A practical and novel “standard addition” strategy to screen pharmacodynamic components in traditional Chinese medicine using Heishunpian as an example†
Yubo Li,
Yuan Wang,
Bin Yang,
Yuming Wang,
Zhiguo Hou,
Aizhu Li,
Yanyan Xu,
Liang Ju,
Huanyu Wu and
Yanjun Zhang*
Tianjin State Key Laboratory of Modern Chinese Medicine, School of Traditional Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, 312 Anshan West Road, Tianjin 300193, China. E-mail: tianjin_tcm001@sina.com; Fax: +86-22-59596223; Tel: +86-22-59596223
First published on 18th February 2015
Abstract
The study of pharmacodynamic components in traditional Chinese medicine (TCM) is very important for future drug development and quality control of TCM. The present study established a new method for screening pharmacodynamic components (PCs) in TCM based on the strategy of standard addition (SA). The novel strategy was then applied to screen anti-inflammatory PCs in Heishunpian (HSP), the processed product of Aconitum carmichaeli Debx. We initially screened target components (TCs, possible PCs) by analyzing the correlation between UPLC-Q-TOF-MS fingerprints of chemical components and the anti-inflammatory efficacies of different HSP extracts. On the basis of spectrum-effect relationship (SER) analysis and TC determination, we added TC quantitatively into HSP extracts, evaluated the pharmacodynamic contribution ratios of TCs related to the original TCM using a mouse ear edema model, and compared them with the contribution ratio of the TC standard. The anti-inflammatory PCs of HSP were then defined. Results showed that hypaconitine, deoxyaconitine and chasmanine were anti-inflammatory PCs in HSP with positive relations with HSP efficacy. Thus, SA was used to systematically evaluate the effect of chemical ingredients in TCM. The proposed method presents simple operation, strong feasibility and reliability, and provides a new approach for screening PCs in TCM in a manner that highlights the complexity and multi-component effects of TCM.
1 Introduction
Traditional Chinese medicine (TCM) is an important area of study, especially in Asian countries, and has received increased attention and wider acceptance in recent decades because of its efficacy and minimal side effects.1–3 The chemical components in TCM that are responsible for its clinical effects are called pharmacodynamic compounds (PCs). In contrast to single-component chemical drugs, PCs of TCM comprise a complex system with multiple components. Therefore, identifying the PCs of TCM can ensure the safety and efficacy of herbs and their derivatives. However, considering the large number of components of TCM and their various mechanisms of action, studies on PCs in TCM have been relatively few, which has greatly hindered TCM modernization, industrialization, and globalization.4–6Current strategies for studying PCs in TCM include separation of the individual chemical components and evaluation of each component's activity.7,8 This strategy, however, disregards the entirety and systematic action of TCM and fails to reflect the characteristics of multi-components and multi-targets in TCM. An alternate strategy involves pharmacological activity-oriented tracking,7 in which the efficacy of each component obtained from each stage of separation is evaluated. Unfortunately, this method is labor-intensive and ignores the wholeness and systematic mode of action of TCM. Another strategy involves computer-aided virtual screening,9 which predicts PCs using computer-based algorithms. This approach, however, relies on programmed models and specialized databases, and the reliability and accuracy of prediction results require further validation. Finally, spectrum-effect analysis10,11 combines pharmacodynamic activity and chemical fingerprints with a mathematical model to screen PCs. However, this method only reflects the relationship between ingredients and pharmacodynamics and also requires further validation.
Taking reference to the mode of “gene knock-out” or “gene deletion”, Li and Xiao recently established a target constituent knock-out/knock-in strategy wherein the target component (TC) in the herbs was eliminated by a specific technology.12,13 PCs were screened by comparing pharmacological activities among TCs, negative samples, and whole herbs. This approach offers higher accuracy and takes the complex environment of TCMs into account.14,15 However, its requires a rapid separation technology with good extraction efficiency, high yield, and no residues to be eliminated.16 Hence, this strategy requires a higher technology condition. Therefore, there is an urgent need for a strategy with general applicability based on the characteristics of TCM to be established.
The recovery test, which involves adding TC standards directly to samples, is used to examine the accuracy of component determination.17 Using this principle, the present article established a “standard addition (SA)” strategy to screen PCs that takes into account the entirety of components in TCM. Research on anti-inflammatory PCs in Heishunpian (HSP, the processed product of Aconitum carmichaeli Debx) was taken as an example. First, the correlation between the fingerprints and efficacy of different HSP extracts was analyzed. In this step, TCs were preliminarily identified. Next, the contents of TCs in different extracts were measured, and the content range was calculated. Finally, the TC standard was added to TCM extracts, and the contribution ratios of TCs were assessed. The ingredient that could significantly change the effect of TCM and contributed greatly to the effect of the whole TCM was considered to be a PC. This strategy allows accurate PC screening and embodies the systematic nature of TCM. Thus, this paper introduces a novel method for investigating the PCs of TCM.
2 Experiments
2.1 Chemicals and materials
10 batches of Heishunpian (HSP, the processed product of Aconitum carmichaeli Debx) were purchased from specialized companies and medicine markets, and authenticated by Dr Lu Zhang at Tianjin University of Traditional Chinese Medicine (Tianjin, China). These samples were sealed and deposited at the specimen laboratory in the School of Traditional Chinese Materia Medica, Tianjin University of Traditional Chinese Medicine, China, at room temperature. Keep dry indoor to avoid samples becoming damp. Because HSP is a sort of toxic herb, we store it separately from others so as not to interfere with them. After identifying and analyzing the relative content of chemical components in each batches of HSP, we selected one batch to use in subsequent experiments at last. The batch selected can stand for the majority of market HSP and was processed by specialized company of Chinese herbal medicine. It was bought from Hua Miao Engineering Technology Development Center of Traditional Chinese Medicine (Beijing, China), (no. 305231). The basis of selecting is shown in ESI.† Four standards, namely, chasmanine, mesaconitine, hypaconitine, and deoxyaconine, were purchased from Chengdu Herbpurify Co., Ltd. (Chengdu, China). Purity of all the standards was more than 98% as determined by HPLC. HPLC-grade acetonitrile, formic acid, and alcohol for UPLC-Q-TOF-MS analysis were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other analytical grade chemicals, such as methanol and absolute ethyl alcohol, were obtained from Concord Science and Technology Co., Ltd. (Tianjin, China). Ultrapure water was produced using a Milli-Q system (Millipore, Bedford, MA, USA).2.2 Animals
Male Kunming mice (SPF, 20 ± 2 g) were provided by the Military Academy of Medical Sciences Laboratory Animal Center (Beijing, China) and raised in the Institute of Radiation Medicine Chinese Academy of Medical Sciences (Tianjin, China). All mice were fed with standard laboratory food and water ad libitum for at least 5 days prior to the experiments in an environmentally controlled room (12 h/12 h dark/light cycle; 25 ± 1 °C, 50 ± 5% humidity). The study was approved by the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine under permit number TCM-2013-12-A06. All procedures were conducted in accordance with the Chinese national legislation and local guidelines.2.3 Instruments and UPLC-Q-TOF-MS conditions
A Waters Acquity UPLC Class I series (Premier from Waters) setup equipped with a quat pump, an autosampler, a DAD detector, and a column compartment was used for analysis. Samples were separated on a Waters ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 µm; Waters, USA). The mobile phases contained water with 0.1% formic acid (eluent A) and acetonitrile with 0.1% formic acid (eluent B) at a flow rate of 0.3 mL min−1. The following linear gradient was applied: 0–2 min, 1% B; 2–5 min, 1–7% B; 5–15 min, 7–10% B; 15–20 min, 10–20% B; 20–31 min, 20–30% B; 31–36 min, 30–50% B; and 36–38 min, 50–99% B. The column temperature was maintained at 40 °C, and the sample injection volume was 2 µL.A Waters Xevo G2 Q-Tof setup was connected to the Waters Acquity UPLC Class I system via an electrospray ionization interface. Ultra-high purity helium (He) was used as the collision gas, and high-purity nitrogen (N2) was used as the nebulizing gas. Data were collected in positive ion mode via full-scan mass analysis from m/z 100 to m/z 1000. Other operating parameters were as follows: capillary voltage, 3.0 kV; drying gas temperature, 350 °C; flow rate, 800 L h−1; and nebulizing gas pressure, 35 psi. The Leu-enkephalin ion at m/z 556.2771 was used to calibrate mass accuracy.
