A phthalimide-based fluorescent probe for thiol detection with a large Stokes shift†
Xingjiang Liu‡
a,
Li Gao‡a,
Liu Yanga,
Lifen Zoua,
Wenqiang Chena and
Xiangzhi Song*abc
aCollege of Chemistry & Chemical Engineering, Central South University, 410083 Changsha, Hunan Province, P. R. China. E-mail: xzsong@csu.edu.cn; Fax: +86-731-88836954; Tel: +86-731-88836954
bState Key Laboratory for Powder Metallurgy, Central South University, Changsha, Hunan Province, P. R. China 410083
cState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, Liaoning Province, P. R. China 116024
First published on 3rd February 2015
Abstract
A phthalimide-based dye, probe 1, was developed as a novel fluorescent probe for thiol detection with excellent selectivity and high sensitivity based on the combination of photo induced electron transfer (PET) and excited state intramolecular proton transfer (ESIPT) mechanisms. The probe can detect thiols quantitatively and selectively with a large Stokes shift (161 nm) and the detection limit (S/N = 3) is as low as 0.8 nM. Furthermore, this probe was successfully used for imaging thiols in living SH-SY5Y cells.
Introduction
Thio-containing amino acids play important physiological roles in a number of biological systems.1 For example, cysteine (Cys) is an essential amino acid involved in protein folding by virtue of its ability to form inter- and intra-chain disulfide bonds with other cysteine residues. Cys deficiency can lead to many health problems such as skin lesions, hair depigmentation, liver damage, slow growth in children, and liver damage.2 High levels of homocysteine (Hcy) are harmful and cause a great risk of heart disease, stroke and complications of diabetes.3 Similarly, glutathione (GSH) is an important endogenous antioxidant and detoxifying agent which can protect the cells from damage by reactive oxygen species, electrophiles and xenobiotics.4 Therefore, it is of great importance to develop methods for the rapid, sensitive and selective detection of thiols. Due to its high sensitivity, good selectivity, easy-to-operation and non-invasiveness, fluorescent probes are the powerful tools for imaging thiols in living cells.5 In the past several years, many fluorescent probes have been developed for the thiol detection based on various sensing mechanisms including photo induced electron transfer (PET),6 intramolecular charge transfer (ICT),7 excited state intramolecular proton transfer (ESIPT),8 Forster resonance energy transfer (FRET),9 and so on. Although fluorescent probes employing one sensing mechanism can qualitatively and quantitatively detect thiols, the combination of two or more mechanisms in one fluorescent probe can bring more advantages such as higher sensitivity and better selectivity.10 For example, Jiang et al. reported a probe to distinguish Cd2+ from Zn2+ based on the combination of PET and ICT mechanisms, which was very difficult to achieve via a single sensing mechanism.11 In our earlier work, we developed a red-emitting fluorescent probe based on PET and ESIPT mechanisms which has a good sensitivity and selectivity for thiol detection.123-Hydroxyphthalimide is a small fluorophore possessing many favorable optical properties such as emission in green region, good photo stability, relatively high fluorescent quantum yield and easy synthesis. Importantly, upon excited, 3-hydroxyphthalimide displays an excited state intramolecular proton transfer (ESIPT) process in which the acidic hydroxyl proton transfers to the imide carbonyl oxygen, resulting in a large Stokes shift. These above traits suggest that 3-hydroxyphthalimide might be a good scaffold for the design of fluorescent probes.13 So far, there is no fluorescent probe reported on the basis of phthalimide derivatives.
