N. Bowman, Adv. Funct. Mater., 2014, 24, 2572 CrossRef CAS; (b) W. Tang and M. L. Becker, Chem. Soc. Rev., 2014, 43, 7013 RSC; (c) X. Xiong and H. Chen, Chin. J. Org. Chem., 2013, 33, 1437 CrossRef CAS; (d) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed; (e) G. C. Tron, T. Pirali, R. A. Billington, P. L. Canonico, G. Sorba and A. A. Genazzani, Med. Res. Rev., 2008, 28, 278 CrossRef CAS PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS PubMed.
- For some selected examples, see: (a) C. Shao, X. Wang, J. Xu, J. Zhao, Q. Zhang and Y. Hu, J. Org. Chem., 2010, 75, 7002 CrossRef CAS PubMed; (b) C. Deraedt, N. Pinaud and D. Astruc, J. Am. Chem. Soc., 2014, 136, 12092 CrossRef CAS PubMed; (c) K. B. Mishra and V. K. Tiwari, J. Org. Chem., 2014, 79, 5752 CrossRef CAS PubMed; (d) H. K. Akula and M. K. Lakshman, J. Org. Chem., 2012, 77, 8896 CrossRef CAS PubMed; (e) J. T. Fletcher and J. E. Reilly, Tetrahedron Lett., 2011, 52, 5512 CrossRef CAS PubMed; (f) S. Dörner and B. Westermann, Chem. Commun., 2005, 2852 RSC; (g) Y. Jiang, D. Kong, J. Zhao, Q. Qi, W. Li and G. Xu, RSC Adv., 2014, 4, 1010 RSC; (h) A. Pathigoolla, R. P. Pola and K. M. Sureshan, Appl. Catal., A, 2013, 453, 151 CrossRef CAS PubMed.
- For some selected examples, see: (a) A. Marra, A. Vecchi, C. Chiappe, B. Melai and A. Dondoni, J. Org. Chem., 2008, 73, 2458 CrossRef CAS PubMed; (b) S. Özçubukçu, E. Ozkal, C. Jimeno and M. A. Pericàs, Org. Lett., 2009, 11, 4680 CrossRef PubMed; (c) I. Cano, M. C. Nicasio and P. J. Pérez, Org. Biomol. Chem., 2010, 8, 536 RSC; (d) J. García-Álvarez, J. Díez and J. Gimeno, Green Chem., 2010, 12, 2127 RSC; (e) T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Org. Lett., 2004, 6, 2853 CrossRef CAS PubMed; (f) W. G. Lewis, F. G. Magallon, V. V. Fokin and M. G. Finn, J. Am. Chem. Soc., 2004, 126, 9152 CrossRef CAS PubMed; (g) V. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12696 CrossRef CAS PubMed; (h) V. O. Rodionov, S. I. Presolski, D. Diaz Diaz, V. V. Fokin and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12705 CrossRef CAS PubMed; (i) S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2008, 47, 8881 CrossRef PubMed; (j) S. Díez-González, Catal. Sci. Technol., 2011, 1, 166 RSC; (k) S. Guo, M. H. Lim and H. V. Huynh, Organometallics, 2013, 32, 7225 CrossRef CAS; (l) H. D. Velázquez, Y. R. García, M. Vandichel, A. Madder and F. Verpoort, Org. Biomol. Chem., 2014, 12, 9350 RSC.
- (a) T. Miao and L. Wang, Synthesis, 2008, 363 CAS; (b) S. Chassaing, M. Kumarraja, A. S. S. Sido, P. Pale and J. Sommer, Org. Lett., 2007, 9, 883 CrossRef CAS PubMed; (c) S. Chassaing, A. S. S. Sido, A. Alix, M. Kumarraja, P. Pale and J. Sommer, Chem.–Eur. J., 2008, 14, 6713 CrossRef CAS PubMed; (d) C. Girard, E. Önen, M. Aufort, S. Beauvière, E. Samson and J. Herscovici, Org. Lett., 2006, 8, 1689 CrossRef CAS PubMed.
