Synthesis and characterization of novel dumbbell shaped copolymers poly(ethylene oxide)x-b-polystyrene-b-poly(ethylene oxide)x with tunable side arms by combination of efficient thiol-ene addition reaction with living polymerization mechanism
Lingdi Chen,
Jian Huang,
Xuepu Wang,
Chengjiao Lu,
Hongdong Zhang* and
Guowei Wang*
State Key Laboratory of Molecular Engineering of Polymers, Collaborative Innovation Center of Polymers and Polymer Composite Materials, Department of Macromolecular Science, Fudan University, Shanghai 200433, China. E-mail: gwwang@fudan.edu.cn; Fax: +86 21 6564 0293; Tel: +86 21 6564 3049
First published on 10th March 2015
Abstract
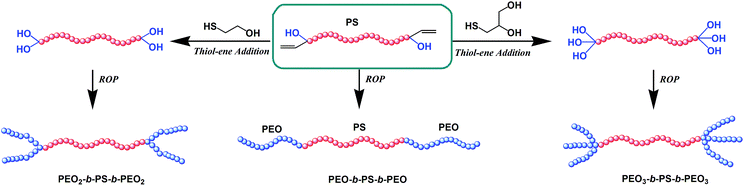
A series of novel dumbbell shaped copolymers poly(ethylene oxide)x-b-polystyrene-b-poly(ethylene oxide)x (PEOx-b-PS-b-PEOx, x = 1, 2, 3) composed of different numbers of hydrophilic PEO and hydrophobic PS segments were prepared by combination of living anionic polymerization (LAP) and ring opening polymerization (ROP) mechanisms, and the efficient thiol-ene addition reaction was also adopted. First, the functional polystyrene with one hydroxyl group and one allyl group at each end (AGE-PS-AGE) was synthesized by the LAP of St monomers followed by the capping reaction with allyl glycidyl ether (AGE). Subsequently, by thiol-ene addition reaction with 2-mercaptoethanol (ME) and 3-mercapto-1,2-propanediol (MP), one allyl group on the AGE-PS-AGE was transformed into one or two hydroxyl groups to synthesize the functional polystyrene with two hydroxyl groups at both ends (ME-PS-ME) or three hydroxyl groups at both ends (MP-PS-MP). Then, the copolymers PEOx-b-PS-b-PEOx were achieved by ROP of EO monomers using AGE-PS-AGE, ME-PS-ME and MP-PS-MP as macro-initiators. The target copolymers and their precursors were fully characterized by GPC, 1H NMR and 13C NMR measurements. The crystallization behavior of copolymers with different topologies and compositions was investigated by DSC, XRD and POM instruments, and the results showed that the topologies (compared to the compositions) tend to make the primary contribution to the crystallization behavior.
Introduction
In recent years, with the rapid development of living/controlled polymerization mechanisms and efficient coupling methods, a variety of polymers with complicated architectures and compositions have been realized by certain synthetic routes.1 The unique architectures include graft,2 hyper-branched,3 cyclic,4 dendritic,5 star-shaped,6 and so on,7 and the compositions can be covered by plenty of segments derived from any polymerizable monomers. All these achievements can be attributed to the increasing interest on their unique physical properties in solution and bulk, as well as their versatile applications, including in biomedical materials,8 nanotechnology,9 composite materials10 and supra-molecular science.11Among all the compositions, polystyrene (PS) is a typical hydrophobic and amorphous segment, and poly(ethylene oxide) (PEO) is a hydrophilic and crystalline segment. Because of these characteristics, the copolymers containing both PS and PEO segments have been widely studied and utilized for various applications. For example, common models are the copolymers PS-b-PEO, PS-b-PEO-b-PS or PEO-b-PS-b-PEO with the simplest topologies. Research priorities for these copolymers have been focused on the microphase separated morphology,12 thermodynamic properties,13 crystallization behaviors,14 and aggregate morphologies.15 As for their applications, these copolymers can serve as template for porous materials16 and micro/nanopatterns17 based on their interesting self-assembly morphologies.18 However, up to now, copolymers composed of PEO and PS segments with complicated architectures and defined molecular weights are still rarely researched, which might be attributed to the somewhat difficult synthesis procedure of these copolymers.
For copolymers with complicated architectures, a mere polymerization mechanism usually cannot reach the target. Some efficient coupling reactions also play important roles in the synthesis of these copolymers. Until recently, innovative coupling reactions with “click” character include the thiol-bromide reaction,19 thiol-ene addition reaction,20 thiol-yne addition reaction,21 Atom Transfer Radical Coupling (ATRC) reaction,22 Glaser coupling,23 Suzuki reaction,24 Copper Catalyzed Azide/Alkyne Click (CuAAC) chemistry and Diels–Alder (DA) [4 + 2] reaction,1f and so on. Because of their high efficiencies and particular versatility, these reactions have been widely used in polymer chemistry. Among these coupling reactions, the thiol-ene addition reaction has been widely adopted because of its high efficiency and tolerance to water and functional groups, as well as its easy operation under photochemical initiation. Applications include nanotechnology,25 composites,26 biochemistry,27 and adhesives.28 Correspondingly, various monomers containing vinyl and thiol groups were designed and their thiol-ene addition reactions were well researched.29 For example, Hawker,30 Son31 and Malmstrom32 have synthesized several dendrimers via this versatile thiol-ene addition reaction. Hawker,33 Kornfield,34 Schlaad35 and Sengupta36 prepared several functionalized polymers (such as PS, poly(butadiene) (PB), and poly(propylene) (PP)) by using this technique under feasible and mild conditions, and telechelic polymers were also realized by Sumerlin37 and Maynard.38 Thus, this thiol-ene “click” chemistry has actually been proved to be an efficient, robust and orthogonal tool in polymer chemistry.
In this contribution, considering the above limitation on copolymers composed of PEO and PS segments with different architectures and defined molecular weights, we aim to synthesize some novel dumbbell shaped copolymers PEOx-b-PS-b-PEOx (x = 1, 2, 3) with tunable side arms (Scheme 1). The PS segment with controlled molecular weight could be realized by the typical living anionic polymerization (LAP) mechanism, and the PEO segment could be synthesized by the ring opening polymerization (ROP) mechanism. Also, thiol-ene “click” chemistry is adopted to modify the functionalized intermediate to tune the initiating sites (corresponding to the tunable arms of the target copolymers) on macro-initiators. On the other hand, in previous works, some researchers have studied the crystallization behavior of copolymers composed of PEO and PS segments.39 These works were all focused on the effect of composition on their crystallization behavior, and the topologies were less considered.40 Thus, in this contribution, as another important task, the crystallization behavior of the synthesized copolymers with similar compositions but different topologies are also investigated and compared.