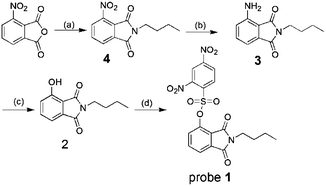
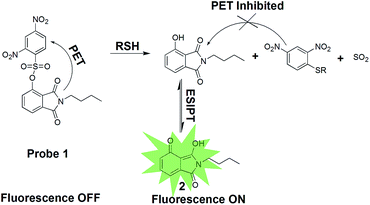
In this work, 2,4-dinitrobenzenesulfonate of 3-hydroxyphthalimide, probe 1, was developed as a fluorescent probe for the detection of biological thiols. The synthetic route of probe 1 is shown in Scheme 1. Probe 1 is non-fluorescent due to an effective PET process from phthalimide moiety to 2,4-dinitrobenzenesulfonate moiety.14 We expected that probe 1 could be converted into N-butyl-3-hydroxyphthalimide, reference dye 2, through selective cleavage by thiols, which emits strong green fluorescence upon excited via an ESIPT process, as shown in Scheme 2.
Results and discussion
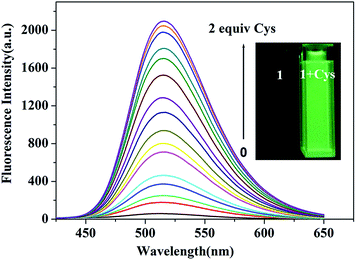
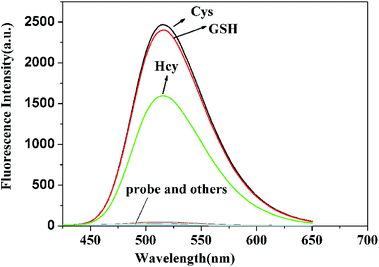
The spectral measurements of probe 1 were conducted in HEPES buffer solution (10.0 mM, pH = 7.4) containing 20% CH3CN. Reference dye 2 is strongly fluorescent (Φfl = 0.27) with a maximum at 516 nm and a large Stokes shift (161 nm) which is desirable for fluorescence microscopy studies wherein clearly separate excitation and emission bands can minimize the background noise (shown in Fig. S1†). In contrast, probe 1 exhibits negligible fluorescence (Φfl < 0.01) because of the PET process and prohibitions of ESIPT process produced by the 2,4-dinitrobenzenesulfonate moiety, as seen in Scheme 2. In order to investigate the ability to detect thiols, titration experiment with various concentrations of Cys was performed on the solution of probe 1 (10.0 μM) (shown in Fig. 1). The addition of Cys to the solution of probe 1 resulted in a strong green fluorescence (λemmax = 516 nm), indicating that Cys could effectively cleave dinitrobenzenesulfonate moiety. The fluorescence intensity grows with increasing the concentration of Cys and the enhancement can be up to 75 folds. A good linear relationship between the fluorescence intensity at 516 nm and the concentration of Cys (0.0–9.0 μM) was obtained with R = 0.99379. The calculated detection limit was 0.8 nM, which was sufficiently low for the detection of Cys in biological samples, as shown in Fig. 2. Similar results were obtained when we repeated the above fluorescence experiments using Hcy and GSH in replace of Cys, as shown in Fig. S2–S7.† The detection limits for Hcy and GSH are 1.1 nM and 0.6 nM, respectively. These results suggest that probe 1 can quantitatively detect thiols.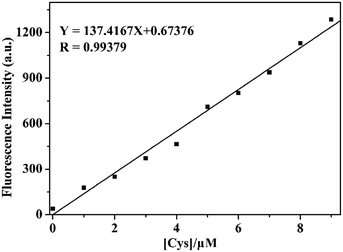 | ||
| Fig. 2 The linear relationship between the emission intensity of probe 1 (10.0 μM) at 516 nm and the concentration of Cys (0.0–9.0 μM) in HEPES buffer (10.0 mM, pH = 7.4) containing 20% CH3CN. | ||
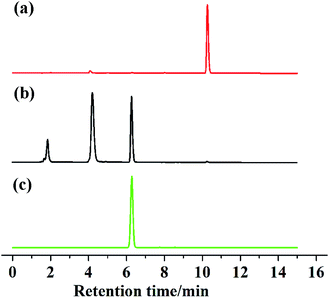