- (a) K. Namitharan, M. Kumarraja and K. Pitchumani, Chem.–Eur. J., 2009, 15, 2755 CrossRef CAS PubMed; (b) Y. Wang, J. Liu and C. Xia, Adv. Synth. Catal., 2011, 353, 1534 CrossRef CAS; (c) S. Mohammed, A. K. Padala, B. A. Dar, B. Singh, B. Sreedhar, R. A. Vishwakarma and S. B. Bharate, Tetrahedron, 2012, 68, 8156 CrossRef CAS PubMed; (d) K. Yamaguchi, T. Oishi, T. Katayama and N. Mizuno, Chem.–Eur. J., 2009, 15, 10464 CrossRef CAS PubMed; (e) K. Kamata, Y. Nakagawa, K. Yamaguchi and N. Mizuno, J. Am. Chem. Soc., 2008, 130, 15304 CrossRef CAS PubMed; (f) Y. Masuyama, K. Yoshikawa, N. Suzuki, K. Hara and A. Fukuoka, Tetrahedron Lett., 2011, 52, 6916 CrossRef CAS PubMed; (g) R. B. N. Baig and R. S. Varma, Green Chem., 2013, 15, 1839 RSC; (h) K. R. Reddy, K. Rajgopal and M. L. Kantam, Catal. Lett., 2007, 114, 36 CrossRef CAS; (i) F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Org. Biomol. Chem., 2011, 9, 6385 RSC; (j) N. Mukherjee, S. Ahammed, S. Bhadra and B. C. Ranu, Green Chem., 2013, 15, 389 RSC.
- (a) G.-C. Kuang, H. A. Michaels, J. T. Simmons, R. J. Clark and L. Zhu, J. Org. Chem., 2010, 75, 6540 CrossRef CAS PubMed; (b) W. S. Brotherton, H. A. Michaels, J. T. Simmons, R. J. Clark, N. S. Dalal and L. Zhu, Org. Lett., 2009, 11, 4954 CrossRef CAS PubMed; (c) P. Appukkuttan, W. Dehaen, V. V. Fokin and E. Van der Eycken, Org. Lett., 2004, 6, 4223 CrossRef CAS PubMed.
- Y. Jiang, D. Kong, J. Zhao, W. Zhang, W. Xu, W. Li and G. Xu, Tetrahedron Lett., 2014, 55, 2410 CrossRef CAS PubMed.
- (a) Y. Yoshida, S. Matsui, Y. Takagi, M. Mitani, T. Nakano, H. Tanaka, N. Kashiwa and T. Fujita, Organometallics, 2001, 20, 4793 CrossRef CAS; (b) S. Matsui, T. P. Spaniol, Y. Takagi, Y. Yoshida and J. Okuda, J. Chem. Soc., Dalton Trans., 2002, 4529 RSC; (c) V. V. Grushin and W. J. Marshall, Adv. Synth. Catal., 2004, 346, 1457 CrossRef CAS; (d) P. J. Figiel, A. Sibaouih, J. U. Ahmad, M. Nieger, M. T. Räisänen, M. Leskelä and T. Repo, Adv. Synth. Catal., 2009, 351, 2625 CrossRef CAS; (e) B. Vidjayacoumar, D. J. H. Emslie, J. M. Blackwell, S. B. Clendenning and J. F. Britten, Chem. Mater., 2010, 22, 4854 CrossRef CAS; (f) C. S. B. Gomes, S. A. Carabineiro, P. T. Gomes, M. T. Duarte and M. A. N. D. A. Lemos, Inorg. Chim. Acta, 2011, 367, 151 CrossRef CAS PubMed.
- Y. Jiao, N. Yan, J. Xie, X. Ma, P. Liu and B. Dai, Chin. J. Chem., 2013, 31, 267 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data for all products. See DOI: 10.1039/c4ra13423k |
| This journal is © The Royal Society of Chemistry 2015 |