Experimental
Materials
Styrene (St, 99%, Sinopharm Chemical Reagent Co. (SCR)) was washed with 10% NaOH aqueous solution followed by water three times successively, dried over anhydrous MgSO4 for 24 h, further dried over CaH2 and distilled under reduced pressure before use. Allyl glycidyl ether (AGE, Aldrich, 99%) was dried over CaH2 and distilled under reduced pressure before use. Ethylene oxide (EO, SCR, 99%) was dried over CaH2 and distilled before use. Naphthalene (SCR, AR) was purified by sublimation. Tetrahydrofuran (THF, SCR, 99%) was refluxed and distilled from potassium naphthalenide solution. Azobisisobutyronitrile (AIBN, SCR, 99%), n-butyllithium (n-BuLi, 1.6 M solution in hexane, J&K), 2-mercaptoethanol (ME, Aldrich, 98%) and 3-mercapto-1,2-propanediol (MP, Aldrich, 98%) were used as received. Diphenylmethyl potassium (DPMK) with a concentration of 0.75 mol L−1 was prepared as described elsewhere.15b All other reagents and solvents were purchased from SCR and used as received.Characterization
Gel permeation chromatography (GPC) of the polymers was performed in THF at 35 °C with an elution rate of 1.0 mL min−1 on an Agilent 1100 equipped with a G1310A pump, a G1362A refractive index detector, and a G1314A variable wavelength detector. One 5 μm LP gel column (500 E, molecular range 500–2 × 104 g mol−1) and two 5 μm LP gel mixed bed columns (molecular range 200–3 × 106 g mol−1) were calibrated by PS standards. 1H NMR spectra were recorded on a Bruker (400 MHz) spectrometer in CDCl3 with tetramethylsilane (TMS) as the internal standard. The MALDI-TOF MS measurement was performed using a Perspective Biosystem Voyager-DE STR MALDI-TOF (matrix-assisted laser desorption/ionization time-of-flight) mass spectrometer (PE Applied Biosystems, Framingham, MA). The accelerating voltage, grid voltage and delay time were optimized for each sample and all the spectra were recorded in reflectron mode. Matrix solution of dithranol (20 mg mL−1), end-functionalized polymer (10 mg mL−1) and cationizing salt of silver trifluoroacetate (10 mg mL−1) in THF were mixed in the ratio of matrix![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cationizing salt
cationizing salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polymer = 10
polymer = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 0.8 μL of mixed solution was deposited on the sample holder (well-plate). Differential scanning calorimetry (DSC) was carried on a DSC Q2000 thermal analysis system (Shimadzu, Japan). The samples were first heated from −80 °C to 150 °C at a heating rate of 10 °C min−1 under nitrogen atmosphere, followed by cooling to −80 °C at 10 °C min−1 after stopping at 150 °C for 5 min, and finally heating to 150 °C at 10 °C min−1 after stopping at −80 °C for 5 min. X-ray diffraction (XRD) measurements were carried out using an XPert PRO (PANalytical) with Cu Kα (1.541 Å) radiation (40 kV, 40 mA). Samples were exposed at a scanning rate of 2θ = 5 °C min−1 between 2θ values of 10° to 40°. Crystal growth was observed under a polarized optical microscope (POM, Leica, DM 2500P). The concentration of copolymer was 5 mg mL−1, dichloromethane (CH2Cl2) was used as solvent, and all the measurements were carried out at 25 °C.
2, and 0.8 μL of mixed solution was deposited on the sample holder (well-plate). Differential scanning calorimetry (DSC) was carried on a DSC Q2000 thermal analysis system (Shimadzu, Japan). The samples were first heated from −80 °C to 150 °C at a heating rate of 10 °C min−1 under nitrogen atmosphere, followed by cooling to −80 °C at 10 °C min−1 after stopping at 150 °C for 5 min, and finally heating to 150 °C at 10 °C min−1 after stopping at −80 °C for 5 min. X-ray diffraction (XRD) measurements were carried out using an XPert PRO (PANalytical) with Cu Kα (1.541 Å) radiation (40 kV, 40 mA). Samples were exposed at a scanning rate of 2θ = 5 °C min−1 between 2θ values of 10° to 40°. Crystal growth was observed under a polarized optical microscope (POM, Leica, DM 2500P). The concentration of copolymer was 5 mg mL−1, dichloromethane (CH2Cl2) was used as solvent, and all the measurements were carried out at 25 °C.
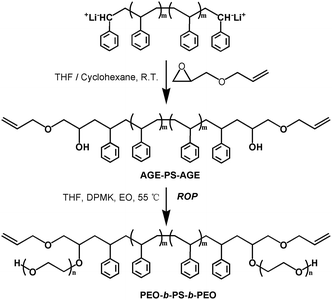
Synthesis of polystyrene functionalized with allyl glycidyl ether (AGE-PS-AGE)
The sample of AGE-PS-AGE was obtained by the LAP of St monomers initiated by lithium naphthalenide followed by the end-capping reaction with AGE agent (Scheme 2). The lithium naphthalenide initiator was first synthesized from naphthalene and lithium according to our previous work,41 and the concentration of 0.71 mol L−1 was found by titration using hydrochloric acid (0.1 mol L−1). Typically, cyclohexane (700 mL), styrene (50.0 mL, 0.43 mol), and THF (6.0 mL) were sequentially introduced into a 1 L ampule. In order to consume the remaining impurities in the ampule, n-Bu−Li+ solution was firstly added dropwise until the mixture turned yellowish, and then the needed lithium naphthalenide solution (35.0 mL, 24.9 mmol) was added rapidly. After the reaction was stirred at 25 °C for 50 min, the AGE agent (10.0 mL, 84.5 mmol) dissolved in THF (20 mL) was added, and the red solution changed into faint yellow immediately. The solution was stirred for another 2.0 h at 25 °C and terminated with acidic methanol (0.1 HCl in CH3OH). After all the solvents were evaporated, the product was recovered by precipitation three times in methanol and dried under vacuum at 45 °C. Yield: 44.6 g (98%). 1H NMR (CDCl3) δ (ppm): 1.11–2.25 (C6H5CHCH2–, –CH2CHOH), 3.50–4.00 (–CH(OH), –CH2OCH2–), 5.08–5.25 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 5.74–5.86 (CH2
CH–), 5.74–5.86 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.28–7.22 (–C6H5–). Mn, GPC = 3700 g mol−1, PDI = 1.10, MMALDI-TOF MS = 2500 g mol−1.
CH–), 6.28–7.22 (–C6H5–). Mn, GPC = 3700 g mol−1, PDI = 1.10, MMALDI-TOF MS = 2500 g mol−1.
Synthesis of polystyrene functionalized with 2-mercaptoethanol and 3-mercapto-1,2-propanediol (ME-PS-ME and MP-PS-MP)
The functionalized precursors of ME-PS-ME and MP-PS-MP were achieved by the thiol-ene addition reaction (Scheme 3). Typically, AGE-PS-AGE (10.0 g, 2.7 mmol), AIBN (1.64 g, 10 mmol) and ME (2.5 mL, 25 mmol) were dissolved in 80.0 mL dimethylformamide (DMF) in a 200 mL ampoule. After the reaction system was degassed by three cycles of freeze–pump–thaw, the ampoule was filled with nitrogen and then maintained at 65 °C for 24 h. Finally, the solvents were removed by reduced distillation, and the ME-PS-ME was obtained by precipitation three times in methanol and dried under vacuum at 45 °C. Yield: 9.8 g (96%). 1H NMR (CDCl3) δ (ppm): 1.20–2.10 (C6H5CHCH2–, –CH2CH2CH2–, –CH2CHOH), 2.54–2.69 (–CH2SCH2–), 2.98–3.95 (–CH2(OH), –CH2OCH2–, –CH2CHOH), 6.33–7.25 (–C6H5–).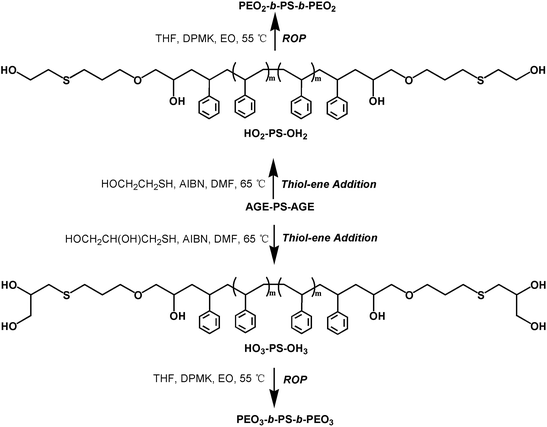 | ||
| Scheme 3 The synthetic procedure of copolymers PEO2-b-PS-b-PEO2, PEO3-b-PS-b-PEO3 and their precursor. | ||
Similarly, the synthetic procedure of MP-PS-MP was the same as that of ME-PS-ME, except that the ME agent was replaced by the MP agent. 1H NMR (CDCl3) δ (ppm): 1.16–2.20 (C6H5CHCH2–), 2.52–2.72 (–CH2SCH2–), 2.92–3.95 (–CH2(OH), –CHOH, –CH2OCH2–), 6.28–7.22 (–C6H5–).
