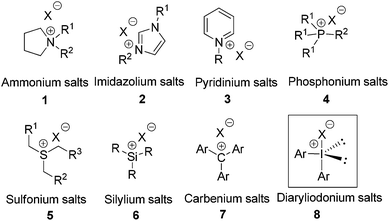
Diaryliodonium salts as efficient Lewis acid catalysts for direct three component Mannich reactions†
Yanxia Zhangab,
Jianwei Han*b and
Zhen-Jiang Liu*a
aSchool of Chemical and Environmental Engineering, Shanghai Institute of Technology, 100 Haiquan Road, Shanghai 201418, P. R. China. E-mail: zjliu@sit.edu.cn; Fax: +86-21-60877231; Tel: +86-21-60877227
bShanghai-Hong Kong Joint Laboratory in Chemical Synthesis, Shanghai Institute of Organic Chemistry, The Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai 200237, P. R. China. E-mail: jianweihan@sioc.ac.cn; Fax: +86-21-54925383; Tel: +86-21-54925551
First published on 27th February 2015
Abstract
Diaryliodonium(III) salts, as highly active and versatile Lewis acid catalysts for the direct three component Mannich reaction under solvent free conditions, have been investigated. The Mannich products were isolated in good to excellent yields in a very clean manner. Additionally, the catalyst could be recycled without significant loss of its catalytic activity.
As a vital part of green chemistry, catalysis has been identified to be one of the most valuable contributors to improve synthetic efficiency while minimizing chemical waste.1 As a result, recent decades have witnessed an explosive growth in this field with the development of various novel catalysts.2 Lewis acids are considered to be the most popular catalytic species in a broad variety of transformations.3 Apart from a number of metal-based Lewis acid catalysts, many organic ionic salts bearing cationic centers as electron pair acceptors have shown highly catalytic activity in the form of ionic liquids and ion-pair catalysts.4 So far, several major classes of cations including ammonium, imidazolium, pyridinium, phosphonium, sulfonium, silylium and carbeniums have been well documented (1–7, Fig. 1).5 However, hypervalent halogen cations have received much less attention to explore their Lewis acid catalytic activity, although oxidations of various alcohols and phenols, α-functionalizations of carbonyl compounds, cyclizations, and rearrangements catalyzed by hypervalent iodines have been well documented.6 In 2012, we reported a cationic bromonium, which was generated from N-halosuccinimide with triphenylphosphine oxide, as an efficient Lewis acid catalyst for Nazarov cyclization of dihydropyran derivatives.7
Diaryliodonium(III) salts (8, Fig. 1) have been extensively used in organic synthesis. As one of the most versatile hypervalent iodine compounds, diaryliodonium(III) salts have been employed as electrophilic arylating agents in a wide range of reactions and the interest in iodine(III)-mediated reactions has recently increased considerably due to their structural properties.8 Iodine(III) compounds are electrophilic at iodine, because of the node in the non-bonding orbital of the hypervalent bond. With this consideration, cationic iodine, which has a low-lying LUMO that can accept an electron pair, is able to form adducts with electronegative atoms in the substrate, such as oxygen, nitrogen, sulfur and halogens. This interaction has partial charge transfer character, thus activating the substrates towards nucleophilic attack for catalysis. Moreover, diaryliodonium salts are air- and moisture-stable compounds, whose chemical properties are similar to transition metal derivatives of Hg, Pb and Pd complexes, but without the toxicity and environmental problems associated with these heavy metals.8 Herein, we would like to describe our results on the evaluation of diaryliodonium(III) salts as catalysts in three component Mannich reactions. To the best of our knowledge, there is no report concerning diaryliodonium salts as Lewis acid catalysts in organic synthesis.
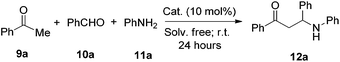
Although a number of Mannich-type reactions have been reported,9 the reaction of acetophenone (9a), benzaldehyde (10a) and aniline (11a) still remains a good model to evaluate newly developed Lewis acid catalysts due to the easy availability of starting materials.10 Initially, we explored diphenyliodonium triflate as a catalyst in this reaction to probe the potential catalytic activity. To our delight, the desired product of 12a was isolated in 83% yield with a 10 mol% catalyst loading, using chloroform as the solvent at ambient temperature. To establish the optimal reaction conditions, various solvents were screened (see ESI†), and it was found that solvent free conditions gave the best yield of 86%. The model reaction could be monitored using phase transition phenomena as shown in Fig. 2; the reaction mixture had totally solidified after 24 hours in the presence of 10 mol% diphenyliodonium triflate as catalyst. As a comparison, the reaction did not occur if no catalyst was employed, as confirmed by thin layer chromatography (TLC). In order to compare the activity with common acid catalysts, several Brønsted acids such as trifluoromethanesulfonic acid (TfOH), hydrochloric acid (conc. HCl) and camphorsulfonic acid (CSA), and the Lewis acid calcium chloride were tried in the Mannich reaction under the standard solvent free conditions. It is noteworthy that one equivalent of each acid was used as a promoter to improve the efficiency. TfOH and HCl also provided excellent yields of 91% and 92% while the rest of the acids gave moderate to good yields of 57–85% (entries 2–5, Table 1).
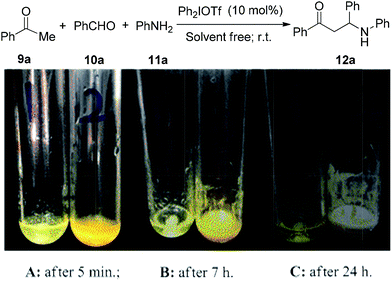 | ||
| Fig. 2 Photographs for comparison of Mannich reactions with/without iodonium catalysis. Tube 1: without catalyst; Tube 2: 10 mol% diphenyliodonium triflate as the catalyst. | ||
| Entry | Cat. | Ar1(Ar2) of 8 | X | % Yieldb |
|---|---|---|---|---|
| a Unless otherwise specified, reaction conditions: 9a (1 mmol), 10a (1 mmol), 11a (1 mmol) in the presence of catalyst (0.1 mmol) at room temperature for 24 hours.b Isolated yield.c Yield in brackets was obtained with recycled catalyst.d The loading of acid was one equivalent.e Conc. HCl was used in excess amounts. | ||||
| 1 | 8a | (Ph)2 | OTf | 86 |
| 2d | TfOH | — | — | 91 |
| 3d | TsOH | — | — | 85 |
| 4e | HCl | — | — | 92 |
| 5d | CaCl2 | — | — | 57 |
| 6 | 8b | (Ph)2 | Br | 55 |
| 7 | 8c | (Ph)2 | BF4 | 65 |
| 8 | 8d | (Ph)2 | OTs | 80 |
| 9 | 8e | (Ph)2 | PF6 | 71 |
| 10 | 8f | (4-FC6H4)2 | OTf | 85 |
| 11 | 8g | (4-ClC6H4)2 | OTf | 81 |
| 12 | 8h | (4-BrC6H4)2 | OTf | 77 |
| 13 | 8i | (4-tBuC6H4)2 | OTf | 77 |
| 14 | 8j | (4-MeOC6H4)2 | OTf | 56 |
| 15 | 8k | (3-NO2C6H4)2 | Br | 54 |
| 16 | 8l | 4-MeOC6H4(4-NO2C6H4) | OTs | 89 |
| 17 | 8m | C6H4C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(Ph) C(Ph) |
OTf | 87 |
| 18 | 8n | C6F5(Mesityl) | OTs | 93 (83)c |
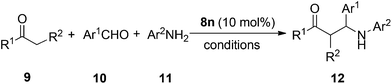
Next, we investigated the influence of the counteranions of the diphenyliodonium salts on the catalytic activity, as shown in Table 1. BF4−, TsO− and PF6− anions gave comparable yields (entries 7–9, Table 1), however, Br− as the anion gave a lower yield of 55% (entry 6, Table 1). Subsequently, we examined the structural diversity of various diaryliodonium salts as Lewis acid catalysts. Functional groups including halogen, tert-butyl, and methoxyl groups on the para-position of the aromatic ring were evaluated in the model reaction. Generally, catalysts 8f–i afforded the products in good yields (entries 10–13, Table 1). The electron-donating methoxyl group of catalyst 8j gave a lower yield of 56% (entry 14, Table 1). Iodonium bromide 8k, [(3-NO2C6H4)2I]Br, as a catalyst was also applied in the reaction, but only 54% yield of 12a was achieved. Unsymmetrical iodonium salts were also employed as the catalysts; it was pleasing to find that 8l–n can catalyze the reaction more efficiently than 8a. Alkynyl-mesityliodonium triflate 8m also afforded the desired product in a good yield of 87%. To our surprise, the yield could be improved to 93% with the use of 8n, which has a pentafluorobenzene ring in the catalyst structure. Furthermore, in general, the iodonium salts have excellent solubility in water. The catalyst of 8n was recycled by water extraction and evaporation. A yield of 83% was achieved by using the recycled iodonium salts under the standard conditions; this result can meet the requirements of green chemistry as it is without obvious loss of activity.
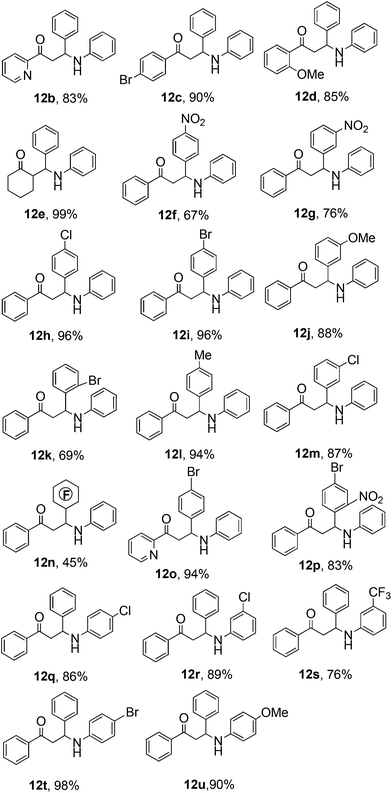
With 8n as the optimized catalyst in hand, we explored the scope of the three component Mannich reaction with a range of various aldehydes, ketones and amines as substrates. In all cases, the three component Mannich reaction proceeded smoothly in the presence of 10 mol% of iodonium salts to give the corresponding products in good to excellent yields at room temperature (Table 2). It is noted that acetophenones, benzaldehydes and anilines carrying either electron-donating or electron-withdrawing substituents reacted well. Substrates with various substituents on the ortho-, meta-, and para-positions of the aromatic ring were well tolerated in this catalytic protocol. However, steric factors affected the reactivity, for example, the products of 12k were obtained in a moderate yield of 69% (Table 2). Interestingly, 4-bromo-2-nitrobenzaldehyde gave 12p in a good yield of 83%. Pentafluorobenzaldehyde was employed as the aldehyde component to checking the fluorous interactions with the fluoro-containing catalyst 8n, however, only 45% yield of 12n was achieved with the standard procedure. For the aldehyde and aniline derivatives, substrates with the electron-donating methoxy group afforded better results. For example, 4-methoxyaniline as a reactant gave product 12u in an excellent yield of 90%. As expected, cyclohexanone as a more reactive partner gave the desired product 12e in a quantitative yield. The results of this study on the various substrates are summarized in Table 2. Overall, it can be seen that the scope and generality of the present method has been shown with respect to various aldehydes and anilines.
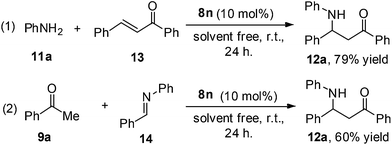
The mechanism of the classic Mannich reaction has been described as an enolized carbonyl species, realized from the active hydrogen compounds after catalysis of an acid, attacking an iminium ion, formed from an amine and an aldehyde, to yield a Mannich base.11 However, a subject of considerable discussion is the possibility of the formation of chalcones from an enolized carbonyl species with benzaldehydes. These chalcones could then react with aniline to afford the desired Mannich bases. In connection with this discussion, two control experiments were carried out with this catalytic protocol. The reaction of aniline with chalcone gives the Mannich base 12a in 79% yield [Scheme 1, eqn (1)]. Another reaction between acetophenone and imine 14 was attempted; the product 12a was obtained in a slightly lower yield of 60% [Scheme 1, eqn (2)]. Of note, the formation of chalcone was observed during this reaction [Scheme 1, eqn (2)]. Therefore, thus far, no single mechanism which can account for all the experimental facts has been suggested.
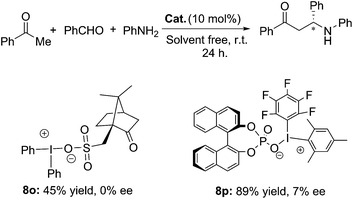
The asymmetric version of the catalytic Mannich reaction is a useful method for the preparation of enantioenriched α-aminocarbonyl molecules which possess favourable pharmacological properties.12 Diaryliodonium salts with a chiral anion have been reported for asymmetric inductions.13 Hence, the asymmetric variations of the Mannich reaction were investigated as depicted in Scheme 2. D-Camphorsulfonate and (R)-BINOL-phosphate as chiral anions were employed for the iodonium catalysts in the title reaction. Unfortunately, 8o afforded the desired product 12a in 45% yield without enantioselectivity. An enantiomeric excess value of 7% was obtained with 8p as the catalyst, although the yield of 12a in 89% is satisfactory (Scheme 2).
In summary, we have firstly demonstrated that diaryliodonium(III) salts are highly active and versatile Lewis acid catalysts for the direct three component Mannich reaction. The products are isolated in good to quantitative yields in a very clean manner. The high yields and recyclable catalyst for this reaction show potential for using hypervalent iodine species as Lewis acid catalysts in the development of novel sustainable processes, both in industry and academia. Furthermore, the catalytic activity could be tuned by the changing aromatic groups around the iodocation. It is anticipated that chiral versions of the catalysis will be discovered by using chiral hypervalent iodine(III) compounds or by using the strategy of asymmetric counteranion-directed catalysis as described above. Therefore, we are continuing to explore the scope and limitation of iodocation catalysis by tuning the Lewis acidity to gain more understanding of reactivity and to further extend the reaction scope. These results will be reported in due course.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (NSFC, nos 21472213, 21202186, 21102093), The Chinese Academy of Sciences–Croucher Funding Scheme for Joint Laboratories and the Innovation Program of Shanghai Municipal Education Commission (no. 14YZ144).Notes and references
- J. Han, Org. Chem. Curr. Res., 2012, 1, e114 CrossRef PubMed.
- Lewis Acids in Organic Synthesis, ed. H. Yamamoto, Wiley-VCH, Weinheim, 2000 Search PubMed.
- A. Corma and H. García, Chem. Rev., 2003, 103, 4307 CrossRef CAS PubMed; S. Kobayashi and K. Manabe, Acc. Chem. Res., 2002, 35, 209 CrossRef PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; K. Brak and E. N. Jacobsen, Angew. Chem., Int. Ed., 2013, 52, 534 CrossRef PubMed.
- V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615 CrossRef CAS PubMed; R. Sheldon, Chem. Commun., 2001, 2399 RSC; Q. Zhang, S. Zhang and Y. Deng, Green Chem., 2011, 13, 2619 RSC; Z.-L. Tang, Chin. J. Org. Chem., 2006, 26, 1059 Search PubMed; S. Shirakawa, P. J. Lombardi and J. L. Leighton, J. Am. Chem. Soc., 2005, 127, 9974 CrossRef PubMed; J. Bah and J. Franzén, Chem.–Eur. J., 2014, 20, 1066 CrossRef PubMed.
- For a recent review, see: F. V. Singh and T. Wirth, Chem.–Asian J., 2014, 9, 950 CrossRef CAS PubMed and the references therein.
- F. Guo, L. Wang, S. Mao, C. Zhang, J. Yu and J. Han, Tetrahedron, 2012, 68, 8367 CrossRef CAS PubMed.
- L. F. Silva and B. Olofsson, Nat. Prod. Rep., 2011, 28, 1722 RSC; M. S. Yusubov and V. V. Zhdankin, Curr. Org. Synth., 2012, 9, 247 CrossRef CAS.
- M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef.
- K. Kundu and S. K. Nayak, RSC Adv., 2012, 2, 480 RSC; L. Wang, J. Han, J. Sheng, H. Tian and Z. Fan, Catal. Commun., 2005, 6, 201 CrossRef CAS PubMed; L.-M. Wang, J.-W. Han, J. Sheng, Z.-Y. Fan and H. Tian, Chin. J. Org. Chem., 2005, 25, 591 Search PubMed.
- S. V. Lieberman and E. C. Wagner, J. Org. Chem., 1949, 14, 1001 CrossRef CAS; E. R. Alexander and E. J. Underhill, J. Am. Chem. Soc., 1949, 71, 4014 CrossRef.
- H. Ishitani, M. Ueno and S. Kobayashi, J. Am. Chem. Soc., 2000, 122, 8180 CrossRef CAS; Q.-X. Guo, H. Liu, C. Guo, S.-W. Luo, Y. Gu and L.-Z. Gong, J. Am. Chem. Soc., 2006, 128, 15892 CrossRef PubMed.
- P.-O. Norrby, T. B. Petersen, M. Bielawski and B. Olofsson, Chem.–Eur. J., 2010, 16, 8251 CrossRef CAS PubMed; N. Jalalian and B. Olofsson, Tetrahedron, 2010, 66, 5793 CrossRef PubMed; M. Ochiai, Y. Kitagawa, N. Takayama, Y. Takaoka and M. Shiro, J. Am. Chem. Soc., 1999, 121, 9233 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00209e |
| This journal is © The Royal Society of Chemistry 2015 |