2.4 Spectrum-effect relationship analysis for initial screening of target components
| I = (Dc − Dt)/Dc × 100% |
2.5 Quantitative analysis of target components in different extracts
2.6 Screening of pharmacodynamic components by standard addition
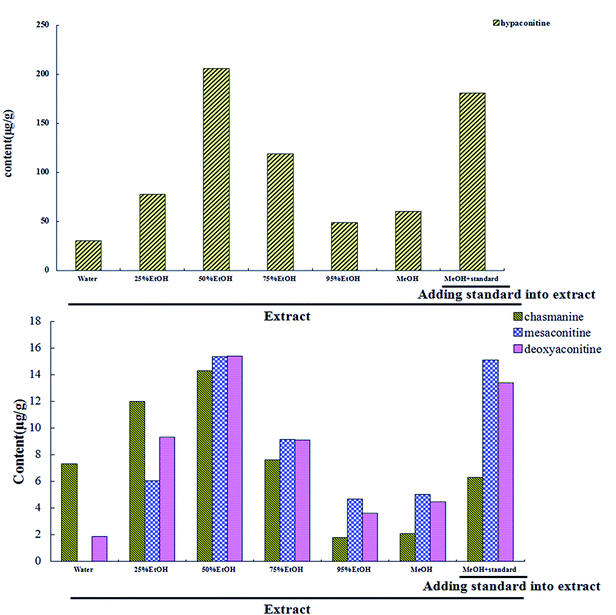
Background drug. According to the results of quantitative analysis of TCs in different extracts, we selected methanol extract as the background drug.
TC standard. Each standard (chasmanine, mesaconitine, hypaconitine and deoxyaconitine) was dissolved to 1 mg mL−1 and further diluted to the appropriate concentration with water at the required time.
SA drug. We ensured that the content of TCs was maintained within the usage range of the SER model after addition. Referring to the dose escalation scheme of previous pharmacological experiments,21 we added twice the amount of the TC standard to background drug, respectively.
3 Result and discussion
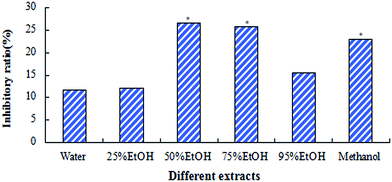
3.1 Spectrum-effect relationship analysis to screen target components
Different inherent components of TCM contribute different clinical effects. However, the relationship between the efficacy and chemical compositions of TCM is still vague. SER defines the link between chemical fingerprints and specific efficacy data by using multivariate statistical tools.22 This method combines both chemical analysis and pharmacological studies, and can be used to screen the PCs of TCM.23 SER has recently been widely used for screening PCs of TCM.10,11,23,24 In this study, we chose to investigate the inflammatory TCs of HSP by the SER method.| No. | RT (min) | [M + H]+ | Identification |
|---|---|---|---|
| 1 | 5.33 | 394.2593 | Columbianine |
| 2 | 6.95 | 486.2701 | Mesaeonine |
| 3 | 6.52 | 424.2687 | Senbusine B |
| 4 | 7.47 | 378.2643 | Carmichaeline |
| 5 | 7.68 | 408.2741 | Isotalatizidine |
| 6 | 8.11 | 358.238 | Songorine |
| 7 | 8.71 | 330.2066 | Hetisine |
| 8 | 10.73 | 454.2803 | Fuziline |
| 9 | 11.91 | 438.2854 | Neoline |
| 10 | 12.08 | 342.1692 | Fuzitine |
| 11 | 13.93 | 344.2586 | Bullatine A |
| 12 | 15.29 | 422.2894 | Talatizamine |
| 13 | 18.06 | 452.3002 | Chasmanine |
| 14 | 19.66 | 464.3007 | 14-Acetyltalatizamine |
| 15 | 19.86 | 606.2925 | 10-OH-Benzoylmesaeonine |
| 16 | 22.57 | 590.2958 | Benzoylmesaconine |
| 17 | 23.86 | 604.3152 | Benzoyaeonine |
| 18 | 24.9 | 574.3009 | Benzoylhypaconine |
| 19 | 26.43 | 588.3168 | Dehydration-10-OH-benzoylmesaeonine |
| 20 | 28.87 | 632.3055 | Mesaconitine |
| 21 | 29.17 | 662.3168 | 10-OH-Aeonitine |
| 22 | 31.15 | 616.3127 | Hypaconitine |
| 23 | 31.35 | 572.3218 | Dehydrated benzoylmesaconine |
| 24 | 32.99 | 602.3331 | Dehydrated-10-OH-aeonitine |
| 25 | 33.37 | 630.3278 | Deoxyaconitine |
By conducting PLS regression analysis, we established a relationship between HSP fingerprints and its inhibitory effect against xylene-induced ear edema in mice, and identified the TCs. Depending on VIP value, chasmanine, mesaconitine, hypaconitine, deoxyaconitine, columbianine, and mesaconine were screened as TCs of HSP.
Many components, including several high content components, have not been screened by SER to date. Our results indicated that the change of efficacy might not have been caused by change of these components. Many studies have found that diester-diterpenoid alkaloids (DDAs) in HSP display anti-inflammatory activity, although the contents of such compounds are usually lower. However, very few reports exist on monoester-diterpenoid alkaloids (MDAs), which are more abundant.34,35 Using paw swelling in mice and other inflammatory models, Nesterova et al. also showed that mesaconitine, hypaconitine, and other ingredients displayed evident anti-inflammatory actions.36–38
Therefore, the results of SER can be believed to some extent. The anti-inflammatory effects of these higher content components will be investigated in future experiments. However, multivariate statistical methods are artificially defined algorithms with fixed principles and formulas. The chemical components and pharmacological effects of TCM are very complex. Multivariate statistical techniques may not be comprehensive enough to describe the relationship between the chemical components and pharmacological effects of TCM. That is to say, due to the limitations of statistical techniques and complication in chemical compounds and action mechanism of TCM, SER does have some disadvantages. For this reason, we only selected SER as a way to screen TCs initially, and proposed the SA technique to further confirm SER results.
3.2 Quantitative analysis of target components in different extracts
As we know, the action of an ingredient is related to its content. Therefore, it is necessary to clear the content range of TCs. Moreover, determining the content of TCs in HSP is the foundation of an SA strategy. Here, we determined four TCs with large VIP values and defined the usage range of the model established by SER analysis.3.3 Screening of pharmacodynamic components by standard addition
PCs of TCM are important in clinical treatment and form the basis of many TCM studies. Whether a TC is an anti-inflammatory PC of HSP must be verified. In order to confirm the TCs screened by SER, we added each TC standard to the original extract of HSP, and then evaluated the activity of the original extract and TC with extract simultaneously. After clarifying the influence of each TC to overall HSP, we confirmed the PCs of HSP.| Ca = (Ia − I)/I/n |
| Cs = (Is − I)/I/n |
The efficacy of TCs is shown in Table 2. The consequences were the mean of 3 repeated experiments, which errors were less than 15%. Mesaconitine, hypaconitine, and deoxyaconitine in the standard solution group could significantly inhibit xylene-induced ear edema in mice (P < 0.05); by contrast, chasmanine showed no such inhibition. Each SA group showed significant inhibition of ear edema (P < 0.05). Mesaconitine, hypaconitine, and deoxyaconitine alone exhibited positive contributions to the anti-inflammatory action, whereas chasmanine alone showed no such contribution. After addition, hypaconitine, deoxyaconitine, and chasmanine showed positive contributions to the anti-inflammatory action, whereas mesaconitine showed negative contribution.
| Group no. | Dose | Weight difference (g) | Inhibition ratio (%) | Contribution ratio (%) |
|---|---|---|---|---|
| a Values are expressed as mean ± S.E.M. There are 10 animals in each group, and this experiment was repeated 3 times. *P < 0.05, compared with control, **P < 0.01, compared with control; #P < 0.05, compared with HSP (SPSS 17.0, independent t-test).b HSP: Heishunpian, the processed product of Aconitum carmichaeli Debx.c Inhibitory ratio (I), I = (Dc − Dt)/Dc × 100%, where Dc and Dt represent the degrees of swelling of the control and test groups, respectively.d Contribution ratio (C) of TCs: Ca = (Ia − I)/I/n, Cs = (Is − I)/I/n, where Ca and Ia are the contribution and inhibitory ratios, respectively, of SA groups (Group no. 7, 8, 9, 10), while Cs and Is are the contribution and inhibitory ratios, respectively, of standard control groups (Group no. 3, 4, 5, 6); I is the inhibitory ratio of the background drug group (Group no. 2), and n is the content multiple of addition. | ||||
| 1 | Control | 0.01320 ± 0.001564 | — | — |
| 2 | HSP | 0.009158 ± 0.001983** | 30.62 | — |
| 3 | Chasmanine | 0.01357 ± 0.001345 | −2.78 | −4.54 |
| 4 | Mesaconitine | 0.009880 ± 0.001911** | 25.15 | 41.07 |
| 5 | Hypaconitine | 0.01083 ± 0.001009* | 17.97 | 29.33 |
| 6 | Deoxyaconitine | 0.01085 ± 0.001791* | 17.80 | 29.07 |
| 7 | HSP + chasmanine | 0.008200 ± 0.001121** | 37.88 | 11.85 |
| 8 | HSP + mesaconitine | 0.01020 ± 0.001441* | 22.73 | −12.89 |
| 9 | HSP + hypaconitine | 0.006643 ± 0.002535**# | 49.68 | 31.11 |
| 10 | HSP + deoxyaconitine | 0.008750 ± 0.002203** | 33.71 | 5.05 |
Hypaconitine alone showed significant anti-inflammatory effects, and addition of hypaconitine to the original HSP extract significantly enhanced its efficacy (P < 0.05) and contribution ratio in the entire TCM environment. Hypaconitine can thus be regarded as one of the anti-inflammatory PCs of HSP. Chasmanine alone did not significantly inhibit mice ear swelling, whereas deoxyaconitine alone did. However, when both components were added directly, HSP efficacy increased. This result revealed a significant relation of these components with efficacy, and the two components may also be regarded as anti-inflammatory PCs. Mesaconitine alone could inhibit mice ear swelling significantly, but the inhibition ratio was reduced when the component was quantitatively added to the extract. This result showed that mesaconitine was negatively correlated with anti-inflammatory effects and could not be an anti-inflammatory PC of HSP.
Thus, the results showed that the overall complex environment of TCM greatly impacted the action of individual components. Overall efficacy changes may be caused by synergism or antagonism among the complex chemical components of TCM during absorption, distribution, metabolism, and excretion. The SA strategy employed in this work revealed that hypaconitine, deoxyaconitine, and chasmanine can be identified as anti-inflammatory PCs in HSP but mesaconitine can not.
In our work, the TCs were screened by SER, and the SA method was used to verify the results. We found that some TCs, such as mesaconitine, may not be PCs. The SA results obtained in our experiments may be more reliable. We also compared the SA and the approach that separated individual chemical components and evaluated each component activity. As we know, the references were obtained from the separation of special factories or research institutes. After evaluating their activity, we found that individual chemical components cannot represent the whole extracts of TCM. Therefore, research on TCM should not ignore its wholeness and system.
Li et al. proposed “target constituent knock-out” strategy to screen PCs in TCM. This approach completely considers the complex environment of TCM and interaction of the bioactive components in TCM.12,13 To confirm our results, we also tried to isolate mesaconitine from the HSP as much as possible and compared the anti-inflammatory efficacy of mesaconitine sample, negative sample (lacking mesaconitine) and overall HSP. However, we encountered some problems when isolating mesaconitine using the existing separation or analysis techniques. We found that some chemical components of HSP were unsteady, and their transformation, hydrolysis and pyrolysis took place easily. According to the literature, the chemical components of HSP can be divided into several types, namely, DDAs, MDAs, alkylolamine-diterpenoid alkaloid (ADAs), lipo-alkaloids (LDAs) and other type of alkaloids. DDAs are unsteady and can be easily turned into other compounds; while mesaconitine belonged to DDAs.38–41 Removing some of the unstable compounds was difficult. Thus, it appears that the “target components knock-out strategy” is more suitable for studying Chinese medicines whose chemical compositions are relatively stable and simple. It may not be suitable for TCMs containing complex unstable ingredients. The SA strategy that we proposed for screening PCs also embodies the wholeness and system of TCM and effectively avoids the difficulty in removing TCs. To a certain degree, the SA strategy makes up for the “target components knock-out” strategy; it can be used to screen PCs of TCM with complex unstable compounds.
In order to systematically screen PCs of HSP, we will try to use other methods to isolate mesaconitine in future studies. To avoid mesaconitine turning into other compounds, the operational condition, such as the temperature and polarity of the solution, must be strictly controlled during the experiment. Because the purity of removed samples and the completeness of negative samples must be ensured, accurate identification of mesaconitine and the negative sample must be done.
4 Conclusions
We established a practical and novel SA strategy to study PCs in TCM. SA can systematically evaluate the effect of chemical ingredients in the whole TCM and represent the complexity and multi-component effects of TCM. In addition, SA features simple operation, strong feasibility and reliability, and applies to most TCM or its preparation. PC determination in TCM has great significance for future drug development and quality control of TCM. The SA strategy not only makes up for the deficiencies of other methods but also solves critical issues of PC identification. Thus, the present work provides a novel method for studying PCs in TCM.Acknowledgements
This project was supported by the National Basic Research Program of China (973 Program) (2011CB505300, 2011CB505302) and the National Undergraduate Training Programs for Innovation and Entrepreneurship of China.Notes and references
- T. H. Xue and R. Roy, Science, 2003, 300, 740 CrossRef CAS PubMed.
- P. Li, L. W. Qi, E. H. Liu, J. L. Zhou and X. D. Wen, Trends Anal. Chem., 2008, 27, 66 CrossRef CAS PubMed.
- D. Normile, Science, 2003, 299, 188 CrossRef CAS PubMed.
- J. C. Tilburt and T. J. Kaptchuk, Bull. W. H. O., 2008, 86, 594 CrossRef.
- R. Stone, Science, 2008, 319, 709 CrossRef CAS PubMed.
- D. A. Guo, A. P. Lu and L. Liu, J. Ethnopharmacol., 2012, 141, 547 CrossRef PubMed.
- X. D. Huang, L. Kong, X. Li, X. G. Chen, M. Guo and H. F. Zou, J. Chromatogr. B, 2004, 812, 71 CrossRef CAS PubMed.