Synthesis of dumbbell shaped copolymer PEOx-b-PS-b-PEOx (x = 1, 2, 3)
The copolymer PEO-b-PS-b-PEO was obtained by the ROP of EO monomers using AGE-PS-AGE as macro-initiator (Scheme 3). Typically, dry AGE-PS-AGE (6.0 g, 1.6 mmol) was dissolved in 200 mL THF and charged into a 500 mL dry ampoule, and the calculated DPMK solution (4.0 mL, 3.0 mmol) was added dropwise by a syringe under magnetic stirring. Then the ampoule was placed into an ice bath and cold EO (15.0 mL, 0.29 mol) monomers were added quickly, and the solution was heated to 55 °C and stirred for 96 h. The solution was finally terminated by acid methanol (0.1 HCl in CH3OH) and the solvents were evaporated. Subsequently, the copolymers PEO-b-PS-b-PEO were precipitated in cold petroleum ether (30–60 °C) slowly three times and dried under vacuum at 45 °C for 12 h to a constant weight. Yield: 9.8 g (96%). 1H NMR (CDCl3) δ (ppm): 3.59–3.70 (–CH2CH2O–), 6.28–7.22 (–C6H5–). Mn, GPC = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, PDI = 1.10, Mn, NMR = 11
500 g mol−1, PDI = 1.10, Mn, NMR = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.
000 g mol−1.
Similarly, the copolymers PEO2-b-PS-b-PEO2 and PEO3-b-PS-b-PEO3 were also prepared by the ROP of EO monomers from macro-initiators of ME-PS-ME and MP-PS-MP, respectively (Scheme 3). By changing the feed molar ratio of macro-initiators to EO monomers, copolymers with different compositions could be realized.
Results and discussion
Synthesis and characterization of amphiphilic dumbbell shaped copolymers PEOx-b-PS-b-PEOx
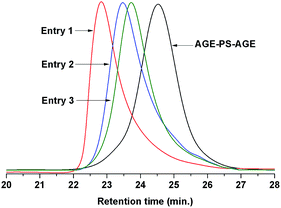
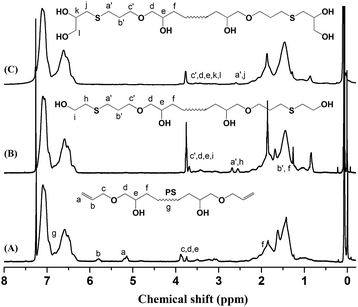
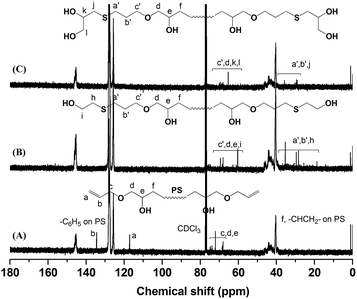
The key precursor AGE-PS-AGE with one active hydroxyl group and one allyl group at each end was designed and synthesized by LAP mechanism and the subsequent end-capping reaction with an oxirane ring on AGE agent. This versatile precursor could be further selectively modified by thiol-ene addition reaction. Subsequently, by combination of ROP mechanism, the target copolymers PEOx-b-PS-b-PEOx (x = 1, 2, 3) with different numbers of arms and compositions could be conveniently realized.Firstly, the difunctional living +Li-PS−Li+ species grown from lithium naphthalenide were carefully end-capped with AGE agent. According to previous work by Quirk,42 they synthesized several functionalized polymers with high efficiencies (usually >95.0%) by capping the living species with an oxirane ring on a substituted epoxy, such as 1-butene oxide, 3,4-epoxy-1-butene, styrene oxide, and so on. Also, in our work on the functionalization of PS−Li+ with 1-ethoxyethyl glycidyl ether (EEGE),43 the capping reaction was again realized with a quantitative efficiency. All these references gave the result that the capping reaction between the living species and oxirane ring was rather efficient, quantitative and accompanied by almost no side reactions, which was mainly due to the high aggregation degree of lithium alkoxides and their disability to initiate the further polymerization of the oxirane ring. In this work, because of the inertness of allyl group, the living species just attacked the oxirane ring on AGE, and a new hydroxyl group was simultaneously generated (Scheme 2). Once the AGE agent was added into the red system of the +Li-PS−Li+ solution, one could observe that the color of the system changed into light yellow immediately, which meant that alkoxide (–O−Li+) species was actually formed. In order to ensure the high efficiency of the end-capping reaction, excess AGE agent (nine-fold) was fed. The successful LAP of St monomers was evidenced by the monomodal peak and symmetrical GPC curve with a narrow molecular weight distribution (PDI = 1.10) (Fig. 1). Also, the 1H NMR (Fig. 2A) and 13C NMR (Fig. 3A) spectra of the synthesized AGE-PS-AGE were monitored. In Fig. 2A, except for the characteristic resonance signals of aromatic protons (–C6H5–) on the PS chain seen at 6.28–7.22 ppm, the resonance signals appearing at 5.16 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) and 5.78 ppm (CH2
CH–) and 5.78 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) confirmed the successful introduction of the allyl group onto PS. Also, signals ascribed to methane and methylene protons (–CH(OH)CH2OCH2CH
CH–) confirmed the successful introduction of the allyl group onto PS. Also, signals ascribed to methane and methylene protons (–CH(OH)CH2OCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) were observed at 3.50–4.00 ppm. In Fig. 3A, the characteristic resonance signals of carbon atoms (–C6H5–) were seen at 124–130 ppm, and the characteristic resonance signals of carbon atoms (CH2
CH2) were observed at 3.50–4.00 ppm. In Fig. 3A, the characteristic resonance signals of carbon atoms (–C6H5–) were seen at 124–130 ppm, and the characteristic resonance signals of carbon atoms (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) and (CH2
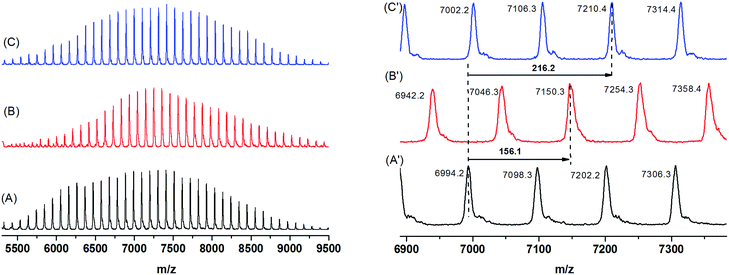
CH–) and (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) were seen at 117 ppm and 134 ppm, respectively. Thus, the NMR results actually proved the smooth coupling reaction of AGE agent with living +Li-PS−Li+ species and the successful introduction of the allyl group onto PS. Furthermore, the functionalized AGE-PS-AGE with defined structure was again analyzed by MALDI-TOF MS (Fig. 4). As shown in Fig. 4A, a series of mono-peaks were observed for AGE-PS-AGE. When the mass spectrum was expanded (Fig. 4A′), the peak at m/z = 6994.2 (a monoisotopic mass peak) was attributed to the functionalized AGE-PS-AGE [C6H10O2–(C8H8)64–C6H10O2·Ag+ = 6994.2, cal. 6994.39], and the m/z spacing of 104.1 between adjacent peaks was the mass of St monomeric unit. The absence of any minor peak series confirmed that the capping reaction at both +Li-PS−Li+ ends was rather successful and a high efficiency was achieved. According to the integral areas at 5.16 ppm (CH2
CH–) were seen at 117 ppm and 134 ppm, respectively. Thus, the NMR results actually proved the smooth coupling reaction of AGE agent with living +Li-PS−Li+ species and the successful introduction of the allyl group onto PS. Furthermore, the functionalized AGE-PS-AGE with defined structure was again analyzed by MALDI-TOF MS (Fig. 4). As shown in Fig. 4A, a series of mono-peaks were observed for AGE-PS-AGE. When the mass spectrum was expanded (Fig. 4A′), the peak at m/z = 6994.2 (a monoisotopic mass peak) was attributed to the functionalized AGE-PS-AGE [C6H10O2–(C8H8)64–C6H10O2·Ag+ = 6994.2, cal. 6994.39], and the m/z spacing of 104.1 between adjacent peaks was the mass of St monomeric unit. The absence of any minor peak series confirmed that the capping reaction at both +Li-PS−Li+ ends was rather successful and a high efficiency was achieved. According to the integral areas at 5.16 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 5.78 ppm (CH2
CH–), 5.78 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.28–7.22 ppm and the absolute molecular weight of AGE-PS-AGE obtained by MALDI-TOF MS, the capping efficiency of the AGE agent to +Li-PS−Li+ was calculated as 98.5%.
CH–), 6.28–7.22 ppm and the absolute molecular weight of AGE-PS-AGE obtained by MALDI-TOF MS, the capping efficiency of the AGE agent to +Li-PS−Li+ was calculated as 98.5%.
 | ||
| Fig. 4 The MALDI-TOF MS of (A and A′) AGE-PS-AGE (Mn, GPC = 7500 g mol−1), (B and B′) ME-PS-ME, and (C and C′) MP-PS-MP. | ||
Subsequently, by efficient thiol-ene addition reaction, one allyl group could be transformed into one or two hydroxyl groups. Using DMF as solvent and AIBN as catalyst, the ME-PS-ME with two hydroxyl groups at each end was obtained by the reaction between 2-mercaptoethanol and the above AGE-PS-AGE (Scheme 3). From the 1H NMR spectrum of ME-PS-ME (Fig. 2B), the disappearance of the resonance signals at 5.16 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) and 5.78 ppm (CH2
CH–) and 5.78 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) confirmed that the allyl groups were completely consumed. Also, the appearance of signals at 2.54 ppm and 2.69 ppm assigned to the methylene protons connected to sulfur atom (–CH2–S–CH2–) further confirmed the successful introduction of 2-mercaptoethanol. Similarly, the MP-PS-MP was obtained by the thiol-ene addition reaction between AGE-PS-AGE and 3-mercapto-1,2-propanediol. The complete disappearance of resonance signals at 5.16 ppm and 5.78 ppm assigned to the allyl group confirmed that an almost 100% efficiency of thiol-ene addition reaction was realized. From the 13C NMR spectra of ME-PS-ME (Fig. 3B) and MP-PS-MP (Fig. 3C), the complete disappearance of characteristic signals ascribed to carbon atoms at 117 ppm (CH2
CH–) confirmed that the allyl groups were completely consumed. Also, the appearance of signals at 2.54 ppm and 2.69 ppm assigned to the methylene protons connected to sulfur atom (–CH2–S–CH2–) further confirmed the successful introduction of 2-mercaptoethanol. Similarly, the MP-PS-MP was obtained by the thiol-ene addition reaction between AGE-PS-AGE and 3-mercapto-1,2-propanediol. The complete disappearance of resonance signals at 5.16 ppm and 5.78 ppm assigned to the allyl group confirmed that an almost 100% efficiency of thiol-ene addition reaction was realized. From the 13C NMR spectra of ME-PS-ME (Fig. 3B) and MP-PS-MP (Fig. 3C), the complete disappearance of characteristic signals ascribed to carbon atoms at 117 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) and 134 ppm (CH2
CH–) and 134 ppm (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) and the new occurrence of signals ascribed to the carbon atoms on the ME or MP groups also gave the same information that the allyl groups at the PS end were completely transformed into the corresponding target groups. Again, the efficient transformations from AGE-PS-AGE to ME-PS-ME and MP-PS-MP were confirmed by MALDI-TOF MS measurement (Fig. 4). As shown in Fig. 4, an increase of 156.1 m/z from peak [C6H10O2–(C8H8)64–C6H10O2·Ag+ = 6994.2, cal. 6994.39] to peak [C2H6OS–C6H10O2–(C8H8)64–C6H10O2–C2H6OS·Ag+ = 7150.3] was attributed to the mass of two introduced 2-mercaptoethanol units, and the increase of 216.2 m/z from peak [C6H10O2–(C8H8)64–C6H10O2·Ag+ = 6994.2, cal. 6994.39] to peak [C3H8O2S–C6H10O2–(C8H8)64–C6H10O2–C3H8O2S·Ag+ = 7210.4] was attributed to the mass of two introduced 3-mercapto-1,2-propanediol units. Thus, the perfectly coincident results from MALDI-TOF MS and NMR gave the information that the macro-initiators of ME-PS-ME and MP-PS-MP were successfully synthesized.
CH–) and the new occurrence of signals ascribed to the carbon atoms on the ME or MP groups also gave the same information that the allyl groups at the PS end were completely transformed into the corresponding target groups. Again, the efficient transformations from AGE-PS-AGE to ME-PS-ME and MP-PS-MP were confirmed by MALDI-TOF MS measurement (Fig. 4). As shown in Fig. 4, an increase of 156.1 m/z from peak [C6H10O2–(C8H8)64–C6H10O2·Ag+ = 6994.2, cal. 6994.39] to peak [C2H6OS–C6H10O2–(C8H8)64–C6H10O2–C2H6OS·Ag+ = 7150.3] was attributed to the mass of two introduced 2-mercaptoethanol units, and the increase of 216.2 m/z from peak [C6H10O2–(C8H8)64–C6H10O2·Ag+ = 6994.2, cal. 6994.39] to peak [C3H8O2S–C6H10O2–(C8H8)64–C6H10O2–C3H8O2S·Ag+ = 7210.4] was attributed to the mass of two introduced 3-mercapto-1,2-propanediol units. Thus, the perfectly coincident results from MALDI-TOF MS and NMR gave the information that the macro-initiators of ME-PS-ME and MP-PS-MP were successfully synthesized.
Finally, the target copolymers PEOx-b-PS-b-PEOx were obtained by the ROP of EO monomers using the above AGE-PS-AGE, ME-PS-ME and MP-PS-MP as macro-initiators and DPMK as deprotonation agent (Scheme 3). Typically, because of the rapid exchange ratio (which was faster than the propagation ratio) between living species –O−K+ and the dormant –OH, the ROP mechanism was endowed with a living character. According to the literature,44 primary and secondary hydroxyls could give the uniform growth of PEO chains under certain polymerization conditions. Using the similar polymerization system in this contribution, all the hydroxyl groups had the ability to initiate the polymerization of EO monomers, and the designed architecture of PEOx-b-PS-b-PEOx with defined numbers of arms could be obtained. From Fig. 1, it could be observed that the GPC traces of the obtained PEO-b-PS-b-PEO, PEO2-b-PS-b-PEO2 and PEO3-b-PS-b-PEO3 all have monomodal peaks and low PDIs, which also gave the conclusion that the ROP mechanism proceeded smoothly. However, an asymmetrical GPC trace with a tail at longer elution time was always detected due to the strong adsorption of the PEO segment with the column in THF solvent (which was not a good solvent for the PEO segment), rather than the existence of PS homopolymers, and a similar phenomenon was also reported in our previous work43 and other’s work.45 In order to further confirm this consumption, the copolymers were verified by the TLC method using THF as developing agent. The absence of any PS signal in the front edge under 254 nm on the UV detector confirmed that there was actually not any PS homopolymer existing in the copolymers. The composition of the copolymers was further studied by the 1H MNR spectrum. In order to avoid the formation of micelles in selective solvent,2a,45 the 1H NMR measurement of the copolymers was carried out in CDCl3 solvent, which was a good solvent for both the PEO and PS segments.46 As shown in Fig. 5, the characteristic resonance signal of aromatic protons (–C6H5–) on the PS segment at 6.28–7.22 ppm and that of methylene protons (–CH2CH2O–) on the PEO segment at 3.05–3.70 ppm were discriminated well. On the other hand, the molecular weight of PEOx-b-PS-b-PEOx determined by GPC measurement using THF as an eluent was unreliable because THF was not a good solvent for the PEO segment and the PEOx-b-PS-b-PEOx might aggregate into micellar structures by self-association in THF; this phenomenon was also reported in ref. 2a and 47 and our previous work.43 Alternatively, according to the above 1H MNR spectrum and the already known absolute molecular weight of the AGE-PS-AGE precursor, the accurate molecular weight of the copolymers could be derived from the formula:
 | (1) |
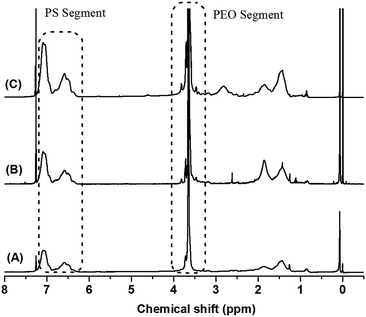 | ||
| Fig. 5 1H NMR spectra of PEO3-b-PS-b-PEO3 with the same PS segment but different length of PEO segments: (A) Entry 3, (B) Entry 4, and (C) Entry 5 (in CDCl3). | ||
Here, APEO and APS represent the integral areas of the resonance signals of aromatic protons (–C6H5–) at 6.28–7.22 ppm and methylene protons (–CH2CH2O–) at 3.59–3.70 ppm, respectively. Mn, MALDI-TOF MS, PS is the absolute molecular weight of the AGE-PS-AGE precursor obtained from MALDI-TOF MS measurement. The values of 104 and 44 are the molecular weights of the St and EO monomeric units, respectively. R is equal to 0 (for PEO-b-PS-b-PEO), 78 (for PEO2-b-PS-b-PEO2) or 108 (for PEO3-b-PS-b-PEO3), and the values of 78 and 108 correspond to the molecular weights of the introduced ME and MP residues, respectively.
Interestingly, comparing the molecular weights of the copolymers obtained from GPC with that from the NMR measurement, one can also discern that the apparent molecular weights from the GPC measurement had low linear dependence on their absolute molecular weights from NMR measurement. For example, PEO-b-PS-b-PEO in Entry 1 and PEO2-b-PS-b-PEO2 in Entry 2 had a difference of 600 g mol−1 in their absolute molecular weights from NMR measurement, however, there was a difference of 3800 g mol−1 from their GPC measurement. Alternatively, the samples in Entry 2, Entry 3, Entry 4 and Entry 5 had very similar apparent molecular weights from GPC measurement, however, there was much difference between their absolute molecular weights. These phenomena could be attributed to the varied length of the PEO segments and the increasing branching of copolymers from PEO-b-PS-b-PEO to PEO2-b-PS-b-PEO2 and PEO3-b-PS-b-PEO3. Also, these phenomena might give the information that the topologies of copolymers actually induced some special properties of the copolymers, and the crystallization behavior of these synthesized copolymers will be discussed in the following section.
The crystallization behavior of copolymers PEOx-b-PS-b-PEOx
Typically, the PS is an amorphous segment, while the PEO is a crystalline segment.48 As shown in Table 1, PS and PEO segments with different lengths were designed and synthesized by the versatile LAP and ROP mechanisms. As an important target, the crystallization behaviors of these synthesized copolymers were investigated and compared in this contribution.| Entry | Samples | Mn, GPCa (g mol−1) | PDIa | Mn, NMRb (g mol−1) | DPPSc | DPPEOd | Percentage of PEO segmente (%) |
|---|---|---|---|---|---|---|---|
| a Determined by GPC with THF as solvent using PS standards.b The molecular weights of copolymers were calculated according to 1H NMR using formula (1).c The degree of polymerization (DPPS) was calculated according to the formula: DPPS = Mn, MALDI-TOF MS, PS/104.d The degree of polymerization (DPPEO) was calculated according to the formula: DPPEO = (Mn, NMR, (PEOx-b-PS-b-PEOx) − Mn, MALDI-TOF MS, PS)/44.e The percentage of PEO segment (%) was calculated according to the formula: %PEO = DPEOx44/Mn, NMR, (PEOx-b-PS-b-PEOx).f Determined by MALDI-TOF MS measurement. | |||||||
| AGE-PS-AGE | 3700 | 1.14 | 2500f | 23 | |||
| 1 | PEO-PS-PEO | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.11 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
23 | 193 | 78.02 |
| 2 | PEO2-PS-PEO2 | 7700 | 1.16 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
23 | 180 | 76.80 |
| 3 | PEO3-PS-PEO3 | 7600 | 1.10 | 9100 | 23 | 150 | 73.40 |
| 4 | PEO3-PS-PEO3 | 7400 | 1.13 | 8100 | 23 | 127 | 68.99 |
| 5 | PEO3-PS-PEO3 | 7000 | 1.09 | 6000 | 23 | 80 | 59.54 |
| AGE-PS-AGE | 7900 | 1.14 | 7500f | 71 | |||
| 6 | PEO-PS-PEO | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.17 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
71 | 330 | 66.29 |
| 7 | PEO-PS-PEO | 9100 | 1.09 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
71 | 132 | 44.03 |
| 8 | PEO2-PS-PEO2 | 7900 | 1.11 | 9400 | 71 | 43 | 20.40 |
| 9 | PEO3-PS-PEO3 | 9800 | 1.14 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
71 | 95 | 36.15 |
The crystallization behavior of PEOx-b-PS-b-PEOx was firstly studied by XRD measurement. All the samples were measured at room temperature without annealing. Typically, the linear PEO showed two intense diffraction peaks at 19.1° and 23.3°.49 As shown in Fig. 6, with a similar percentage of the PEO segment but varied numbers of side PEO arms, the intensity of the diffraction peaks for the PEO crystallite decreased from the copolymer PEO-b-PS-b-PEO (Entry 1) to PEO2-b-PS-b-PEO2 (Entry 2) and PEO3-b-PS-b-PEO3 (Entry 3). For these three samples with the similar percentages of the PEO segment, the lengths of the PEO arms were shortened with the increase of the number of side arms, and correspondingly, the shorter PEO segment and the increased terminal PEO ends severely interrupted the crystallization abilities of the copolymers.
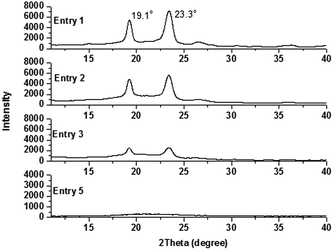 | ||
| Fig. 6 X-ray diffraction patterns for PEO-b-PS-b-PEO (Entry 1), PEO2-b-PS-b-PEO2 (Entry 2), and PEO3-b-PS-b-PEO3 (Entry 3 and Entry 5). | ||
Alternatively, when the numbers of PEO arms were fixed but their percentages were varied, the crystallization behavior of the copolymers led to another result. Comparing the XRD trace for Entry 3 with that for Entry 5, no diffraction signals could be observed in the XRD trace for the latter when the PEO content was merely decreased to 59.54%. However, comparing the XRD trace for Entry 6 with that for Entry 7, strong signals could still be discriminated at 19.1° and 23.3° even though the PEO content was decreased to 44.03% for Entry 7 (Fig. 7). These results preliminarily gave the conclusion that the degree of branching has more effect on the crystallization behavior than the percentage of the PEO segment.
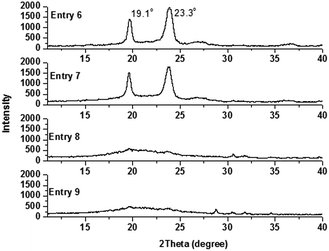 | ||
| Fig. 7 X-ray diffraction patterns for PEO-b-PS-b-PEO (Entry 6 and Entry 7), PEO2-b-PS-b-PEO2 (Entry 8), and PEO3-b-PS-b-PEO3 (Entry 9). | ||
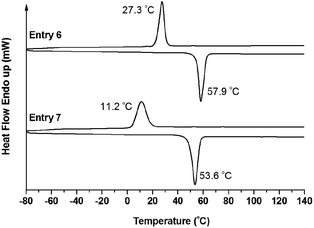
Furthermore, the crystallization behavior of the copolymers was verified by DSC measurement. The crystallization temperature (Tc) was obtained from the cooling run, and the melting temperature (Tm) was obtained from the second heating run. Usually, for the linear PEO homopolymer with a molecular weight of about Mn = 5000 g mol−1, Tm and Tc were observed at 63.9 °C and 43.9 °C, respectively.50 As shown in Fig. 8, for the sample of PEO-b-PS-b-PEO (Entry 1), Tc and Tm were clearly discriminated at 32.9 °C and 53.6 °C, respectively, which were both lower than for the PEO homopolymer. However, for the sample of PEO2-b-PS-b-PEO2 (Entry 2), which had a similar percentage of the PEO segment but a different number of side PEO arms compared to the sample in Entry 1, except for a decreased Tm peak at 31.3 °C and a Tc peak at −21.0 °C observed in the heating curve, there was almost not any Tc detected in the cooling run. We concluded that, because of the increased complexity of the architecture of PEO2-b-PS-b-PEO2 in Entry 2, the PEO segment could not be arranged into a crystalline form in the cooling program, and the insufficient crystallization of the PEO segment was maintained in the sample. Alternatively, in the following heating program, some of the PEO segment in the amorphous phase might again be folded into a crystalline form under certain temperatures by a thermal crystallization procedure. This phenomenon has also been reported in ref. 51. Similarly, the sample of PEO3-b-PS-b-PEO3 (Entry 3) also gave a Tc at −32.5 °C corresponding to thermal crystallization and a typical Tm at 32.3 °C in the heating curve, and there was also not any Tc observed in the cooling curve.
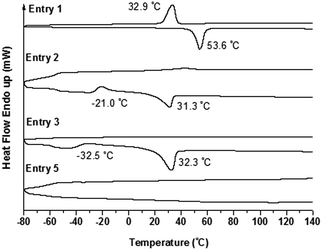 | ||
| Fig. 8 DSC traces of copolymer PEO-b-PS-b-PEO (Entry 1), PEO2-b-PS-b-PEO2 (Entry 1), and PEO3-b-PS-b-PEO3 (Entry 3 and Entry 5). | ||
Again, for the samples with the same number of PEO arms but varied percentage of the PEO segment, the DSC results of the PEO3-b-PS-b-PEO3 series also gave a different tendency to those of the PEO-b-PS-b-PEO series. For example, comparing the DSC curve for Entry 3 with that for Entry 5 (Fig. 8), no peaks could be discriminated in the DSC curve for the latter, Entry 5, because of the decreased percentage of the PEO segment (59.54%). However, comparing the DSC curve for Entry 6 with that for Entry 7 (Fig. 9), both samples gave DSC curves with defined Tm and Tc peaks (even though the percentage of the PEO segment in the latter, Entry 7, decreased to 44.03%) because of the linear architecture of PEO-b-PS-b-PEO.
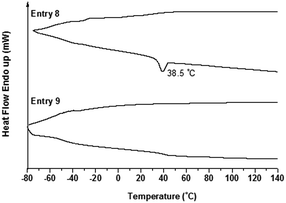
From Fig. 10, for the DSC curves of Entry 8 and Entry 9, there was almost no obvious signal for the crystallization behavior (such as Tm and Tc peaks) of the samples, except that there was a weak Tm peak at 38.5 °C, which was rather consistent with the results from their XRD measurements (Fig. 7). This might be attributed to the largely lowered PEO content or increased number of side arms, as well as the increased length of the PS segment. On the other hand, it was worth noting that even with the increased length of PS segment (7500 g mol−1) for samples in Entry 8 and Entry 9, no trace of the glass transition temperature (Tg) for the PS segment could be discriminated.
Thus, the DSC results again gave the information that the degree of branching would have a greater effect on the crystallization behavior of the copolymers than the percentage of individual segments.
Finally, the crystallization behavior was also investigated by POM measurement (Fig. 11). From the POM micrograph of PEO-b-PS-b-PEO (Entry 1), we clearly observed that the big spherulites of the PEO segments could be formed in 30 minutes. With the increase of the number of PEO arms, the crystallization behavior was significantly affected. Obviously, from the sample of PEO-b-PS-b-PEO (Entry 1) to PEO2-b-PS-b-PEO2 (Entry 2) and PEO3-b-PS-b-PEO3 (Entry 3), a longer crystallization time was needed and only smaller spherulites could be observed.
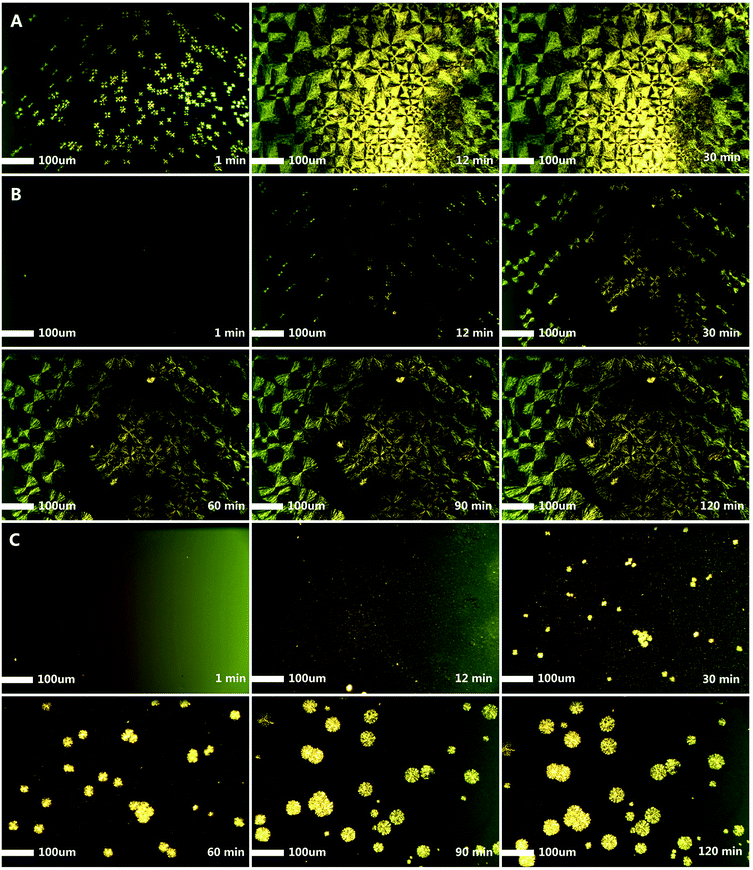 | ||
| Fig. 11 Optical microscopy images of copolymers (scale bar = 100 μm): (A) PEO-b-PS-b-PEO (Entry 1), (B) PEO2-b-PS-b-PEO2 (Entry 2), and (C) PEO3-b-PS-b-PEO3 (Entry 3). | ||
All the above results indicated that the degree of branching and compositions actually exerted some important effects on the crystallization behaviors of synthesized dumbbell shaped copolymers PEOx-b-PS-b-PEOx. The existence of a PS segment largely interrupted the crystallization behavior of the PEO segment, and the existence of a crystalline PEO segment largely restricted the mobility of the PS segment and affected its Tg. Comprehensively, the degree of branching derived from various complicated topologies might make the primary contribution to the crystallization behavior.
Conclusions
A series of well-defined dumbbell shaped copolymers PEOx-b-PS-b-PEOx (x = 1, 2, 3) consisting of PEO and PS segments were successfully synthesized by LAP and ROP mechanisms and the efficient thiol-ene addition reaction. This versatile synthetic route might be further explored to synthesize other copolymers with complicated architectures. Also, the crystallization behaviors of the copolymers with similar compositions but different topologies, or conversely, with different compositions but the same topologies, were investigated and compared. The preliminary results confirmed that the topologies of copolymers actually made the primary contribution to the crystallization behavior.Acknowledgements
We appreciate the financial support of this research by the Natural Science Foundation of China (21274024).Notes and references
- (a) A. Vazaios, D. J. Lohse and N. Hadjichristidis, Macromolecules, 2005, 38, 5468–5474 CrossRef CAS; (b) L. Andruzzi, A. Hexemer, X. F. Li, C. K. Ober, E. J. Kramer, G. Galli, E. Chiellini and D. A. Fischer, Langmuir, 2004, 20, 10498–10506 CrossRef CAS PubMed; (c) F. J. Xu, Y. Song, Z. P. Cheng, X. L. Zhu, C. X. Zhu, E. T. Kang and K. G. Neoh, Macromolecules, 2005, 38, 6254–6258 CrossRef CAS; (d) V. Percec, T. Guliashvili, J. S. Ladislaw, A. Wistrand, A. Stjerndahl, M. J. Sienkowska, M. J. Monteiro and S. Sahoo, J. Am. Chem. Soc., 2006, 128, 14156–14165 CrossRef CAS PubMed; (e) H. F. Gao and K. Matyjaszewski, J. Am. Chem. Soc., 2007, 129, 6633–6639 CrossRef CAS PubMed; (f) W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15–54 CrossRef CAS.
- (a) H. Zhang and E. Ruckenstein, Macromolecules, 2000, 33, 814–819 CrossRef CAS; (b) K. Se, H. Yamazaki, T. Shibamoto, A. Takano and T. Fujimoto, Macromolecules, 1997, 30, 1570–1576 CrossRef CAS.
- M. Gauthier, L. Tichagwa, J. S. Downey and S. Gao, Macromolecules, 1996, 29, 519–527 CrossRef CAS.
- C. Lee, H. Lee, W. Lee, T. Chang and J. Roovers, Macromolecules, 2000, 33, 8119–8121 CrossRef.
- J. Teng and E. R. Zubarev, J. Am. Chem. Soc., 2003, 125, 11840–11841 CrossRef CAS PubMed.
- N. Hadjichristidis, M. Pitsikalis, S. Pispas and H. Iatrou, Chem. Rev., 2001, 101, 3747–3792 CrossRef CAS PubMed.
- Y. Yagci and M. A. Tasdelen, Prog. Polym. Sci., 2006, 31, 1133–1170 CrossRef CAS PubMed.
- S. E. Stiriba, H. Kautz and H. Frey, J. Am. Chem. Soc., 2002, 124, 9698–9699 CrossRef CAS PubMed.
- R. Djalali, S. Y. Li and M. Schmidt, Macromolecules, 2002, 35, 4282–4288 CrossRef CAS.
- M. Zhang, M. Drechsler and A. H. E. Muller, Chem. Mater., 2004, 16, 537–543 CrossRef CAS.
- L. H. He, J. Huang, Y. M. Chen, X. J. Xu and L. P. Liu, Macromolecules, 2005, 38, 3845–3851 CrossRef CAS.
- L. Huang, H. Yuan, D. B. Zhang, Z. Zhang, J. Guo and J. M. Ma, Appl. Surf. Sci., 2004, 225, 39–46 CrossRef CAS PubMed.
- L. M. Wu and Q. C. Zou, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 2015–2022 Search PubMed.
- (a) Y. Gao and H. L. Liu, J. Appl. Polym. Sci., 2007, 106, 2718–2723 CrossRef CAS; (b) C. Y. Luo, X. Han, Y. Gao, H. L. Liu and Y. Hu, J. Macromol. Sci., Part B: Phys., 2010, 49, 440–453 CrossRef CAS.
- (a) S. Y. Cheng, Z. S. Xu and J. J. Yuan, Acta Chim. Sin., 2000, 58, 368–370 CAS; (b) R. Francis, D. Taton, J. L. Logan, P. Masse, Y. Gnanou and R. S. Duran, Macromolecules, 2003, 36, 8253–8259 CrossRef CAS.
- (a) T. Kimura and Y. Yamauchi, Langmuir, 2012, 28, 12901–12908 CrossRef CAS PubMed; (b) D. Chandra, M. Bekki, M. Nakamura, S. Sonezaki, T. Ohji, K. Kato and T. Kimura, J. Mater. Chem., 2011, 21, 5738–5744 RSC.
- (a) Y. J. Cheng, M. Wolkenhauer, G. G. Bumbu and J. S. Gutmann, Macromol. Rapid Commun., 2012, 33, 218–224 CrossRef CAS PubMed; (b) T. Ghoshal, M. T. Shaw, C. T. Bolger, J. D. Holmes and M. A. Morris, J. Mater. Chem., 2012, 22, 12083–12089 RSC.
- (a) N. V. Salim, T. L. Hanley, L. Waddington, P. G. Hartley and Q. P. Guo, Macromol. Rapid Commun., 2012, 33, 401–406 CrossRef CAS PubMed; (b) Y. Zhang, H. Li, Y. Q. Liu and J. Wang, Chem. Commun., 2012, 48, 8538–8540 RSC.
- (a) B. M. Rosen, G. Lligadas, C. Hahn and V. Percec, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3931–3939 CrossRef CAS; (b) B. M. Rosen, G. Lligadas, C. Hahn and V. Percec, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3940–3948 CrossRef CAS.
- (a) J. W. Chan, C. E. Hoyle and A. B. Lowe, J. Am. Chem. Soc., 2009, 131, 5751–5753 CrossRef CAS PubMed; (b) V. T. Huynh, G. J. Chen, P. D. Souza and M. H. Stenzel, Biomacromolecules, 2011, 12, 1738–1751 CrossRef CAS PubMed; (c) B. Yu, J. W. Chan, C. E. Hoyle and A. B. Lowe, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3544–3557 CrossRef CAS.
- (a) A. Dondoni, Angew. Chem., Int. Ed., 2008, 47, 8995–8997 CrossRef CAS PubMed; (b) C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- T. Sarbu, K. Y. Lin, J. Ell, D. J. Siegwart, J. Spanswick and K. Matyjaszewski, Macromolecules, 2004, 37, 3120–3127 CrossRef CAS.
- P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632–2657 CrossRef CAS.
- (a) R. Kandre, K. Feldman, H. E. H. Meijer, P. Smith and A. D. Schluter, Angew. Chem., Int. Ed., 2007, 46, 4956–4959 CrossRef CAS PubMed; (b) W. G. Huang, L. J. Su and Z. J. Bo, J. Am. Chem. Soc., 2009, 131, 10348–10349 CrossRef CAS PubMed.