To further confirm the sensing mechanism, 1H NMR, mass spectral analysis and High Performance Liquid Chromatography (HPLC) analysis were performed on probe 1 with Cys. As shown in Fig. 3, the reaction product of probe 1 with Cys and reference dye 2 had the very similar 1H NMR spectra. Compared with the 1H NMR spectrum of probe 1, the protons at 8.71, 8.64 and 8.59 ppm, assigned to 2,4-dintrobenzenesulfonyl group in probe 1, disappeared in the 1H NMR spectrum of the isolated reaction product. The mass spectra of probe 1 displayed a peak at m/z = 450.0597. However, the reaction product of probe 1 with Cys displayed a new peak at m/z = 219.0890 which was identical to the molar weight of reference dye 2 (Fig. S16 and S17†). HPLC is an effective method widely used for the separation and analysis of mixtures of organic compounds. Thus, we conducted HPLC analysis on reference dye 2, probe 1 and the reaction product of probe 1 with Cys (shown in Fig. 4). Probe 1 displayed a single peak with a retention time at 10.266 min while reference dye 2 produced a single peak with a retention time at 6.278 min. Upon the addition of Cys to the solution of probe 1, the peak at 10.266 min disappeared with the appearance of a new peak at 6.281 min. These results proved that thiols could selectively cleave the 2,4-dinitrobenzenesulfonate moiety in probe 1 and generate reference dye 2.
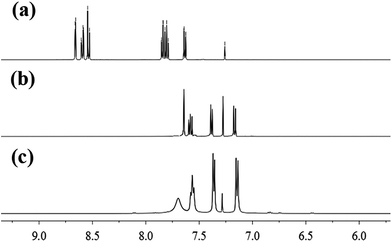 | ||
| Fig. 3 Partial 1H NMR spectra of probe 1 (a), the isolated product of probe 1 with Cys (b) and reference dye 2 (c) in CDCl3. | ||
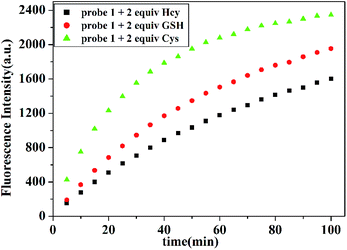
In addition, time-dependent fluorescence response of probe 1 with Cys, Hcy and GSH were also investigated respectively. As depicted in Fig. 5, the reaction of probe 1 (10.0 μM) with thiols is almost finished within 100 min. Within 5 min, fluorescence signals can be detected when the concentration of Cys is equal or above 20.0 μM. The reaction rates of probe 1 with GSH and Hcy are much slower than that for Cys, which may be attributed to the steric hindrance of Hcy and GSH.
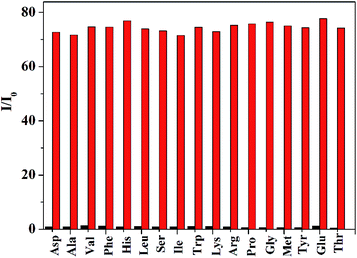
To evaluate the selectivity performance of probe 1 for the detection of thiols, we investigated the fluorescence behavior of probe 1 toward other physiologically relevant amino acids including Asp, Ala, Val, Phe, His, Leu, Ser, Ile, Trp, Lys, Arg, Pro, Gly, Met, Tyr, Glu and Thr at identical conditions. As shown in Fig. 6, only Hcy, GSH and Cys could produce a prominent fluorescence enhancement. In contrast, other amino acid resulted in negligible fluorescence signal. Moreover, competitive assays indicated that the co-existence of typical thiol-free amino acids has no impact on the detection of thiols for probe 1 (shown in Fig. 7), suggesting that this probe exhibited a good selectivity toward thiols.
We further investigated the pH influence on fluorescence behavior of probe 1 for the detection of Hcy, GSH and Cys. It can be seen in Fig. 8 that probe 1 was essentially non-fluorescent within a wide pH value from 2.0 to 11.0, indicating the good stability of probe 1. Enormous fluorescence enhancement was observed upon addition of Hcy, GSH and Cys within a pH range from 5.0 to 10.0, which implied that probe 1 had the ability to detect thiols in physiological conditions.