- Y. Jiang, B. David, P. F. Tu and Y. Barbin, Anal. Chim. Acta, 2010, 657, 9 CrossRef CAS PubMed.
- B. K. Shoichet, Nature, 2004, 432, 862 CrossRef CAS PubMed.
- W. J. Kong, Y. L. Zhao, X. H. Xiao, J. B. Wang, H. B. Li, Z. L. Li, C. Jin and Y. Liu, Anal. Chim. Acta, 2009, 634, 279 CrossRef CAS PubMed.
- C. Tistaert, B. Dejaegher, N. Nguyen Hoai, G. Chataigné, C. Rivière, V. Nguyen Thi Hong, M. Chau Van, J. Quetin-Leclercq and Y. Vander Heyden, Anal. Chim. Acta, 2009, 649, 24 CrossRef CAS PubMed.
- Y. Liu, J. L. Zhou, P. Liu, S. Sun and P. Li, J. Chromatogr. A, 2010, 1217, 5239 CrossRef CAS PubMed.
- W. J. Kong, J. B. Wang, Q. C. Zang, C. Jin, Z. W. Wang, X. Y. Xing, Y. Y. Wu, Y. L. Zhao, M. H. Yang and X. H. Xiao, J. Chromatogr. B, 2011, 879, 3565 CrossRef CAS PubMed.
- L. Y. Dong, Y. Luo, B. F. Cheng, Y. S. Zhang, N. Zhang, Y. Y. Hou, M. Jiang, G. A. Luo and G. Bai, Biomed. Chromatogr., 2013, 27, 960 CrossRef CAS PubMed.
- T. Uto, I. Tuvshintogtokh and Y. Shoyama, Curr. Drug Discovery Technol., 2011, 8, 16 CrossRef CAS.
- D. Yan, J. X. Li, Y. Xiong, C. G. Zhang, J. Y. Luo, Y. M. Han, R. L. Wang, C. Jin, H. Qian, J. G. Li, L. L. Qiu, C. Peng, Y. L. Lin, X. A. Song and X. H. Xiao, Sci. Rep., 2014, 4, 3668, DOI:10.1038/srep03668.
- H. Wei, L. N. Sun, Z. G. Tai, S. H. Gao, W. Xu and W. S. Chen, Anal. Chim. Acta, 2010, 662, 97 CrossRef CAS PubMed.
- J. H. Shang, X. H. Cai, T. Feng, Y. L. Zhao, J. K. Wang, L. Y. Zhang, M. Yan and X. D. Luo, J. Ethnopharmacol., 2010, 129, 174 CrossRef CAS PubMed.
- H. Hosseinzadeh, M. Khoshdel and M. Ghorbani, J. Acupunct. Meridian Stud., 2011, 4, 242 CrossRef PubMed.
- D. Z. Yang, Y. Q. An, X. L. Jiang, D. Q. Tang, Y. Y. Gao, H. T. Zhao and X. W. Wu, Talanta, 2011, 85, 885 CrossRef CAS PubMed.
- S. Y. Xu, R. L. Bian and X. Chen, The method of pharmacology experiment, People's medical publishing house, Beijing, China, 2001 Search PubMed.
- G. L. Xu, M. Xie, X. Y. Yang, Y. Song, C. Yan, Y. Yang, X. Zhang, Z. Z. Liu, Y. X. Tian, Y. Wang, R. Jiang, W. R. Liu, X. H. Wang and G. M. She, Molecules, 2014, 19, 17897 CrossRef CAS PubMed.
- J. X. Wang, X. Tong, P. B. Li, M. H. Liu, W. Peng, H. Cao and W. W. Su, J. Ethnopharmacol., 2014, 155, 405 CrossRef CAS PubMed.
- J. Wang, H. W. Kong, Z. M. Yuan, P. Gao, W. D. Dai, C. X. Hu, X. Lu and G. W. Xu, J. Pharm. Biomed. Anal., 2013, 83, 57 CrossRef CAS PubMed.
- P. S. Xie, S. B. Chen, Y. Z. Liang, X. H. Wang, R. T. Tian and R. Upton, J. Chromatogr. A, 2006, 1112, 171 CrossRef CAS PubMed.
- W. J. Kong, Y. L. Zhao, X. H. Xiao, C. Jin and Z. L. Li, Phytomedicine, 2009, 16, 950 CrossRef CAS PubMed.
- S. Wold, M. Sjöström and L. Eriksson, Chemom. Intell. Lab. Syst., 2001, 58, 109 CrossRef CAS.
- B. S. Elif Seyma Bayrak, B. S. Kamuran Turksoy, P. D. Ali Cinar, P. D. R. N. Lauretta Quinn, M. D. Elizabeth Littlejohn and P. D. Derrick Rollins, J. Diabetes Sci. Technol., 2013, 7, 206 CrossRef PubMed.
- Y. Akimoto, K. Yugi, S. Uda, T. Kudo, Y. Komori, H. Kubota and S. Kuroda, PLoS One, 2013, 8, DOI:10.1371/journal.pone.0072780.
- S. Thiangthuma, B. Dejaegher, M. Goodarzi, C. Tistaert, A. Y. Gordien, N. Nguyen Hoai, M. Chau Van, J. Quetin-Leclercq, L. Suntornsuk and Y. Vander Heydena, J. Chromatogr. B, 2012, 910, 114 CrossRef PubMed.
- T. Mehmood, K. H. Liland, L. Snipen and S. Sæbø, Chemom. Intell. Lab. Syst., 2012, 118, 62 CrossRef CAS PubMed.
- R. Gosselin, D. Rodrigue and C. Duchesne, Chemom. Intell. Lab. Syst., 2010, 100, 12 CrossRef CAS PubMed.
- I. G. Chong and C. H. Jun, Chemom. Intell. Lab. Syst., 2005, 78, 103 CrossRef CAS PubMed.
- J. S. Wang, R. Heijden, S. Spruit, T. Hankermeier, K. Chan, J. Greef, G. W. Xu and M. Wang, J. Ethnopharmacol., 2009, 126, 31 CrossRef PubMed.
- J. Singhuber, M. Zhu, S. Prinz and B. Kopp, J. Ethnopharmacol., 2009, 126, 18 CrossRef PubMed.
- H. Hikino, H. Takata, M. Fujiwara, C. Konno and K. Ohuchi, Eur. J. Pharmacol., 1982, 82, 65 CrossRef CAS.
- Y. V. Nesterova, T. N. Povetieva, N. I. Suslov, G. N. Zyuz'kov, S. G. Aksinenko, S. V. Pushkarskii and A. V. Krapivin, Bull. Exp. Biol. Med., 2014, 156, 665 CrossRef CAS PubMed.
- M. Zhang, Y. Long, Y. Sun, Y. K. Wang, Q. Li, H. J. Wu, Z. J. Guo, Y. H. Li, Y. B. Niu, C. Li, L. Liu and Q. B. Mei, Eur. J. Pharmacol., 2011, 651, 187 CrossRef CAS PubMed.
- Y. Yang, X. J. Yin, H. M. Guo, R. L. Wang, R. Song, Y. Tian and Z. J. Zhang, Chin. J. Nat. Med., 2014, 12, 542 Search PubMed.
- H. Yue, Z. F. Pi, H. L. Li, F. R. Song, Z. Q. Liu and S. Y. Liu, Phytochem. Anal., 2008, 19, 141 CrossRef CAS PubMed.
- H. Sun, B. Ni, A. H. Zhang, M. Wang, H. Dong and X. J. Wang, Analyst, 2012, 137, 170 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00461f |
| This journal is © The Royal Society of Chemistry 2015 |