- (a) E. C. Hagberg, M. Malkoch, Y. Ling, C. J. Hawker and K. R. Carter, Nano Lett., 2007, 7, 233–237 CrossRef CAS PubMed; (b) V. S. Khire, A. W. Harant, A. W. Watkins, K. S. Anseth and C. N. Bowman, Macromolecules, 2006, 39, 5081–5086 CrossRef CAS.
- (a) A. W. Harant, V. S. Khire, M. S. Thibodaux and C. N. Bowman, Macromolecules, 2006, 39, 1461–1466 CrossRef CAS; (b) V. S. Khire, T. Y. Lee and C. N. Bowman, Macromolecules, 2008, 41, 7440–7447 CrossRef CAS; (c) L. A. Connal, C. R. Kinnane, A. N. Zelikin and F. Caruso, Chem. Mater., 2009, 21, 576–578 CrossRef CAS.
- (a) V. S. Khire, D. S. W. Benott, K. S. Anseth and C. N. Bowman, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 7027–7039 CrossRef CAS; (b) A. E. Rydholma, C. N. Bowmana and K. S. Anseth, Biomaterials, 2005, 26, 4495–4506 CrossRef PubMed; (c) A. Dondoni, Angew. Chem., Int. Ed., 2008, 47, 8995–8997 CrossRef CAS PubMed.
- M. Sangermano, R. Bongiovanni, G. Malucelli, A. Priola, A. Harden and N. Rehnber, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2583–2590 CrossRef CAS.
- (a) H. Y. Wei, A. F. Senyurt, S. Jonsson and C. E. Hoyle, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 822–829 CrossRef CAS; (b) J. Shin, S. Nazarenko and C. E. Hoyle, Macromolecules, 2008, 41, 6741–6746 CrossRef CAS; (c) L. Kwisnek, S. Nazarenko and C. E. Hoyle, Macromolecules, 2009, 42, 7031–7041 CrossRef CAS.
- K. L. Killops, L. M. Campos and C. J. Hawker, J. Am. Chem. Soc., 2008, 130, 5062–5064 CrossRef CAS PubMed.
- C. Rissing and D. Y. Son, Organometallics, 2009, 28, 3167–3172 CrossRef CAS.
- C. Nilsson, N. Simpson, M. Malkoch, M. Johansson and E. Malmstrom, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1339–1348 CrossRef CAS.
- L. M. Campos, K. L. Killops, R. Sakai, J. M. J. Paulusse, D. Damiron, E. Drockenmuller, B. W. Messmore and C. J. Hawker, Macromolecules, 2008, 41, 7063–7070 CrossRef CAS.
- R. L. A. David and J. A. Kornfield, Macromolecules, 2008, 41, 1151–1161 CrossRef CAS.
- A. Gress, A. Volkel and H. Schlaad, Macromolecules, 2007, 40, 7928–7933 CrossRef CAS.
- J. S. Parent and S. S. Sengupta, Macromolecules, 2005, 38, 5538–5544 CrossRef CAS.
- M. Li, P. De, S. R. Gondi and B. S. Sumerlin, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5093–5100 CrossRef CAS.
- Z. P. Tolstyka, J. T. Kopping and H. D. Maynard, Macromolecules, 2008, 41, 599–606 CrossRef CAS.
- (a) S. Peleshanko, J. Jeong, R. Gunawidjaja and V. V. Tsukruk, Macromolecules, 2004, 37, 6511–6522 CrossRef CAS; (b) U. S. Jeng, Y. S. Sun, H. Y. Lee, C. H. Hsu and K. S. Liang, Macromolecules, 2004, 37, 4617–4622 CrossRef CAS.
- B. Lotz and A. J. Kovacs, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 1969, 10, 820 CAS.
- Y. N. Zhang, G. W. Wang and J. L. Huang, Macromolecules, 2010, 4, 10343–10347 CrossRef.
- (a) R. P. Quirk, Q. Ge, M. A. Arnould and C. Wesdemiotis, Macromol. Chem. Phys., 2001, 202, 1761–1767 CrossRef CAS; (b) R. P. Quirk and G. M. Lizarraga, Macromolecules, 1998, 31, 3424–3430 CrossRef CAS; (c) R. P. Quirk, H. Hasgawa, D. L. Gomochak, C. Wesdemiotis and K. Wollyung, Macromolecules, 2004, 37, 7146–7155 CrossRef CAS; (d) R. P. Quirk and W. C. Chen, Macromol. Chem., 1982, 183, 2071–2076 CrossRef CAS; (e) R. P. Quirk, D. L. Gomochak, C. Wesdemiotis and M. A. Arnold, J. Polym. Sci., Part A:Polym. Chem., 2003, 41, 947–957 CAS; (f) R. P. Quirk and J. J. Ma, J. Polym. Sci., Part A: Polym. Chem., 1988, 26, 2031–2037 CrossRef CAS; (g) R. P. Quirk, D. L. Pickel and H. Hasegawa, Macromol. Symp., 2005, 226, 69–77 CrossRef CAS.
- (a) G. W. Wang and J. L. Huang, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 1136–1150 CrossRef CAS; (b) G. W. Wang and J. L. Huang, Macromol. Rapid Commun., 2007, 28, 298–304 CrossRef CAS.
- (a) X. S. Feng, D. Taton, E. L. Chaikof and Y. Gnanou, J. Am. Chem. Soc., 2005, 127, 10956–10966 CrossRef CAS PubMed; (b) X. S. Feng, D. Taton, E. L. Chaikof and Y. Gnanou, Macromolecules, 2009, 42, 7292–7298 CrossRef CAS.
- (a) F. Calderara, Z. Hruska, G. Hurtrez, T. Nugay and G. Riess, Makromol. Chem., 1993, 194, 1411–1420 CrossRef CAS; (b) M. Teodorescu, M. Dimonie, C. Draghici and G. Vasilievici, Polym. Int., 2004, 53, 1987–1993 CrossRef CAS; (c) R. P. Quirk, J. Kim, C. Kausch and M. Chun, Polym. Int., 1996, 39, 3–10 CrossRef CAS.
- (a) J. L. Hedrick, M. Trollsas, C. J. Hawker, B. Atthoff, H. Claesson, A. Heise, R. D. Miller, D. Mecerreyes, R. Jerome and P. Dubois, Macromolecules, 1998, 31, 8691–8705 CrossRef CAS; (b) A. Heise, J. L. Hedrick, M. Trollsas, R. D. Miller and C. W. Frank, Macromolecules, 1999, 32, 231–234 CrossRef CAS.
- S. Angot, D. Taton and Y. Gnanou, Macromolecules, 2000, 33, 5418–5426 CrossRef CAS.
- (a) A. Boschetti-de-Fierro, A. J. Mueller and V. Abetz, Macromolecules, 2007, 40, 1290–1298 CrossRef CAS; (b) N. Lin and A. Dufresne, Macromolecules, 2013, 46, 5570–5583 CrossRef CAS; (c) C. He, J. Sun, T. Zhao, Z. Hong, X. Zhuang, X. Chen and X. Jing, Biomacromolecules, 2006, 7, 252–258 CrossRef CAS PubMed; (d) C. He, J. Sun, J. Ma, X. Chen and X. Jing, Biomacromolecules, 2006, 7, 3482–3489 CrossRef CAS PubMed.
- G. Maglio, G. Nese, M. Nuzzo and R. Palumbo, Macromol. Rapid Commun., 2004, 25, 1139–1144 CrossRef CAS.
- C. Y. Liu, K. Lv, B. Huang, C. L. Hou and G. W. Wang, RSC Adv., 2013, 3, 17945–17953 RSC.
- H. Yu, A. Natansohn, M. A. Singh and I. Torriani, Macromolecules, 2001, 34, 1258–1266 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |