Development and application of a novel screening method and experimental use of the mutant bacterial strain Clostridium beijerinckii NCIMB 8052 for production of butanol via fermentation of fresh cassava
Haifeng Sua,
Yun Zhaob,
Maolin Wang*b and
Yuanjian Xu*a
aEnviromentally-Begnin Chemical Process Research Center, Division of Ecological & Enviromental Research on the Three Gorges, Chongqing Institute of Green and Interligent Technology, Chinese Academy of Science, P. R. China
bKey Laboratory of Bio-resources and Eco-environment of Ministry of Education, College of Life Sciences, Sichuan University, Chengdu 610064, Sichuan, P. R. China. E-mail: xuyuanjian@cigit.ac.cn; mlwang@scu.edu.cn; Fax: +86-23-65935819
First published on 15th January 2015
Abstract
Classic chemical mutagenesis has a demonstrated potential to create a strain of Clostridium with improved fermentation performance for obtaining high butanol yield. However, screening methods to select the desired bacterial phenotype from large populations are not currently feasible or readily available. In this paper, a simple, novel screening method to select target strains by using trypan blue dye as an indicator in solid starch media to detect the target mutant bacterium was devised. A well performing mutant strain Clostridium beijerinckii no. 15 was obtained using this screening method. It was derived from wild-type C. beijerinckii NCIMB 8052 by N-methyl-N′-nitro-N-nitrosoguanidine (NTG) mutagenesis, and its fermentative capability for producing butanol was investigated, using hydrolysate of fresh cassava and other substrates. The mutant strain C. beijerinckii no. 15 produced a maximum yield of 15.06 g L−1 butanol and 23.78 g L−1 total solvents, when the fresh cassava substrate was given a simple pretreatment at 121 °C for 1 h. For other substrates, the highest butanol yields: 20.67 g L−1 from glucose, 17.96 g L−1 from liquefied dextrin, and 13.59 g L−1 from liquefied corn powder (P ≤ 0.05) were also achieved. Experimental trials showed that the mutant strain had a significantly greater fermentation ability, and produced a high-yield butanol from fresh cassava hydrolysates. Further research showed that the highest overall yield of butanol resulted from the improvement of enzymatic activity of C. beijerinckii no. 15's α-amylase (P ≤ 0.05). Also, the protein structure of the α-amylase was predicted and its amino acid mutation sites were analyzed. Results revealed that these mutant amino acids may have somehow improved α-amylase activity and thus, led to producing more butanol. Our research will contribute to efforts to develop C. beijerinckii fermentation of fresh cassava to produce butanol.
Introduction
Given the rapidly increasing cost of fossil fuels, numerous programs have been initiated in many countries in order to develop alternative energy options, including the use of biofuels such as hydrogen, alcohol, and renewable thermal energy from biomass.1–8 Microbial fermentation of carbohydrates to generate acetone, butanol, and ethanol is a well-known example.9–12 The solventogenic bacteria Clostridium (e.g., C. acetobutylicum and C. beijerinckii) have been proven for this purpose due to their unique physiological ability to produce butanol via fermentation—no other organism is known to naturally synthesize butanol on a large-scale for use in industrial applications. Several schemes have been proposed for the production of butanol via fermentation of low-cost substrates by solventogenic clostridia.13–16 However, the high-yield strains that would be required are not readily available in many countries because of intellectual property rights. This has motivated widespread efforts to generate an efficient mutant strain from wild types of clostridia using classic chemical mutagenesis or biotechnology.It is very difficult to obtain a mutant strain of C. beijerinckii capable of producing high butanol yields directly from feedstocks. One reliable method for obtaining an ideal mutant strain is classic chemical mutagenesis with N-methyl-N′-nitro-N-nitrosoguanidine (NTG), because transform metabolic pathways to obtained strain with high butanol yield using existing genetic manipulation of clostridia is difficult; this would require metabolic engineering approaches that are quite rare for use with this genus, compared to other model organisms such as Escherichia coli.17–20 However, this classic method is time-consuming, so it is essential to refine the method in order to enable production of butanol on a large-scale. We therefore aimed to develop an effective, efficient screening procedure for isolating mutants with specifically desired traits with trypan blue, such as a strain that produces high butanol yields, for practical application to the fermentation of fresh cassava substrates. Trypan blue dye is a kind of cell activity dyestuff, often used for detecting the integrity of the cell membrane, and is also often used to detect cell survival. Living cells with strong vitality will not be dyed blue but cell of vitality decline will be dyed pale blue. There are numerous studies in the literature where the cell growth was tested by trypan blue stain assay.21–24
Cassava (Manihot esculenta) is a woody shrub of the Spurge family (Euphorbiaceae) that is native to the tropics. It is cultivated widely around the world as a staple food source, as its tuberous roots are rich in starch, and it can grow in poor soils and drought conditions.25–27 Cassava tubers have been used as feedstocks to produce butanol in traditional industrial manufacturing.28 They are usually cut into small blocks, dried, and then mill-crushed into powder for use as a substrate to produce butanol in industrial fermentation. Here, we investigated the potential for using fresh cassava as a cheaper alternative to dried material powder as the direct fermentation substrate for production of butanol. To our knowledge, there is currently no other relevant, published research on this topic.
Some mutant strains of C. acetobutylicum are used commonly to ferment cassava for the production of butanol, because their amylase can hydrolyze starch to produce glucose in industrial applications.29 However, to date, no published reports at large-scale have assessed butanol production via direct fermentation of cassavas by C. beijerinckii NCIMB 8052 in commercial manufacture. The possible reason is that α-amylase of this strain has very low enzyme activity, so starch is not fully broken down, less glucose is produced, and this results in poor butanol yields. We reasoned that this bottleneck could be overcome to enable the application of C. beijerinckii in butanol manufacturing, by using classical chemical mutagenesis to obtain strain, or biotechnological changes to improve the enzymatic activity of amylase. An additional advantage of using this strain is that C. beijerinckii NCIMB 8052 can ferment diverse sugars, particularly disaccharides and other polysaccharoses. Fresh cassava tubers contain many complex sugar compounds, such as lignose and cellulose (accounting for up to 15–30% of their composition) and starch, which only certain microbes are capable of breaking down. The sugar compounds present in fresh cassava are hydrolyzed to xylose, lactose, sorbose, or other disaccharides and polysaccharoses. Hence, we predicted that its ability to use a wide range of substrates should make C. beijerinckii NCIMB 8052 a more desirable strain for industrial butanol fermentation from cassava.
In addition to the inherent fermentation ability of the mutant strain being targeted, the type of fermentation substrate is another important factor that influences the maximum amount of end-products a strain can produce. It is imperative that fresh cassava be pretreated in order to obtain substrate compounds that are more suitable for mutant strains. Therefore, we further evaluated the fermentation capacity of mutant strains grown on various substrates that were produced by pretreating fresh cassava in different ways.
Materials and methods
Experimental design
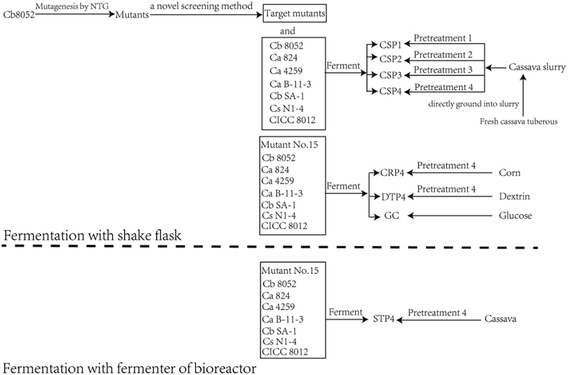
Experiments were conducted following the methodology illustrated in the flowsheet of fermentation processes (Fig. 1). Fresh cassava tuberous were pretreated with four different methods of hydrolysis. The products of hydrolysis were then fermented by the mutant strains, wild-type strains C. beijerinckii NCIMB 8052 and C. acetobutylicum ATCC 824 and the other solventogenic clostridia listed below that are frequently used for fermentation in the manufacture of butanol.Microbial strains for fermentation
Determination and inhibition of total amylase activity
Total amylase activity was determined as follows. The mutant strains were cultured in TGY medium for 3 d until the exponential growth phase (OD600 ∼ 1.5) was reached, and 1 mL of bacterial suspension was then centrifuged to collect cells. The cell pellet was washed three times using sterile water to remove residual glucose, and then suspended in 1 mL phosphate buffer of pH 7.0. All of the bacterial suspension was inoculated into 1 mL soluble sterilized starch solution (STY medium, 6%, w/v), placed in a 37 °C water bath incubator for 3 h, and then centrifuged. The supernatant was measured for the total amount of sugar reduced using the 3,5-dinitrosalicylic acid method.30 The total amount of reducing sugar was used as an index of total amylase activity. To investigate the effect of amylase activity on butanol production, and to confirm the identity of the key enzyme involved in the process, we used 3 mg L−1 of the α-amylase inhibitor trestatin (Hubei Yuan Cheng Pharmaceutical Co. Ltd., Wuhan, China) to inhibit the mutant amylase activity of31,32 when the liquefied fresh cassava and soluble starch were used as fermentation substrates.Pretreatment of fresh cassava
Fresh cassava tubers were purchased from local farmers, cleaned with water, and then ground into slurry. The starch content of fresh cassava slurry was determined,33 adjusted to 10% (w/v), and the slurry was divided equally into pretreatment samples 1 through 4 (Table 2). The starch content of each slurry sample was ∼100 g L−1. The samples were then pretreated with the four different methods of enzymatic hydrolysis, with the following processing procedures:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1, Anked Bioengineering, Wuhan) and 5 mL of pullulanase (400 PUN mL−1, Novozymes; 1 PUN equals the quantity of enzyme used to hydrolyze 1 μg maltose in 1 min) were added to the liquefied solution, which was then saccharified at 50 °C for 20 h before being cooled to room temperature for the next step.
000 U g−1, Anked Bioengineering, Wuhan) and 5 mL of pullulanase (400 PUN mL−1, Novozymes; 1 PUN equals the quantity of enzyme used to hydrolyze 1 μg maltose in 1 min) were added to the liquefied solution, which was then saccharified at 50 °C for 20 h before being cooled to room temperature for the next step.Small-scale production of acetone, butanol, and ethanol (ABE) via fermentation of cassava, corn, dextrin and glucose in triangular flask
The concentrations of dextrin, glucose, maltose, and xylose in the cassava slurry were determined (Table 2). The hydrolysates of cassava produced with the four pretreatment methods were used as fermentation substrates for the mutant strains, so that we could determine the optimal substrates in terms of maximizing butanol yield.The pH of all fermentation substrates (i.e., the four treated slurry samples) was adjusted to 6.0 using 1% (v/v) phosphoric acid and 1 g L−1 Ca(OH)2 after pretreatment. Each of the four samples was further subdivided into four equal portions. Treatment codes were assigned to reflect the unique combinations of bacterial strain and hydrolyzed substrate; for example, treatments with the strain C. beijerinckii no. 15 were labeled as no. 15-1, 15-2, 15-3, and 15-4, which contained hydrolysates from pretreatment method 1, 2, 3, and 4, respectively. The original strains C. beijerinckii NCIMB 8052 and C. acetobutylicum ATCC 824 were labeled as 8052-1, 2, 3, 4 and 824-1, 2, 3, 4, with treatments using other strains named similarly. All substrates were fermented at 37 °C for 146 h. Three replicates were conducted for all experimental treatment combinations.
Dried corn (starch content 69%) was obtained from the local farmer's market and ground into powder. Separately, the corn powder (70 g L−1) and dextrin (70 g L−1) were liquefied using pretreatment 4. Glucose (70 g L−1) was sterilized as another fermentation substrate. We also investigated fermentation of the liquefied cassava, and soluble starch, in the presence of inhibiting α-amylase to confirm which key enzyme affected the butanol yields of the target mutant.
Scale-up of butanol fermentation to bioreactor
The next step was to assess fermentation at a larger scale, moving from using small triangular flasks as the fermenter to a 20 L stirred-tank bioreactor (Holves Company, China). Cassava slurry (10% w/v) was pretreated in the bioreactor using preteatment method 4. We then inoculated mutant no. 15 (500 mL inoculum size, OD600 = 2.36) into the fermentation substrate. N2 gas was injected into the reactor to remove internal oxygen, and the reactor was then sealed. To ensure that the cassava slurry could be fully utilized by the bacteria, the solution was stirred for 5 min every 5 h period after fermentation had progressed for 12 h, and exhaust gases were released every 5 h. The ABE products were measured after fermentation was finished.Prediction of α-amylase protein structure
A pair of primers (1-aataatatcaagattaagct, 2-ttattgagatatagaatagttata) was designed to clone the gene α-amylases with vector NTI software (Informax Vector NTI Suite 8).34 The α-amylases of C. beijerinckii (KEGG, Cbei_0664) and C. beijerinckii no. 15 were amplified with PCR using the high-fidelity enzyme TransStart FastPfu DNA Polymerase (TransGen Biotech Company, Beijing, China). The PCR products were linked to a pEASY-Blunt cloning vector (TransGen Biotech Company) with T4 DNA ligase (TransGen Biotech Company), and the sequences were detected by the BGI Tech. Company (Beijing, China). A homologous protein model of C. beijerinckii no. 15's α-amylases was built using the Discovery studio 2.5 software (Accelrys, USA),35 in order to analyze the amino acid mutation sites and predict the structure of this protein.Analytical procedures
The compounds (acetone, butanol, ethanol) were measured with a gas chromatograph (GC) equipped with a flame ionization detector. The system was a model 6890 GC (Agilent Technologies, Santa Clara, CA, USA) with a model 7673A automatic injector, sampler, and controller (Hewlett-Packard). Alcohol compounds were separated out using a ZB-WAX capillary column (30 m, 0.25 mm inside diameter, 0.25 μm film thickness; Phenomenex Inc., PA, USA). The GC oven temperature was held initially at 40 °C for 5 min, and then raised stepwise, by 15 °C min−1, until it reached 150 °C. It was then raised by 50 °C min−1 up to 250 °C, and held for 4 min. Helium was used as the carrier gas, with an inlet pressure of 9.3 lb in−2. The injector and detector were maintained at 220 °C. A 1 μL volume of supernatant from the culture broth was injected in split-injection mode at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 split ratio. Isobutanol was used as the internal standard.
30 split ratio. Isobutanol was used as the internal standard.
Total residual carbohydrate was determined with the phenol–sulfuric acid method.36–39 The productivity of strain was calculated using the ratio of solvent produced (g)/carbohydrate consumed (g). The starch content of fresh cassava was measured via acid hydrolysis.40
The constituent sugars were detected with DIONEX UltiMate3000 liquid chromatograph in a column packed with Aminex HPX-87H (Hercules, CA, USA: carbohydrate analysis column Aminex HPX-87P Column 300 × 7.8 mm, catalog 125-0098 serial 426070, 5 mM H2SO4, 0.6 mL min−1; column temperature at 65 °C). Run conditions of the RI detector (detecting liquid refractive index): a RI detector temperature of 45 °C. Concentrations of the sugars were determined using extrapolation from standard curves. Butyric and acetic acids were determined with a DIONEX UltiMate3000 liquid chromatograph in a column packed with Aminex HPX-87H and 0.05 mM H2SO4 on Chromosorb WAW. The chromatography was conducted at an injector temperature of 175 °C, detector temperature of 180 °C, and oven temperature of 125 °C.
Statistical analysis
Three replicates of each experiment and assay were carried out unless otherwise indicated. For each treatment, we calculated the mean response variables and their standard deviation (SD). Analysis of variance (ANOVA) was performed and the mean separation was done by the Fisher's Least Significant Difference (P ≤ 0.05) using the SPSS 21.0 program.Results
Screening, characterization of mutants and selected mutants for further investigation
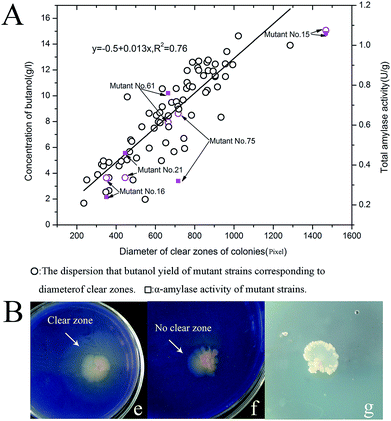
Diameters of the total of 75 colonies that appeared on plates were measured using the software Image-Pro Plus 6 (Media cybernetics, Silver Spring, MD, USA).41,42 We compared the diameters of all colonies against their the highest butanol yield from the cassava hydrolyzed substrates of pretreatment method 4. There was a positive linear correlation between the highest butanol yield and colony diameter (Fig. 5A). These results demonstrated the feasibility of using soluble starch medium containing trypan blue dye to identify and screen target mutants capable of high butanol production from fermentation of liquefied cassava. This approach may enable easier identification of high-yield strains.In addition, we noticed that the different colonies have different brightness of the clear zones.We randomly selected five colonies (C. beijerinckii no. 21, 61, 15, 16 and 75) in order to determine possible relationship between the brightness of the these colonies and their highest butanol yield. The brightness of the clear zones of these colonies was determined by visual observation (Table 6), and the clear zone around the original strain C. beijerinckii NCIMB 8052 was used as a control, for comparison. The result showed the clear zone of the mutant no. 15 was larger and brighter than the other mutant strains.
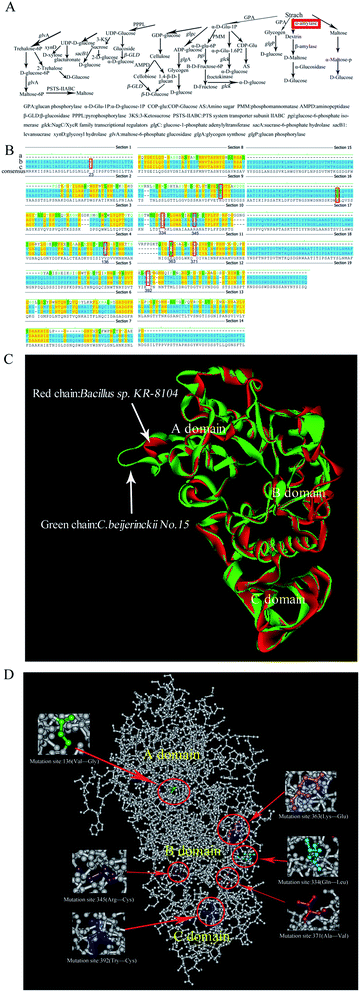
These mutant strains were further cultivated in STY medium (an improved TGY medium that contains soluble starch instead of glucose). Mutant no. 15 had the maximum amylase activity (1.06 U g−1) on this medium, almost 5-fold higher than that of the original parent strain C. beijerinckii NCIMB 8052 (Table 1, P ≤ 0.05). Thus, the α-amylase gene of the mutant no. 15 was cloned and sequenced. Aligning the α-amylase gene sequences of C. beijerinckii NCIMB 8052 with the nucleotide sequence of the α-amylase gene of C. beijerinckii no. 15 showed that the nucleotides had mutated at positions 66, 99, 407, 955, 1033, 1087, 1112, 1176, and 1696, leading to changes in the structure of the corresponding amino acids (Fig. 6Ba–c). Through further prediction of structure of C. beijerinckii no. 15's protein we surmised that enhanced amylase activity might be resulting from mutation of these amino acids. Further analysis showed that compared to α-amylases (3DC0. A) of Bacilhis sp. KR-8104, the amino acid sequences of mutant no. 15's α-amylase protein had 34.1% consensus positions and 25.2% identity positions. The structure of the mutant protein was highly similar to the template KR-8104 α-amylases, based on homology protein modeling (Fig. 6C). Based on all residues, the C-Alpha atom RMSD to reference protein: 3DC0 was 0.6140, and the amino acid residues used reached 401 based on superimposing the sequence alignments. Therefore, we further investigated the ability of mutant no. 15 to produce butanol from the treated fresh cassava by the possible effects of the mutant α-amylase.
| Strain | Total reducing sugar (g L−1) | Total amylase activity (U g−1) |
|---|---|---|
| a All values are means ± SD values within columns followed by the * symbols are significantly different (P ≤ 0.05) compared with that without * symbols. | ||
| C. beijerinckii no. 15 | 6.35 ± 0.32 | 1.06 ± 0.053 |
| C. beijerinckii no. 61 | 4.53 ± 0.15* | 0.76 ± 0.025* |
| C. beijerinckii no. 21 | 2.78 ± 0.24* | 0.46 ± 0.04* |
| C. beijerinckii no. 16 | 1.42 ± 0.15 | 0.24 ± 0.25* |
| C. beijerinckii no. 75 | 1.89 ± 0.26* | 0.32 ± 0.053* |
| C. beijerinckii NCIMB 8052 | 1.33 ± 0.46* | 0.22 ± 0.077* |
| C. acetobutylicum ATCC 824 | 3.33 ± 0.34* | 0.56 ± 0.057* |
| C. beijerinckii SA-1 | 4.63 ± 0.16* | 0.77 ± 0.026* |
| C. acetobutylicum B-11-3 | 4.03 ± 0.67* | 0.67 ± 0.11* |
| C. saccharoperbutylacetonicum N1-4 | 3.67 ± 0.53* | 0.61 ± 0.089* |
| C. acetobutylicum CICC 8012 | 5.67 ± 0.63 | 0.94 ± 0.11 |
| C. acetobutylicum ATCC 4259 | 5.96 ± 0.71 | 0.99 ± 0.12 |
Fermentation characteristics of selected mutants, wild type and other common strains
| Pretreatment | Starcha | Dextrinb | Glucoseb | Maltoseb | Xyloseb |
|---|---|---|---|---|---|
| a Starch content before pretreatment.b Hydrolysates from samples using different pretreatment methods. 1, 2, 3, 4: different pretreatment methods of fresh cassava, Pretreatment method 1, Pretreatment method 2, Pretreatment method 3, Pretreatment method 4. | |||||
| 1 | 100 ± 0.21 | 6.77 ± 0.03 | 71.27 ± 0.43 | 14.43 ± 0.06 | 18.5 ± 0.35 |
| 2 | 100 ± 0.19 | 56.7 ± 0.27 | 14.63 ± 0.8 | 36.27 ± 0.07 | 15.5 ± 0.04 |
| 3 | 100 ± 0.13 | 7.23 ± 0.06 | 12.17 ± 0.9 | 48.07 ± 0.26 | 26.2 ± 0.09 |
| 4 | 100 ± 0.28 | 93.07 ± 0.17 | — | 15.43 ± 0.04 | — |
| Strains | Butanol | Acetone | Ethanol | Butyrate | Acetate | Total solvent | Butanol/total solvent (%) |
|---|---|---|---|---|---|---|---|
| a 1, 2, 3, 4: pretreatment methods of fresh cassavas. NCIMB 8052: wild type strain C. beijerinckii. NCIMB 8052. ATCC 824: wild type strain C. acetobutylicum ATCC 824. ATCC 4259: C. acetobutylicum ATCC 4259. B-11-3: C. acetobutylicum B-11-3. SA-1: C. beijerinckii SA-1. CICC 8012: C. acetobutylicum CICC 8012. N1-4: C. saccharoperbutylacetonicum N1-4.b All values are means ± SD values within columns followed by the * symbols are significantly different (P ≤ 0.05) compared with that without * symbols. | |||||||
| Mutant no. 15-1 | 10.12 ± 1.26* | 4.73 ± 1.61* | 2.34 ± 0.23 | 1.4 ± 0.54* | 0.85 ± 0.03* | 19.44 ± 2.67 | 52.12%* |
| Mutant no. 15-2 | 14.36 ± 2.36* | 6.26 ± 2.34 | 2.16 ± 0.61* | 0.2 ± 0.63* | 0.01 ± 0.002* | 23.48 ± 2.64 | 61.01% |
| Mutant no. 15-3 | 8.87 ± 1.36* | 4.03 ± 1.51* | 0.6 ± 0.28* | 2.3 ± 0.84 | 1.3 ± 0.23 | 17.15 ± 1.63* | 47.05%* |
| Mutant no. 15-4 | 15.06 ± 1.45 | 6.03 ± 2.64 | 2.56 ± 1.23 | 0.15 ± 0.06* | 0.02 ± 0.01* | 23.76 ± 3.16 | 65.21% |
| NCIMB 8052-1 | 7.91 ± 2.31* | 2.51 ± 1.35* | 0.65 ± 0.37* | 0.50 ± 0.29* | 0.40 ± 0.15* | 11.97 ± 2.48* | 63.5%* |
| NCIMB 8052-2 | 5.26 ± 1.45* | 1.62 ± 2.39* | 0.43 ± 0.25* | 0.71 ± 0.21* | 0.52 ± 0.24* | 9.5 ± 2.61* | 55.5%* |
| NCIMB 8052-3 | 7.89 ± 2.34* | 2.61 ± 1.34* | 0.71 ± 0.64* | 0.81 ± 0.45* | 0.67 ± 0.36* | 12.69 ± 3.15* | 58.3%* |
| NCIMB 8052-4 | 4.26 ± 1.26* | 1.16 ± 0.25* | 0.37 ± 0.24* | 0.63 ± 0.36* | 0.43 ± 0.15* | 6.85 ± 2.85* | 57.14%* |
| ATCC 824-1 | 5.8 ± 2.34* | 1.36 ± 0.36* | 0.53 ± 0.41* | 0.46 ± 0.12* | 0.34 ± 0.14* | 8.49 ± 1.64* | 58.82% |
| ATCC 824-2 | 6.9 ± 2.46* | 2.42 ± 0.84* | 0.76 ± 0.36* | 0.57 ± 0.34* | 0.29 ± 0.05* | 10.61 ± 2.64* | 63.3% |
| ATCC 824-3 | 8.2 ± 2.87* | 2.8 ± 0.68* | 0.98 ± 0.39* | 0.62 ± 0.26* | 0.57 ± 0.06* | 13.17 ± 1.94* | 62.5% |
| ATCC 824-4 | 5.4 ± 1.67* | 2.63 ± 1.26* | 0.69 ± 0.15* | 0.43 ± 0.15* | 0.31 ± 0.13* | 9.46 ± 2.64* | 57.08%* |
| SA-1-1 | 12.63 ± 2.75* | 4.36 ± 1.34* | 2.26 ± 0.84* | 1.45 ± 0.62* | 0.92 ± 0.26* | 21.62 ± 2.34 | 58.2%* |
| SA-1-2 | 8.2 ± 2.84* | 3.95 ± 1.36* | 1.98 ± 0.96* | 1.89 ± 0.64* | 1.42 ± 0.37 | 17.44 ± 2.91* | 47.01%* |
| SA-1-3 | 11.63 ± 1.64* | 3.61 ± 1.84* | 2.61 ± 0.85* | 1.56 ± 0.67* | 0.93 ± 0.49* | 23.34 ± 2.97 | 49.82%* |
| SA-1-4 | 4.96 ± 1.26* | 2.68 ± 1.91* | 1.73 ± 1.13* | 1.06 ± 0.42* | 0.92 ± 0.56* | 11.35 ± 1.62* | 43.70%* |
| B-11-3-1 | 6.73 ± 1.74* | 2.69 ± 1.34* | 0.76 ± 0.35* | 0.51 ± 0.16* | 1.69 ± 0.67 | 12.38 ± 2.64* | 51.93%* |
| B-11-3-2 | 11.17 ± 2.36* | 4.24 ± 1.16* | 1.45 ± 0.56* | 0.8 ± 0.34* | 0.39 ± 0.18* | 18.05 ± 2.98* | 64% |
| B-11-3-3 | 5.62 ± 1.4* | 2.63 ± 1.14* | 0.87 ± 0.21* | 0.74 ± 0.28* | 0.64 ± 0.26* | 10.5 ± 1.64* | 53.3%* |
| B-11-3-4 | 4.62 ± 1.74* | 2.32 ± 1.36* | 1.02 ± 0.68* | 1.09 ± 0.13* | 0.95 ± 0.19* | 10.0 ± 3.61* | 46.2%* |
| N1-4-1 | 11.13 ± 2.91* | 6.56 ± 2.34 | 2.55 ± 0.82 | 1.84 ± 0.15* | 0.91 ± 0.34* | 22.99 ± 2.64 | 51.4%* |
| N1-4-2 | 4.62 ± 1.26* | 2.44 ± 1.62* | 0.89 ± 0.32* | 0.15 ± 0.09* | 1.63 ± 0.81 | 9.73 ± 1.26* | 47.4%* |
| N1-4-3 | 5.62 ± 1.48* | 3.52 ± 1.54* | 1.51 ± 0.94* | 1.72 ± 0.64* | 0.74 ± 0.19* | 13.11 ± 2.64* | 42.86%* |
| N1-4-4 | 3.65 ± 1.86* | 1.37 ± 0.35* | 1.29 ± 0.64* | 2.05 ± 1.05* | 1.69 ± 0.93 | 10.05 ± 1.97* | 34.7%* |
| CICC 8012-1 | 10.67 ± 2.54* | 4.92 ± 1.65* | 1.45 ± 0.93* | 0.96 ± 0.18* | 1.78 ± 0.86 | 19.78 ± 3.61 | 53.94%* |
| CICC 8012-2 | 8.62 ± 2.84* | 4.53 ± 1.58* | 2.19 ± 1.12* | 2.2 ± 1.06 | 1.2 ± 0.69* | 18.74 ± 2.94* | 45.99%* |
| CICC 8012-3 | 7.61 ± 1.62* | 3.58 ± 1.62* | 2.94 ± 1.03* | 1.98 ± 0.92* | 0.68 ± 0.38* | 16.79 ± 2.82* | 45.32%* |
| CICC 8012-4 | 12.64 ± 2.67* | 5.25 ± 2.57 | 1.85 ± 1.25* | 1.59 ± 0.64* | 1.26 ± 0.46 | 22.59 ± 2.61 | 55.1%* |
| ATCC 4259-1 | 12.67 ± 3.61* | 5.99 ± 1.63 | 2.29 ± 0.64 | 1.32 ± 0.34* | 0.84 ± 0.37* | 23.11 ± 3.16 | 54.82%* |
| ATCC 4259-2 | 10.62 ± 1.26* | 2.69 ± 1.52* | 1.53 ± 1.52* | 0.98 ± 0.45* | 1.29 ± 0.67 | 17.11 ± 1.94* | 62.06% |
| ATCC 4259-3 | 9.61 ± 2.58* | 3.78 ± 1.68* | 1.89 ± 0.98* | 1.56 ± 0.78* | 0.81 ± 0.36* | 15.55 ± 3.67* | 61.8% |
| ATCC 4259-4 | 13.64 ± 3.61* | 5.59 ± 1.49* | 2.85 ± 1.24* | 1.06 ± 0.91* | 0.49 ± 0.14* | 23.63 ± 3.19 | 57.72%* |
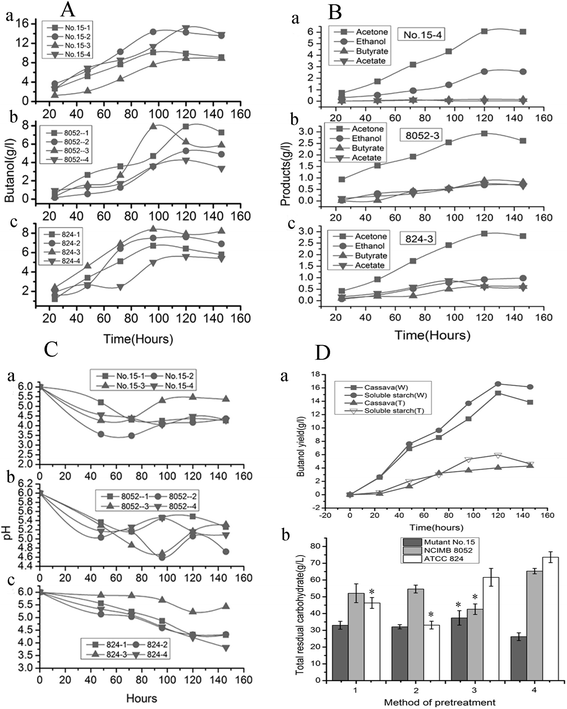
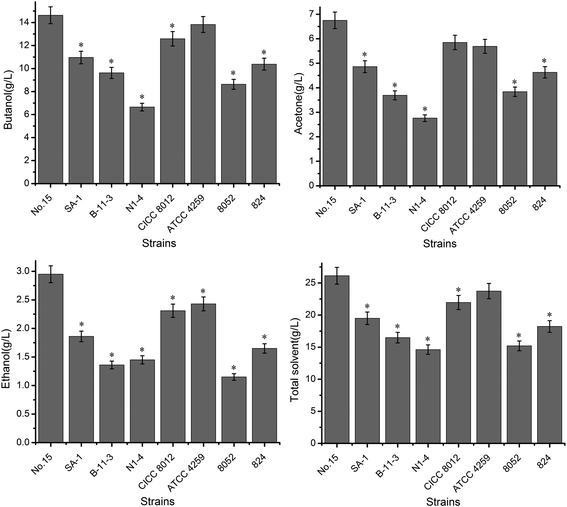
In terms of the highest yield of other main products, mutant no. 15 produced substantially higher levels of acetone than other strains, no matter which substrate was used (Fig. 2B and Table 3; P ≤ 0.05). The highest yields of acetone (6.03 g L−1) and ethanol (2.56 g L−1) were obtained from mutant no. 15 acting on sample 4 (Table 3; P ≤ 0.05). The maximum yield of other compounds (ethanol, butyrate and acetate) was obtained with the original strains C. beijerinckii NCIMB 8052, C. acetobutylicum ATCC 824 and the mutant no. 15 (Fig. 2Ba). The maximum concentrations of acetone and ethanol for all strains were attained after 120 h. The original strains produced more acetate and butyrate than did mutant no. 15 (Fig. 2Bb). Combined, all of these results demonstrate that the method of pretreating fresh cassava had a significant impact on butanol yield. Mutant no. 15 appears to be the most useful for exploring alternative carbohydrate sources that can be used as substrates for value-added fermentation. Therefore, we chose this strain as the candidate in view of investigation result based on small-scale fermentation of cassava hydrolysates, with pretreatment method 4, for further analysis.
| Strains | Substrates | Butanol | Acetone | Ethanol | Butyrate | Acetate | Total solvent | Butanol/total solvent (%) |
|---|---|---|---|---|---|---|---|---|
| a 4: pretreatment method 4 for corn. ATCC 4259: C. acetobutylicum ATCC 4259. B-11-3: C. acetobutylicum B-11-3. SA-1: C. beijerinckii SA-1. CICC 8012: C. acetobutylicum CICC 8012. N1-4: C. saccharoperbutylacetonicum N1-4. ATCC 824: wild type strain C. acetobutylicum ATCC 824. NCIMB 8052: wild type strain C. beijerinckii NCIMB 8052.b All values are means ± SD values within columns followed by the * symbols are significantly different (P ≤ 0.05) compared with that without * symbols. | ||||||||
| Mutant no. 15-4 | Corn | 13.59 ± 2.63 | 4.75 ± 1.62 | 1.95 ± 0.62 | 0.91 ± 0.13 | 0.86 ± 0.34 | 22.06 ± 2.26 | 61.6% |
| Dextrin | 17.96 ± 1.36 | 5.57 ± 1.36 | 2.59 ± 1.13 | 0.53 ± 0.16 | 0.26 ± 0.06 | 26.91 ± 2.16 | 66.71% | |
| NCIMB 8052-4 | Corn | 9.36 ± 1.91* | 3.36 ± 1.54* | 1.27 ± 0.23* | 1.15 ± 0.31 | 1.16 ± 0.43 | 16.30 ± 2.24* | 57.42% |
| Dextrin | 11.36 ± 2.34** | 3.63 ± 1.54** | 1.71 ± 0.61** | 1.15 ± 0.68 | 0.91 ± 0.12 | 18.76 ± 2.64** | 60.55% | |
| ATCC 824-4 | Corn | 11.38 ± 1.37* | 3.97 ± 1.92* | 1.69 ± 0.51* | 0.97 ± 0.21 | 0.85 ± 0.21 | 18.86 ± 2.61* | 60.33% |
| Dextrin | 12.38 ± 2.61** | 3.79 ± 1.62** | 1.96 ± 0.94** | 0.97 ± 0.34 | 0.68 ± 0.16 | 19.78 ± 2.91** | 62.58% | |
| SA-1-4 | Corn | 6.96 ± 2.61* | 2.86 ± 1.32* | 1.37 ± 0.61* | 1.09 ± 0.62 | 0.92 ± 0.16 | 13.2 ± 1.96* | 52.72% |
| Dextrin | 12.36 ± 3.15** | 4.68 ± 1.85 | 1.73 ± 0.67** | 1.11 ± 0.61 | 0.64 ± 0.34 | 20.52 ± 3.12** | 60.23% | |
| B-11-3-4 | Corn | 8.62 ± 1.97* | 2.23 ± 1.03* | 0.96 ± 0.32* | 1.09 ± 0.31 | 0.95 ± 0.34 | 13.85 ± 1.87* | 62.23% |
| Dextrin | 10.39 ± 2.16** | 2.23 ± 1.23** | 1.65 ± 0.52** | 1.26 ± 0.64 | 0.48 ± 0.15 | 16.01 ± 2.46** | 64.89% | |
| N1-4-4 | Corn | 3.65 ± 1.61* | 1.76 ± 0.94* | 1.29 ± 0.53* | 2.05 ± 0.16 | 1.69 ± 0.68 | 10.44 ± 1.96* | 34.96% |
| Dextrin | 4.59 ± 1.62** | 1.67 ± 0.68** | 1.92 ± 0.56** | 1.95 ± 0.43 | 1.07 ± 0.57 | 11.20 ± 1.97** | 40.17% | |
| CICC 8012-4 | Corn | 11.64 ± 1.36* | 5.25 ± 1.89 | 2.85 ± 0.67 | 0.63 ± 0.34 | 0.98 ± 0.16 | 21.35 ± 2.34 | 54.51% |
| Dextrin | 13.46 ± 2.94** | 5.52 ± 1.64 | 2.58 ± 0.81 | 0.85 ± 0.18 | 0.91 ± 0.16 | 23.32 ± 2.36 | 57.71% | |
| ATCC 4259-4 | Corn | 12.96 ± 1.6 | 5.59 ± 1.75 | 1.85 ± 0.38 | 1.06 ± 0.53 | 0.53 ± 0.11 | 21.99 ± 2.98 | 58.92% |
| Dextrin | 16.69 ± 2.86 | 4.94 ± 1.83 | 1.91 ± 0.72** | 1.21 ± 0.45 | 0.36 ± 0.36 | 25.11 ± 2.49 | 66.46% | |
| Strains | Butanol | Acetone | Ethanol | Butyrate | Acetate | Total solvent | Butanol/total solvent (%) |
|---|---|---|---|---|---|---|---|
| a ATCC 4259: C. acetobutylicum ATCC 4259. B-11-3: C. acetobutylicum B-11-3. SA-1: C. beijerinckii SA-1. CICC 8012: C. acetobutylicum CICC 8012. N1-4: C. saccharoperbutylacetonicum N1-4. ATCC 824: wild type strain C. acetobutylicum ATCC 824. NCIMB 8052: wild type strain C. beijerinckii NCIMB 8052.b All values are means ± SD values within columns followed by the * symbols are significantly different (P ≤ 0.05) compared with that without * symbols. | |||||||
| Mutant no. 15 | 20.67 ± 2.63 | 6.79 ± 1.56 | 3.41 ± 1.26 | 1.03 ± 0.16 | 0.85 ± 0.26 | 32.75 ± 2.34 | 63.11% |
| NCIMB 8052 | 11.58 ± 3.18* | 3.63 ± 1.45* | 2.31 ± 1.34 | 1.51 ± 0.18* | 1.06 ± 0.46* | 20.09 ± 3.26* | 52.66%* |
| ATCC 824 | 12.91 ± 3.45* | 5.79 ± 1.68 | 2.64 ± 1.26 | 0.67 ± 0.17* | 0.94 ± 0.34 | 22.95 ± 2.68* | 56.25%* |
| SA-1 | 14.35 ± 3.61* | 6.68 ± 2.35 | 2.49 ± 1.12* | 1.03 ± 0.32 | 1.09 ± 0.53 | 25.64 ± 2.61* | 55.96%* |
| B-11-3 | 12.46 ± 2.84* | 5.23 ± 1.96 | 2.68 ± 0.97 | 2.79 ± 1.23* | 0.85 ± 0.38 | 24.01 ± 2.68* | 51.89%* |
| N1-4 | 10.59 ± 2.45* | 3.67 ± 1.84* | 2.29 ± 1.03* | 1.56 ± 0.67 | 0.63 ± 0.16 | 18.74 ± 2.46* | 56.51%* |
| CICC 8012 | 15.79 ± 2.67* | 5.52 ± 1.61 | 3.85 ± 1.09 | 1.61 ± 0.69* | 0.89 ± 0.27 | 27.66 ± 3.61* | 57.08%* |
| ATCC 4259 | 17.56 ± 2.96* | 6.94 ± 2.34 | 2.52 ± 0.98* | 1.59 ± 0.86 | 0.97 ± 0.39 | 29.58 ± 3.91* | 59.36%* |
The phenomenon that the different pretreatment methods produced different fermentation substrates from the fresh raw cassava was observed, which led to subsequent variation in the productivity of fermentation (Table 7, P ≤ 0.05). The lowest butanol yield was obtained when mutant no. 15 fermented substrates from pretreatment methods 1 and 3 (Fig. 2A and Table 3), indicating that there was an incomplete transition phase when the carbon fluxed from the metabolized sugars to both butyrate and butanol. One cause of the lower butanol yield may be that, in this study, pH values were adjusted to 4.5 and 5.2 during enzymatic hydrolysis with pretreatment methods 1 and 3, respectively, and thus, growth inhibitors from cellulose and outermost epidermal layer that affect microorganisms (such as furans, aliphatic acids, and phenolic compounds)46 may have formed in the low-acid conditions. That reason is that fresh cassavas contain more complex compounds (e.g., hydrolysates of lignin and cellulose) than dried materials (outermost epidermal layer were conventional peeled off before pretreatment) because they completely retain the components present in their intact, outermost epidermal layer lead to the production of inhibitors. Once hydrolyzed these produce more saccharides, and ultimately should yield more butanol after fermentation because the fresh cassava retained all the ingredients. Thus, utilizing fresh cassava to produce butanol has great potential for producing butanol in industrial applications. Our experiment verified the conclusion that the fermentation substrates from pretreatment method 4 were most suitable for producing butanol using mutant no. 15. This illustrates that obtaining the highest butanol yield depends on selecting proper pretreatment methods for fresh cassava.
Discussion
The importance of selecting an appropriate screening method to obtain the target mutant with the highest butanol yield
There has been great progress in the industrial manufacturing of butanol, because of the use of modern biotechnology tools in the genetic improvement of microorganisms for industrial use, such as metabolic engineering and protein-directed evolution. However, to date there has still not been the expected breakthrough of obtaining a strain with high yield via genetic modification of the metabolic pathways of wild-type strains, due to the complex genetic background of Clostridium. For example, in recent years there have been many initiatives to increase butanol yields by transforming C. acetobutylicum and C. beijerinckii with genetic engineering and other tools, but the complex, branched metabolism of this species has posed a technical challenge (Fig. 6A). Previous studies aimed to increase the metabolic flux toward the desired product of butanol by inactivating competing pathways. For example, targeting the acetone biosynthetic pathway reduced or eliminated the unwanted byproduct; however, the butanol titer was also lower than in the wild-type strain.47–49 Besides the efforts with C. acetobutylicum, there have been no reports that the strain with high butanol yield was obtained via breeding for C. beijerinckii to directly ferment fresh cassava in industrial application. Therefore, traditional methods such as classic chemical mutagenesis are still an option for an effective approach to obtain a desired strain capable of producing high butanol yields; the key challenge has been how to find an efficient and simple method to identify and separate out the target strain. Therefore devising such a screening method would represent a significance advance in this field of research.There have been many reports about methods for screening target mutant strains. Using ADP-glucose pyrophosphorylase, and granulose synthase as an index, Reysenbach et al. found that defective mutants in granulose accumulation lacked either one or both enzyme activities necessary to screen the mutant based on metabolism of granulose by C. acetobutylicum.50 The strain C. acetobutylicum ATCC 10132 was isolated using the “tolerance of strains”: the mutants altered in acetic acid synthesis or in the shift to solventogenesis were selected directly via a proton suicide method, after mutagenic treatment with bromide and bromate as the selective agents. On the selection plates, mutants differed in colony phenotype from the parent strain.51 Changes in colony morphology were associated with the degeneration of solvent-producing strains of C. acetobutylicum. Another technique has been developed for predicting the solvent-producing ability of C. acetobutylicum: the most efficient solvent-producing strains gave rise exclusively to colonies with dense centers containing large numbers of spores. The relationship between colony morphology and solvent production thus provides a method for predicting the solvent-producing potential of C. acetobutylicum cultures.52 Amylolytic activity is primarily cell-associated when C. acetobutylicum is grown on glucose or maltose, and primarily extracellular when it is grown on dextrin or starch. Therefore, one method for screening strains is the use of possible changes, regulation and localization of amylolytic enzymes in C. acetobutylicum by mutagenesis.53 The transcription factor-based biosensor for specific activation—by either succinate, adipate, or 1-butanol—was used to screen strains that showed better production.54 A high-throughput screening system was also applied to identify a good strain.55 Although there have been important developments for screening methods by chemical mutagenesis, obtaining mutant strains with high butanol yields is still very much a work in progress.
In our work, we first used trypan blue as a screening indicator on a starch medium to identify target strains. Trypan blue can penetrate into the degenerative cell and integrate with DNA of cell, and can color dead cells, but living cells can prevent the dye from traversing the cell membrane. With the enhancement of intracellular metabolic activity, the ability of the cell to inhibit trypan blue dye from penetrating into the membrane is then stronger. When the strain with the stronger metabolic activity appears on the trypan blue-dyed agar medium, it will exclude trypan blue dye more completely, and thus, produce a brighter clear zone. The wild-type strains were mutated via classic NTG mutagenesis, resulting in a mixture of cell viabilities, which leads to the production of different bright clear zones on trypan blue agar medium (STTY). The strain with stronger growth activity will produce a brighter clear zone (Fig. 5Be–g). Using this method, mutant strains with high butanol production are more likely to be identified; our statistical analysis confirmed the feasibility of this simple, intuitive and effective approach (Fig. 5A). Further assessment showed that the effectiveness of this method was based on differences in the α-amylase activity of the mutants. We investigated the relationship between effect of amylase activity, butanol yield, and brightness of the clear zone on mutant colonies. The highest α-amylase activity, the highest butanol yield and the brightest clear zone were obtained with mutant no. 15 (Tables 1, 4 and 6).
| Mutant and pretreatment method | Brightness of clear zone |
|---|---|
| a B+, B, B−: brightness of the clear zone around colonies was divided into three ranks in accordance with the degree of brightness, where B+++ > B++ > B+. | |
| Mutant C. beijerinckii no. 15 | B+++ |
| Mutant C. beijerinckii no. 75 | B++ |
| Mutant C. beijerinckii no. 61 | B++ |
| Mutant C. beijerinckii no. 21 | B+ |
| Mutant C. beijerinckii no. 16 | B+ |
| C. beijerinckii NCIMB 8052 | B+ |
| Strains | Butanol yieldc | Acetone yieldc | Total solvent yieldc |
|---|---|---|---|
| a The mutant strains.b Pretreatment method of fresh cassava.c Yield was determined by dividing the grams of butanol or total solvent produced by grams of fresh cassava utilized. The productivity of strains were calculated using the ratio of solvent produced (g)/carbohydrate consumed (g). Mutant no. 15: C. beijerinckii no. 15; NCIMB 8052: original strain C. beijerinckii NCIMB 8052; ATCC 824: original strain C. acetobutylicum ATCC 824. *Significant difference (P ≤ 0.05) compared with that without * symbols. | |||
| Mutant no. 15a-1b | 0.1* | 0.047* | 0.023 |
| Mutant no. 15-2 | 0.14 | 0.062 | 0.021 |
| Mutant no. 15-3 | 0.088* | 0.040* | 0.021 |
| Mutant no. 15-4 | 0.151 | 0.0603 | 0.0256 |
| NCIMB 8052-1 | 0.0791* | 0.0251* | 0.0065* |
| NCIMB 8052-2 | 0.0526* | 0.0162* | 0.0043* |
| NCIMB 8052-3 | 0.0789* | 0.0261* | 0.0071* |
| NCIMB 8052-4 | 0.0426* | 0.11* | 0.0037* |
| ATCC 824-1 | 0.058* | 0.0136* | 0.0053* |
| ATCC 824-2 | 0.069* | 0.0242* | 0.0076* |
| ATCC 824-3 | 0.082* | 0.028* | 0.0098* |
| ATCC 824-4 | 0.054* | 0.0264* | 0.0067* |
Considering that the alpha-amylase from the wild type C. beijerinckii has not been purified and crystallized, its protein structure has not been analyzed in the past, and therefore protein spatial structure is still unknown. We make attempts to predict this mutant protein structure based on known protein model, expect to receive some parameters of this protein. As a result, we proceeded to homology modeling to predict similar tertiary structures of alpha-amylase from C. beijerinckii no. 15 based on known three-dimensional structure of alpha-amylase protein. It was found that the three-dimensional structure of α-amylases (3DC0. A) of Bacilhis sp. KR-8104 has the highest similarity compare to the mutant protein. Further prediction of the protein structure of this mutant no. 15's α-amylase suggested that changed positions of the amino acids occurred mainly in the B domain, the mutation position of no. 136's amino acid occurred in the A domain, and no. 392's position occurred in the C domain (Fig. 6D). We predicted the amino acids of the catalytic activity site of the mutant protein by comparing with positions 142, 178, 187, 210, 212, 221, 317, 354 of the catalytic activity site of α-amylase (3DC0.A) of Bacilhis sp. KR-8104. This revealed that mutant site 136 was near position 142 of 3DC0.A, mutant position 334 was near position 317 of 3DC0.A, and the mutant positions 345, 363 and 371 were also near position 317 of 3DC0.A. These mutant amino acids may result in the improvement of mutant α-amylase activity. C. beijerinckii no. 15 had higher amylase activity compared to the other selected strains (Table 1), possibly because to mutation of the amino acids of the mutant strain improved this protein's catalytic activity (Fig. 6B). These results provide a foundation of information for potential further improvements of α-amylase activity via site-specific mutagenesis or other methods of protein engineering, to obtain mutant strains of C. beijerinckii that are capable of producing high butanol yields.
The importance of selecting appropriate pretreatment methods to obtain optimal fermentation substrates that match the characteristics of target mutants
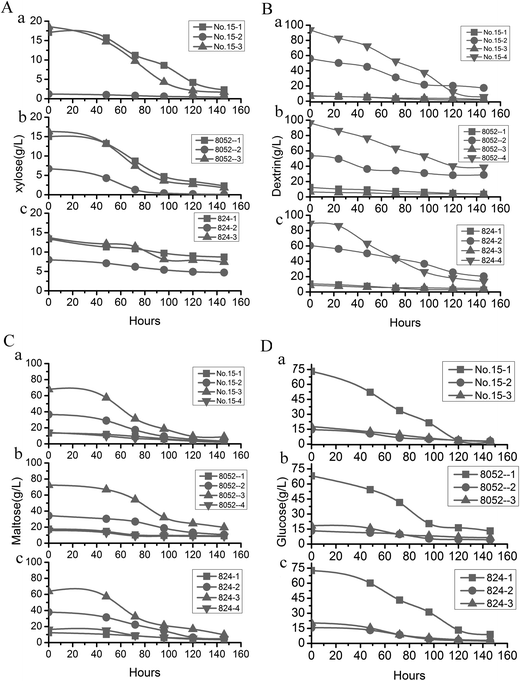
Most fermentation substrates used in the industrial manufacturing of butanol are hydrolysates of starch-rich crops. Researchers have often used different enzymes to hydrolyze starch in order to obtain desired fermentation substrates that match the characteristics of fermentation strains in the food industry or bioenergy industry. For example, (i) plentiful glucose is produced by α-amylase and glucoamylase, (ii) malt oligosaccharides are produced by α-amylase, and (iii) high concentrations of maltose are produced by α-amylase, pullulanase, and β-amylase under optimum conditions.56–60 Different strains often thrive on different types of fermentation substrates in the production of butanol. For example, wild-type C. beijerinckii NCIMB 8052 has a demonstrated ability to ferment glucose or other monosaccharides directly, yet it have a poor performance for directly using starch because it has poor α-amylase activity. In our study, the ideal substrate for mutant strains was unclear. Therefore, it was necessary to evaluate various hydrolysis procedures to determine which would be able to produce high butanol yields. The difference in butanol yields that resulted from the various treatment combinations of substrates and pretreatments with different enzymes was remarkable (Tables 2 and 3), underscoring the importance of identifying the optimal fermentation substrate for obtaining the highest butanol yield.The catabolic and regulatory mechanisms of amylase activity on starch in Clostridium bacteria have been well studied (Fig. 6A): strains with high amylase activity produce more butanol,53,61 so finding a bacterial strain with high amylase activity62 is the key to obtaining high butanol yields. The highest butanol yield that we obtained was with C. beijerinckii no. 15, perhaps simply because α-amylase activity of the strain was improved. That conclusion was verified by combining α-amylase activity and fermentation substrates to obtain the highest butanol yield from pretreatment method 4. The higher amylolytic activity of mutant no. 15 produced dramatically elevated levels of butanol compared to the output of other strains (Tables 1 and 3) because the high α-amylase activity of mutant no. 15 can fully break down dextrin into glucose or other monosaccharides (Fig. 4 and Table 1). In addition, inhibiting α-amylase activity of mutant no. 15 lowered the production of butanol also confirmed that α-amylase activity is the key factor for obtaining high butanol production in this process (Fig. 2Da). The result showed further that it is practical to directly ferment the starch in fresh cassava using the oversimplified procedure of pretreatment method 4, with no requirement for additional hydrolysis enzymes because of the high amylase content. Freshly-picked raw cassavas do not need to be dried and ground into powder before fermentation. Using pretreated fresh cassava as the fermentation substrate bypasses the processes of drying/grinding cassava into powder.63
Until now, an effective mutant strain had not been obtained from wild-type C. beijerinckii NCIMB 8052 to enable the production of high butanol yields from the fermentation of fresh cassava. The purpose of this study was to assess the feasibility of using C. beijerinckii rather than C. acetobutylicum to produce butanol from fresh cassava. Our experimental results showed that the mutant strain C. beijerinckii no. 15 is a promising candidate for fermenting liquefied fresh cassava to produce butanol. Our ongoing work is aimed at increasing the production of solvents from mutant no. 15, by further optimizing the conditions for generating ideal fermentation substrates. This advance will contribute to efforts to produce more affordable, cleaner, and sustainable sources of energy as an alternative to fossil fuels.
Competing interests
The authors declare that they have no competing interests.Acknowledgements
This work was financially supported by two Key Technology R&D Programs of Chongqing (grant number: cstc2012gg-sfgc20001 and cstc2011ggC20014) and the 100-talent program of Chinese Academia of Sciences and the 12th ‘five-year’ key task project in crop breeding of Sichuan Province (SN: 2011yzgg05).References
- P. Durre, Biotechnol. J., 2007, 2, 1525–1534 CrossRef PubMed.
- Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert and A. Darzins, Plant J. Cell Mol. Biol., 2008, 54, 621–639 CrossRef CAS PubMed.
- J. Wang and W. Wan, Int. J. Hydrogen Energy, 2008, 33, 2934–2941 CrossRef CAS PubMed.
- J. Wang and W. Wan, Int. J. Hydrogen Energy, 2008, 33, 6976–6984 CrossRef CAS PubMed.
- H. Tuo, Int. J. Energy Res., 2013, 37, 857–867 CrossRef.
- N. Al-Shorgani, E. Ali, M. Kalil and W. Yusoff, BioEnergy Res., 2012, 5, 287–293 CrossRef.
- J. Isar, H. Joshi and V. Rangaswamy, BioEnergy Res., 2013, 6, 991–999 CrossRef CAS.
- J. Liu, Z. Sun, H. Gerken, Z. Liu, Y. Jiang and F. Chen, Mar. Drugs, 2014, 12, 3487–3515 CrossRef CAS PubMed.
- P. Dürre, Appl. Microbiol. Biotechnol., 1998, 49, 639–648 CrossRef.
- V. Zverlov, O. Berezina, G. Velikodvorskaya and W. Schwarz, Appl. Microbiol. Biotechnol., 2006, 71, 587–597 CrossRef CAS PubMed.
- M. Kumar and K. Gayen, Appl. Energy, 2011, 88, 1999–2012 CrossRef CAS PubMed.
- S. Chatterjee, Biofuels, 2013, 4, 571–573 CrossRef CAS.
- N. Qureshi and T. C. Ezeji, Biofuels, Bioprod. Biorefin., 2008, 2, 319–330 CrossRef CAS.
- P. Dürre, Ann. N. Y. Acad. Sci., 2008, 1125, 353–362 CrossRef PubMed.
- M. Kumar, Y. Goyal, A. Sarkar and K. Gayen, Appl. Energy, 2012, 93, 193–204 CrossRef CAS PubMed.
- D. Liu, Y. Chen, F.-Y. Ding, T. Zhao, J.-L. Wu, T. Guo, H.-F. Ren, B.-B. Li, H.-Q. Niu and Z. Cao, Biotechnol. Biofuels, 2014, 7, 5 CrossRef PubMed.
- S. Atsumi, A. F. Cann, M. R. Connor, C. R. Shen, K. M. Smith, M. P. Brynildsen, K. J. Chou, T. Hanai and J. C. Liao, Metab. Eng., 2008, 10, 305–311 CrossRef CAS PubMed.
- M. P. Brynildsen and J. C. Liao, Mol. Syst. Biol., 2009, 5, 277 CrossRef PubMed.
- C. R. Shen and J. C. Liao, Metab. Eng., 2008, 10, 312–320 CrossRef CAS PubMed.
- G. Yin, Z. Sun, N. Liu, L. Zhang, Y. Song, C. Zhu and F. Wen, Appl. Microbiol. Biotechnol., 2009, 84, 323–333 CrossRef CAS PubMed.
- W. Strober, Current Protocols in Immunology, 2001, pp. A.3B.1–A.3B.2 Search PubMed.
- J. R. Tennant, Transplantation, 1964, 2, 685–694 CrossRef CAS PubMed.
- S. A. Altman, L. Randers and G. Rao, Biotechnol. Prog., 1993, 9, 671–674 CrossRef CAS PubMed.
- H. O. Jauregui, N. T. Hayner, J. L. Driscoll, R. Williams-Holland, M. H. Lipsky and P. M. Galletti, In Vitro, 1981, 17, 1100–1110 CrossRef CAS.
- P. A. Calatayud, E. Llovera, J. F. Bois and T. Lamaze, Photosynthetica, 2000, 38, 97–104 CrossRef CAS.
- A. Burns, R. Gleadow, J. Cliff, A. Zacarias and T. Cavagnaro, Sustainability, 2010, 2, 3572–3607 CrossRef PubMed.
- K. Sriroth, K. Piyachomkwan, V. Santisopasri and C. Oates, Euphytica, 2001, 120, 95–102 CrossRef CAS.
- V. Thang, K. Kanda and G. Kobayashi, Appl. Biochem. Biotechnol., 2010, 161, 157–170 CrossRef CAS PubMed.
- A. Chojecki and H. Blaschek, J. Ind. Microbiol., 1986, 1, 63–67 CrossRef CAS.
- C. Breuil and J. N. Saddler, Enzyme Microb. Technol., 1985, 7, 327–332 CrossRef CAS.
- L. Tappy, A. Buckert, M. Griessen, A. Golay, E. Jequier and J. Felber, Int. J. Obes., 1986, 10, 185–192 CAS.
- K. Yokose, K. Ogawa, T. Sano, K. Watanabe, H. B. Maruyama and Y. Suhara, J. Antibiot., 1983, 36, 1157–1165 CrossRef CAS.
- D. Howling, Int. Biodeterior. Biodegrad., 1989, 25, 15–19 CrossRef CAS.
- G. Lu and E. N. Moriyama, Briefings Bioinf., 2004, 5, 378–388 CrossRef CAS PubMed.
- A. Levit, D. Barak, M. Behrens, W. Meyerhof and M. Niv, in Membrane Protein Structure and Dynamics, ed. N. Vaidehi and J. Klein-Seetharaman, Humana Press, 2012, pp. 179–205 Search PubMed.
- X. Xi, X. Wei, Y. Wang, Q. Chu and J. Xiao, Arch. Biol. Sci., 2010, 62, 669–676 CrossRef.
- G. Cuesta, N. Suarez, M. I. Bessio, F. Ferreira and H. Massaldi, J. Microbiol. Methods, 2003, 52, 69–73 CrossRef CAS.
- P. Rao and T. N. Pattabiraman, Anal. Biochem., 1989, 181, 18–22 CrossRef CAS.
- T. Masuko, A. Minami, N. Iwasaki, T. Majima, S.-I. Nishimura and Y. C. Lee, Anal. Biochem., 2005, 339, 69–72 CrossRef CAS PubMed.
- M. Dubois, K. A. Gilles and J. K. Hamilton, Anal. Chim., 1956, 28, 350–356 CAS.
- S. Kumar and G. Mittal, Food Bioprocess Technol., 2010, 3, 741–751 CrossRef PubMed.
- C. Mandarim-de-Lacerda, C. Fernandes-Santos and M. Aguila, in Histology Protocols, ed. T. D. Hewitson and I. A. Darby, Humana Press, 2010, pp. 211–225 Search PubMed.
- M. Tangney and W. J. Mitchell, J. Mol. Microbiol. Biotechnol., 2000, 2, 71–80 CAS.
- L. Girbal, C. Croux, I. Vasconcelos and P. Soucaille, FEMS Microbiol. Rev., 1995, 17, 287–297 CrossRef CAS PubMed.
- W. J. Mitchell, Anaerobe, 1996, 2, 379–384 CrossRef CAS.
- B. C. Saha, L. B. Iten, M. A. Cotta and Y. V. Wu, Process Biochem., 2005, 40, 3693–3700 CrossRef CAS PubMed.
- Y. S. Jang, J. Y. Lee, J. Lee, J. H. Park, J. A. Im, M. H. Eom, J. Lee, S. H. Lee, H. Song, J. H. Cho, Y. Seung do and S. Y. Lee, mBio, 2012, 3, e00314-12 CrossRef PubMed.
- Y. Jiang, C. Xu, F. Dong, Y. Yang, W. Jiang and S. Yang, Metab. Eng., 2009, 11, 284–291 CrossRef CAS PubMed.
- D. Lehmann, D. Hönicke, A. Ehrenreich, M. Schmidt, D. Weuster-Botz, H. Bahl and T. Lütke-Eversloh, Appl. Microbiol. Biotechnol., 2012, 94, 743–754 CrossRef CAS PubMed.
- A. L. Reysenbach and N. Ravenscroft, Appl. Environ. Microbiol., 1986, 52, 185–190 CAS.
- C. H. Pablo and M. S. Beatriz, Appl. Environ. Microbiol., 1990, 56, 578–580 Search PubMed.
- W. C. Howard and I. Adler, Appl. Environ. Microbiol., 1987, 53, 2496–2499 Search PubMed.
- B. A. Annous and H. P. Blaschek, Appl. Environ. Microbiol., 1990, 56, 2559–2561 CAS.
- J. A. Dietrich, D. L. Shis, A. Alikhani and J. D. Keasling, ACS Synth. Biol., 2012, 2, 47–58 CrossRef PubMed.
- M. Scheel and T. Lütke-Eversloh, Metab. Eng., 2013, 17, 51–58 CrossRef CAS PubMed.
- P. Bernfeld, in Advances in Enzymology and Related Areas of Molecular Biology, John Wiley & Sons, Inc., 2006, pp. 379–428 Search PubMed.
- P. Nigam and D. Singh, Enzyme Microb. Technol., 1995, 17, 770–778 CrossRef CAS.
- M. J. E. C. van der Maarel, J. Biotechnol., 2002, 94, 137–155 CrossRef CAS.
- W. Douglas Crabb and C. Mitchinson, Trends Biotechnol., 1997, 15, 349–352 CrossRef.
- B. E. Haissig and R. E. Dickson, Physiol. Plant., 1979, 47, 151–157 CrossRef CAS PubMed.
- C. Andrew and B. HansP, J. Ind. Microbiol. Biotechnol., 1986, 1, 63–67 CrossRef.
- B. A. Annous and H. P. Blaschek, Appl. Environ. Microbiol., 1991, 57, 2544–2548 CAS.
- S. B. Bankar, S. A. Survase, H. Ojamoa and T. Granström, RSC Adv., 2013, 3, 24734–24757 RSC.
| This journal is © The Royal Society of Chemistry 2015 |