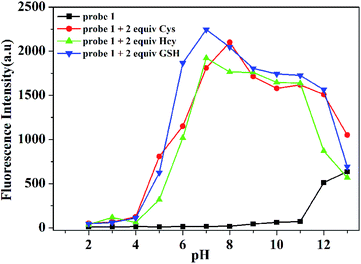 | ||
| Fig. 8 Fluorescence intensity at 516 nm of probe 1 (10.0 μM) (black line) and probe 1 (10.0 μM) with Cys (red line), Hcy (green line) and GSH (blue line) at pH values from 2.0 to 13.0. | ||
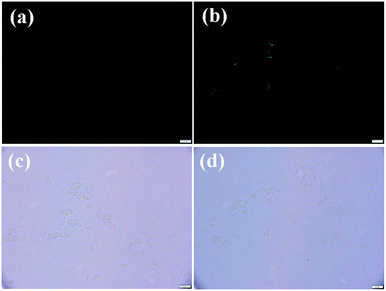
In order to explore the practicability of probe 1, intracellular thiol imaging experiment was performed on this probe in living SH-SY5Y cells, as shown in Fig. 9. When the cells were incubated with probe 1 (10.0 μM) at 37 °C for 30 min, strong green fluorescence signal inside the cells was observed. In contrast, the cells pretreated with a thiol-blocking reagent (N-ethylmaleimide, 1.0 mM) and further incubated with probe 1 produced no fluorescence signal. These results suggested that probe 1 could permeate cell membrane and react with thiols to give easily distinguishable fluorescent signal.
Experimental section
Instruments
Mass spectra were obtained on MICROTOF-Q II mass spectrometer (Bruker Daltonics, Germany). 1H and 13C NMR spectra were recorded on a Bruker 500 NMR spectrometer. Chemical shifts were reported in parts per million using tetramethylsilane (TMS) as the internal standard.All spectral characterizations were carried out in HPLC-grade solvents at 20 °C within a 10 mm quartz cell. UV-vis absorption spectroscopy was measured with a UV-2450 spectrophotometer, and fluorescence spectroscopy was determined on a Hitachi F-7000 spectrometer. The fluorescence quantum yields were measured at 20 °C with coumarin 151 as the reference (Φfl = 0.53 in ethanol).15 Fluorescence imaging was performed with an Olympus IX83 inverted microscope. The pH measurement was carried out on a Leici PHS-3C meter. TLC silica gel plates and silica gel (mesh 200–300) for column chromatography were purchased from Qingdao Ocean Chemicals, China.
Materials and reagents
All chemicals and solvents were obtained from commercial suppliers and used as received. The probe 1 was prepared according to the synthetic route shown in Scheme 1.Synthesis of compound 4
To a solution of 3-nitrophthalic anhydride (1.9361 g, 10 mmol) in acetic acid (20 mL) was slowly added butylamine (1.0951 g, 15 mmol) within 5 min. After stirring at room temperature for 10 min, the resulting mixture was refluxed at 120 °C for 2.5 h. After cooling to room temperature, the reaction mixture was poured into cold water (50 mL). The precipitated solid was collected by filtration and washed with water (3 mL × 3) to give compound 4 as a white solid (2.1252 g, 85.7% yield). 1H NMR (500 MHz, DMSO-d6, TMS) δH 8.27 (d, J = 8.1 Hz, 1H), 8.16 (d, J = 7.5 Hz, 1H), 8.05 (t, J = 7.8 Hz, 1H), 3.57 (t, 2H), 1.63–1.51 (m, 2H), 1.38–1.24 (m, 2H), 0.90 (t, 3H). 13C NMR (125 MHz, DMSO-d6) δC 166.5, 163.9, 144.6, 136.5, 134.1, 128.6, 127.1, 123.5, 38.1, 30.2, 19.9, 13.9.Synthesis of compound 3
Compound 4 (0.4964 g, 2 mmol) was hydrogenated in methanol (15 mL) under reflux at 65 °C for 12 h with 10% Pd/C (0.0496 g, 5 mol%) as a catalyst. Then, the reaction mixture was filtered through celite to remove the catalyst. Next, the filtrate was concentrated in vacuum to give a residue. Pure compound 3 (0.6240 g, 71.5% yield) was obtained by silica gel column chromatography using petroleum ether/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. 1H NMR (500 MHz, CDCl3, TMS) δH 7.42 (dd, J = 8.3, 7.1 Hz, 1H), 7.16 (d, J = 7.6 Hz, 1H), 6.86 (d, J = 8.8 Hz, 1H), 5.13 (s, 1H), 3.65 (t, 2H), 1.63–1.70 (m, 2H), 1.35–1.42 (m, 2H), 0.95 (t, 3H). 13C NMR (125 MHz, CDCl3) δC 170.4, 168.8, 145.2, 135.0, 132.9, 121.0, 112.6, 111.4, 37.4, 30.8, 20.1, 13.7.
1) as eluent. 1H NMR (500 MHz, CDCl3, TMS) δH 7.42 (dd, J = 8.3, 7.1 Hz, 1H), 7.16 (d, J = 7.6 Hz, 1H), 6.86 (d, J = 8.8 Hz, 1H), 5.13 (s, 1H), 3.65 (t, 2H), 1.63–1.70 (m, 2H), 1.35–1.42 (m, 2H), 0.95 (t, 3H). 13C NMR (125 MHz, CDCl3) δC 170.4, 168.8, 145.2, 135.0, 132.9, 121.0, 112.6, 111.4, 37.4, 30.8, 20.1, 13.7.
Synthesis of compound 2
To a solution of compound 3 (0.2180 g, 1 mmol) in 50% sulfuric acid (10 mL) at 0 °C was added the solution of NaNO2 (0.0692 g, 1 mmol) in 2 mL water dropwisely. After stirring for 30 min at 0 °C, the mixture was heated to 90 °C and stirred for 1 h. The reaction mixture was diluted with 15 mL water, then extracted with ethyl acetate (20 mL × 3). The organic layer was combined, washed with brine and dried over anhydrous Na2SO4. After removal of the solvent, the solid was purified by silica gel flash chromatography using petroleum ether/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to afford compound 2 (0.1862 g, 85.4% yield). 1H NMR (500 MHz, CDCl3, TMS) δH 7.70 (s, 1H), 7.56 (t, J = 7.6 Hz, 1H), 7.36 (d, J = 7.2 Hz, 1H), 7.15 (d, J = 8.4 Hz, 1H), 3.65 (t, 2H), 1.68–1.62 (m, 2H), 1.40–1.32 (m, 2H), 0.95 (t, 3H). 13C NMR (125 MHz, CDCl3) δC 170.5, 167.9, 154.6, 136.1, 132.1, 122.5, 115.8, 114.6, 37.5, 30.6, 19.9, 13.4.
1) as eluent to afford compound 2 (0.1862 g, 85.4% yield). 1H NMR (500 MHz, CDCl3, TMS) δH 7.70 (s, 1H), 7.56 (t, J = 7.6 Hz, 1H), 7.36 (d, J = 7.2 Hz, 1H), 7.15 (d, J = 8.4 Hz, 1H), 3.65 (t, 2H), 1.68–1.62 (m, 2H), 1.40–1.32 (m, 2H), 0.95 (t, 3H). 13C NMR (125 MHz, CDCl3) δC 170.5, 167.9, 154.6, 136.1, 132.1, 122.5, 115.8, 114.6, 37.5, 30.6, 19.9, 13.4.
Synthesis of probe 1
To a solution of compound 2 (0.1096 g, 0.50 mmol) and Et3N (0.1066 g, 1.5 mmol) in dichloromethane (10 mL) was added 2,4-dinitrobenzenesulfonylchloride (0.1650 g, 0.62 mmol) in dichloromethane (3 mL) dropwisely at 0 °C. While keeping the temperature at 0 °C, the resulting mixture was stirred for 2 h. The reaction mixture was washed with water and the organic layer was dried over anhydrous Na2SO4. After removal of the solvent under reduced pressure, the resulting residue was further purified by silica gel flash chromatography using ether/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to give probe 1 (0.1605 g, 73.6% yield). HRMS (EI) m/z: calcd for C18H15N3O9S [M + H], 450.0529; found, 450.0597. 1H NMR (500 MHz, CDCl3, TMS) δH 8.71 (d, J = 2.1 Hz, 1H), 8.64 (dd, J = 8.7, 2.2 Hz, 1H), 8.58 (d, J = 8.7 Hz, 1H), 7.92–7.80 (m, 2H), 7.67 (d, J = 8.0 Hz, 1H), 3.56 (d, 2H), 1.65–1.46 (m, 2H), 1.23–1.29 (m, 2H), 0.91 (t, 3H). 13C NMR (125 MHz, CDCl3) δC 166.6, 164.8, 150.9, 148.8, 143.3, 136.2, 134.4, 133.9, 129.5, 126.8, 123.3, 122.9, 120.3, 38.0, 30.4, 20.0, 13.6.
1) as eluent to give probe 1 (0.1605 g, 73.6% yield). HRMS (EI) m/z: calcd for C18H15N3O9S [M + H], 450.0529; found, 450.0597. 1H NMR (500 MHz, CDCl3, TMS) δH 8.71 (d, J = 2.1 Hz, 1H), 8.64 (dd, J = 8.7, 2.2 Hz, 1H), 8.58 (d, J = 8.7 Hz, 1H), 7.92–7.80 (m, 2H), 7.67 (d, J = 8.0 Hz, 1H), 3.56 (d, 2H), 1.65–1.46 (m, 2H), 1.23–1.29 (m, 2H), 0.91 (t, 3H). 13C NMR (125 MHz, CDCl3) δC 166.6, 164.8, 150.9, 148.8, 143.3, 136.2, 134.4, 133.9, 129.5, 126.8, 123.3, 122.9, 120.3, 38.0, 30.4, 20.0, 13.6.
Synthesis of the reaction product of probe 1 with Cys
To a solution of probe 1 (0.0502 g, 0.1 mmol) in acetonitrile (5 mL) was added Cys (0.0242 g, 0.2 mmol) in distilled water (5 mL) at room temperature. After stirring for 4 h. the reaction mixture was extracted with dichloromethane (20 mL × 3). The organic layer was combined and dried over anhydrous Na2SO4. Remove the solvent by rotary evaporation, the obtained residue was purified by silica gel column chromatography using petroleum ether/dichloromethane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent to give the target product (0.0142 g, 64.8% yield). 1H NMR (500 MHz, CDCl3, TMS) δH 7.66 (s, 1H), 7.62–7.57 (m, 1H), 7.39 (d, J = 7.2 Hz, 1H), 7.17 (d, J = 8.4 Hz, 1H), 3.67 (t, 2H), 1.64–1.70 (m, 2H), 1.35–1.43 (m, 1H), 0.97 (t, 3H).
2) as eluent to give the target product (0.0142 g, 64.8% yield). 1H NMR (500 MHz, CDCl3, TMS) δH 7.66 (s, 1H), 7.62–7.57 (m, 1H), 7.39 (d, J = 7.2 Hz, 1H), 7.17 (d, J = 8.4 Hz, 1H), 3.67 (t, 2H), 1.64–1.70 (m, 2H), 1.35–1.43 (m, 1H), 0.97 (t, 3H).
Imaging of SH-SY5Y cells
SH-SY5Y cells were seeded in a 6-well plate in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin. The cells were incubated under an atmosphere of 5% CO2 and 95% air at 37 °C for 24 h. After washing with PBS buffer three times, the cells were incubated with probe 1 (10.0 μM) at 37 °C for 30 min in PBS buffer. Cell images were obtained after washing with PBS buffer three times. For a control experiment, SH-SY5Y cells were pretreated with 1.0 mM of N-ethylmaleimide (NEM) for 30 min at 37 °C and then used.Conclusions
In conclusion, 2,4-dinitrobenzenesulfonate of N-butyl-3-hydroxyphthalimide, probe 1, was developed as a turn-on fluorescent probe toward thiols with excellent selectivity and high sensitivity. Upon the treatment with thiols, this probe produced a strong green fluorescence with a 75-fold enhancement and 161 nm Stokes shift. The sensing strategy was based on the combination of PET and ESIPT mechanisms. Preliminary biological experiments have indicated this probe has the potential to detect and image thiols in biological systems.Acknowledgements
The research was supported by the State Key Laboratory of Fine Chemicals (KF1202) and Hunan Nonferrous Metals Holding Group Co. Ltd.Notes and references
- (a) Z. A. Wood, E. Schroder, J. R. Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32–40 CrossRef CAS; (b) Y. Suzuki, K. Suda, Y. Matsuyama, S. Era and A. Soejima, Clin. Nephrol., 2014, 82, 320–325 CrossRef CAS PubMed; (c) S. Y. Zhang, C. N. Ong and H. M. Shen, Cancer Lett., 2004, 211, 175–188 CrossRef CAS PubMed.
- (a) E. Weerapana, C. Wang, G. M. Simon, F. Richter, S. Khare, M. B. D. Dillon, D. A. Bachovchin, K. Mowen, D. Baker and B. F. Cravatt, Nature, 2010, 468, 790–795 CrossRef CAS PubMed; (b) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- (a) S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino, P. W. F. Wilson and P. A. Wolf, N. Engl. J. Med., 2002, 346, 476–483 CrossRef CAS PubMed; (b) M. Ebbing, K. H. Bonaa, E. Arnesen, P. M. Ueland, J. E. Nordrehaug, K. Rasmussen, I. Njolstad, D. W. Nilsen, H. Refsum, A. Tverdal, S. E. Vollset, H. Schirmer, O. Bleie, T. Steigen, O. Midttun, A. Fredriksen, E. R. Pedersen and O. Nygard, J. Intern. Med., 2010, 268, 367–382 CrossRef CAS PubMed.
- (a) M. H. Lee, J. H. Han, P. S. Kwon, S. Bhuniya, J. Y. Kim, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2012, 134, 1316–1322 CrossRef CAS PubMed; (b) S. Sabelle, P. Y. Renard, K. Pecorella, S. de Suzzoni-Dezard, C. Creminon, J. Grassi and C. Mioskowski, J. Am. Chem. Soc., 2002, 124, 4874–4880 CrossRef CAS PubMed; (c) A. Pastore, G. Federici, E. Bertini and F. Piemonte, FEBS Lett., 2003, 333, 19–39 CAS; (d) A. Pastore, G. Tozzi, L. M. Gaeta, A. Giannotti, E. Bertini, G. Federici, M. C. Digilio and F. Piemonte, J. Pediatr., 2003, 142, 583–585 CrossRef CAS PubMed.
- (a) G. J. Kim, K. Lee, H. Kwon and H. J. Kim, Org. Lett., 2011, 13, 2799–2801 CrossRef CAS PubMed; (b) X. Zhang, X. Ren, Q. H. Xu, K. P. Loh and Z. K. Chen, Org. Lett., 2009, 11, 1257–1260 CrossRef CAS PubMed; (c) H. Li, J. Fan, J. Wang, M. Tian, J. Du, S. Sun, P. Sun and X. Peng, Chem. Commun., 2009, 5904–5906 RSC; (d) P. Wang, J. Liu, X. Lv, Y. Liu, Y. Zhao and W. Guo, Org. Lett., 2012, 14, 520–523 CrossRef CAS PubMed; (e) L. Yi, H. Li, L. Sun, L. Liu, C. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2009, 48, 4034–4037 CrossRef CAS PubMed; (f) X. Yang, Y. Guo and R. M. Strongin, Angew. Chem., Int. Ed., 2011, 50, 10690–10693 CrossRef CAS PubMed; (g) L. Y. Niu, Y. S. Guan, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928–18931 CrossRef CAS PubMed; (h) L. Long, W. Lin, B. Chen, W. Gao and L. Yuan, Chem. Commun., 2011, 47, 893–895 RSC; (i) L. Zhu, Z. Yuan, J. T. Simmons and K. Sreenath, RSC Adv., 2014, 4, 20398–20440 RSC; (j) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC; (k) H. Li, J. Fan and X. Peng, Chem. Soc. Rev., 2013, 42, 7943–7962 RSC.
- (a) Y. Chen, J. Zhao, H. Guo and L. Xie, J. Org. Chem., 2012, 77, 2192–2206 CrossRef CAS PubMed; (b) H. Guo, Y. Jing, X. Yuan, S. Ji, J. Zhao, X. Li and Y. Kan, Org. Biomol. Chem., 2011, 9, 3844–3853 RSC; (c) T. Matsumoto, Y. Urano, T. Shoda, H. Kojima and T. Nagano, Org. Lett., 2007, 9, 3375–3377 CrossRef CAS PubMed.
- (a) S. Ji, J. Yang, Q. Yang, S. Liu, M. Chen and J. Zhao, J. Org. Chem., 2009, 74, 4855–4865 CrossRef CAS PubMed; (b) D. Kand, A. M. Kalle and P. Talukdar, Org. Biomol. Chem., 2013, 11, 1691–1701 RSC.
- (a) S. Goswami, A. Manna, S. Paul, A. K. Das, P. K. Nandi, A. K. Maity and P. Saha, Tetrahedron Lett., 2014, 55, 490–494 CrossRef CAS PubMed; (b) M. Lan, J. Wu, W. Liu, H. Zhang, W. Zhang, X. Zhuang and P. Wang, Sens. Actuators, B, 2011, 156, 332–337 CrossRef CAS PubMed.
- (a) H. Y. Shiu, H. C. Chong, Y. C. Leung, M. K. Wong and C. M. Che, Chem.–Eur. J., 2010, 16, 3308–3313 CrossRef CAS PubMed; (b) L. Long, W. Lin, B. Chen, W. Gao and L. Yuan, Chem. Commun., 2011, 47, 893–895 RSC; (c) K. Sreenath, Z. Yuan, J. R. Allen, M. W. Davidson and L. Zhu, Chem.–Eur. J., 2015, 21, 867–874 CrossRef PubMed.
- (a) O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029–8036 CrossRef CAS PubMed; (b) O. A. Bozdemir, F. Sozmen, O. Buyukcakir, R. Guliyev, Y. Cakmak and E. U. Akkaya, Org. Lett., 2010, 12, 1400–1403 CrossRef CAS PubMed.
- L. Xue, C. Liu and H. Jiang, Org. Lett., 2009, 11, 1655–1658 CrossRef CAS PubMed.
- S. Chen, P. Hou, B. Zhou, X. Song, J. Wu, H. Zhang and J. W. Foley, RSC Adv., 2013, 3, 11543–11546 RSC.
- H. Okamoto, H. Konishi and K. Satake, Chem. Commun., 2012, 48, 2346–2348 RSC.
- (a) D. Maity and T. Govindaraju, Org. Biomol. Chem., 2013, 11, 2098–2104 RSC; (b) L. Peng, Z. Zhou, R. Wei, K. Li, P. Song and A. Tong, Dyes Pigm., 2014, 108, 24–31 CrossRef CAS PubMed; (c) Y. Guan, L. Niu, Y. Chen, L. Wu, C. Tung and Q. Yang, RSC Adv., 2014, 4, 8360–8364 RSC; (d) X. Liu, W. Zhang, C. Li, W. Zhou, Z. Li, M. Yu and L. Wei, RSC Adv., 2015, 5, 4941–4946 RSC; (e) J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. H. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351–5358 CrossRef CAS PubMed.
- G. A. Reynolds and K. H. Drexhage, Opt. Commun., 1975, 13, 222–225 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00255a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |